Research Article - Biomedical Research (2017) Volume 28, Issue 3
Marginatoxin induces human hepatoma BEL-7402 cells apoptosis in vitro and in vivo via activation of Fas/FasL-mediated apoptotic pathway
Xiang Hu1#, De He2#, Chenjie Zhou1, Guolin He1, Zhi Zhang1, Yuan Cheng1, Mingxin Pan1 and Yi Gao1*1Department of Hepatobiliary Surgery, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong, PR China
2Department of General Surgery, the Affiliated Baoan Hospital of Southern Medical University, Shenzhen 518101, Guangdong, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Yi Gao
Department of Hepatobiliary Surgery
Zhujiang Hospital, Southern Medical University, PR China
Accepted date: August 03, 2016
Abstract
Marginatoxin, an aryltetraline lactone lignan isolated from Bupleurum marginatum Wall. ex DC (Apiaceae), showed cytotoxic activity against human hepatocellular carcinoma. However, the cytotoxic mechanisms of marginatoxin against cancer cells remained unclear. This study was designed to investigate the detailed mechanism of its cytotoxic effect on human hepatoma BEL-7402 cells by CCK-8, flow cytometry, western blot and xenograft assays. Marginatoxin caused a dose-dependent growth inhibition of BEL-7402 cells with an IC50 of 15.20 μg/ml. Marginatoxin induced BEL-7402 cells apoptosis identified by annexing V/propidium iodide staining kits with the aid of flow cytometry. Marginatoxin-induced apoptosis was characterized by increasing Fas, FasL and Fas associated protein with death domain expression, and cleavage of caspase-8, caspase-10, caspase-3. Furthermore, the results of xenograft assay suggested that marginatoxin inhibited the BEL-7402 cells-induced tumor growth without effect on body weight of nude mice in vivo by regulating the expression levels of above apoptotic proteins. These results demonstrated that marginatoxin could induce apoptosis of human hepatoma BEL-7402 cells in vitro and in vivo through activation of Fas/FasL-mediated apoptotic pathway. All these findings demonstrated that marginatoxin may be a promising chemopreventive agent for treating hepatocellular carcinoma. Moreover, there is a need to further investigate marginatoxin in this regard.
Keywords
Marginatoxin, Apoptosis, Human hepatoma BEL-7402 cells, Fas/FasL-mediated apoptotic pathway.
Introduction
Liver cancer is the second biggest cause of mortality in the globe [1]. Hepatocellular carcinoma (HCC) occupies up to 90% of all primary liver cancers worldwide [2], and is also one of the four most prevalent malignant diseases in China, Korea, and sub-Africa [3,4]. Surgical treatments, such as liver resection and liver transplantation, are the first-line therapeutic protocols for HCC patients [5]. Reports say that such curative treatment can extend median survival times around 60 months, and patients are prone to show disease recurrence after surgical treatments, and therefore, the postoperative survival rate is not quite promising [6]. Unfortunately, most HCC patient are often diagnosed at a late stage when surgical treatments are least effective, and however chemotherapy has an unique advantage in the treatment of patients with late-stage HCC [7,8]. Due to the development of drug resistance in the treatment of HCC, it is necessary to develop novel chemotherapy drugs for the treatment of HCC. In the past decades, traditional Chinese medicine and their active ingredients have attracted much attention as candidates for the treatment of HCC [9].
Bupleurum marginatum Wall. ex DC (Apiaceae) under the name of "Chaihu", a traditional Chinese medicine which is widely distributed in the southwest of China, can be used to treat cancer, hepatitis, microbial inflections, common cold, malaria, menstrual disorder and so on [10-12]. It was reported that B. marginatum had anti-hepatoma activity, and marginatoxin, an aryltetraline lactone lignan isolated from the aerial part of B. marginatum, showed an inhibitory effect on the proliferation of HepG2 with the IC50 values of 16.14 uM, indicating that marginatoxin may be one of the anti-hepatoma active ingredients of B. marginatum [12]. However, the cytotoxic mechanisms of marginatoxin against human hepatoma cells were still unknown. Therefore, this work was designed to investigate the in vitro and in vivo cytotoxic mechanisms of marginatoxin against human hepatoma BEL-7402 cells. The results of the study showed that the in vitro and in vivo cytotoxic mechanisms of marginatoxin against human hepatoma BEL-7402 cells were related to the activation of Fas/FasL-mediated apoptotic pathway.
Materials and Methods
Reagents and chemicals
Dimethyl sulfide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM), penicillin and streptomycin were purchased from Gibco-BRL (Gaithersburg, MD, USA). Cell counting kit-8 (CCK-8 kit), annexin V-FITC, propidium iodide (PI), and enhanced BCA protein assay kits were obtained from Beyotime Biotechnology (Haimen, China). Primary antibodies against β-actin, Fas, FasL, Fas associated protein with death domain (FADD), cleaved-caspase-8 (ccaspase- 8), c-caspase-10 and c-caspase-3 were purchased from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)- conjugated goat anti-rabbit antibody was obtained from Cell Signaling Technology (Beverly, MA, USA).
Isolation of marginatoxin
B. marginatum were purchased from Chengdu Hehuachi Chinese Herbal Medicine Market (Chengdu, China) in 2014 and identified by Yi Gao. A voucher specimen (no. ZJHSMU2014058) was stored in Zhujiang Hospital, Southern Medical University for future reference.
Marginatoxin (Figure 1) was obtained from the air dried aerial parts of B. marginatum through extraction with methanol, chromatography on a silica gel column and recrystallization in n-hexane: ethyl acetate mixture. Marginatoxin was identified by Nuclear Magnetic Resonance (NMR) and Mass Spectrum (MS), and the NMR and MS data corresponded with those in the literatures [12,13].
Cell culture
The HCC cell line BEL-7402 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). BEL-7402 cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml of penicillin and 100 μg/ml of streptomycin in a humidified atmosphere containing 5% CO2 in air at 37ºC.
Cell proliferation assay
CCK-8 assay was conducted to evaluate the effect of marginatoxin on the proliferation of BEL-7402 cells. In brief, cells were seeded on 96-well plates. After incubation for 4 h, BEL-7402 cells were treated with marginatoxin at the doses of 0, 2, 5, 10, 15, 20, 25 and 30 μg/ml for 48 h, and the group treated with 0 μg/ml marginatoxin was regarded as the control group. Then CCK-8 solution was added into every well, followed by incubation for 4 h. The optical densities (OD) were determined at 450 nm using Bio-Rad Model 680 microplate reader (Hercules, CA, USA). The cytotoxic effect of marginatoxin on BEL-7402 cells was evaluated by the inhibition rate, calculated as in Equation 1.
Inhibition rate (%) = [(OD control - OD treatment)/OD control] × 100 → (1)
Apoptosis detection
According to the manufacturer’s instructions, cells undergoing apoptosis were identified by an annexin V-FITC and PI staining kits. During the induction of apoptosis, phosphatidylserine, which is normally located in the inner leaflet of cell membranes [14], is exposed on the cell surface. Then annexin V binds to phosphatidylserine and can be used to distinguish the earliest stages of apoptosis. PI, which cannot enter cells with intact membranes, is used to tell late apoptotic cells from early apoptotic cells [15]. After treatment with marginatoxin (0, 5, 10 and 20 μg/ml) for 48 h, BEL-7402 cells were harvested, washed with Phosphate-Buffered Saline (PBS) solution, and then resuspended in ice-cold binding buffer. Annexin V-FITC and PI solutions were added and then incubated for 30 min in the dark. Stained cells were analyzed by flow cytometry.
Western blot
After treated with marginatoxin (0, 5, 10 and 20 μg/ml) for 48 h, cells were washed thrice with cold PBS and lysed in lysis buffer. The above lysates were centrifuged at 12000 rpm for 5 min at 4ºC to obtain total protein, and then the total protein concentrations were determined by the enhanced BCA protein assay kit. About 40 μg of total proteins were resolved by 12% SDS-PAGE, followed by transferring to the PVDF membrane. After the membrane was blocked in Tris-buffered saline plus Tween 20 (TBS-T) containing 5% fat-free milk, the membrane was incubated with a selected primary antibody and then with HRP-conjugated goat anti-rabbit antibody. Immunoreactive proteins were detected by chemiluminescence.
Xenograft assay
Sixteen nude mice (5-6 weeks old, 20 ± 2 g) were obtained from SLRC Laboratory Animal Company (Shanghai, China) and were used for in vivo studies. The mice were housed and well maintained. All animal treatments were strictly consistent with international ethical guidelines and national institutes of health guide concerning the care and use of laboratory animals [16]. Mice received food and water ad libitum.
Nude mice were randomly assigned into either control or marginatoxin group. BEL-7402 cells suspension (2 × 106 cells/ nude mouse) was subcutaneously injected in the right flank of each mouse. When the BEL-7402 cells-induced tumor grew to appropriate diameter (2-3 mm), the nude mice in the control and marginatoxin group were orally administrated with 0.5% DMSO and 40 mg/kg marginatoxin once a day for 20 days, respectively. Tumor dimensions (width and length) and body weights of nude mice were measured on days 5, 10, 15 and 20 using vernier caliper and electronic balance. Then, the mice of 40 mg/kg marginatoxin group were euthanized, and their tumor tissues were collected for western blot. The tumor volume was calculated as in Equation 2.
Tumor volume (mm3) = width2 (mm2) × length (mm) × 0.5 → (2)
Statistical analysis
All experiments were done in triplicate and the data are expressed as mean ± standard deviation. Differences among different groups were analyzed by one-way analysis of variance (ANOVA) with the aid of SPSS 21.0 software. Difference was considered as statistically significant at P<0.05.
Results
Marginatoxin inhibits proliferation of BEL-7402 cells
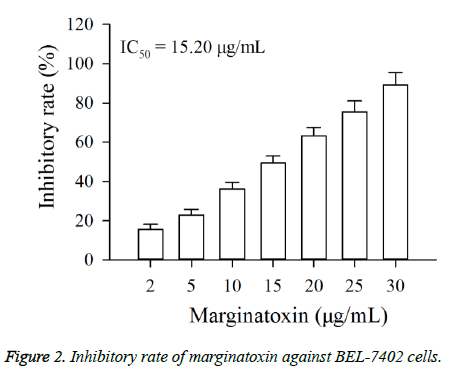
The cytotoxic effect of marginatoxin on BEL-7402 cells was evaluated by CCK-8 assay. As shown in Figure 2, exposure of BEL-7402 cells to increasing concentrations (2, 5, 10, 15, 20, 25 and 30 μg/ml) of marginatoxin for 48 h leaded to a dosedependent growth inhibition. The IC50 value at the 48 h incubation was 15.20 μg/ml.
Induction of apoptosis by marginatoxin on BEL-7402 cells
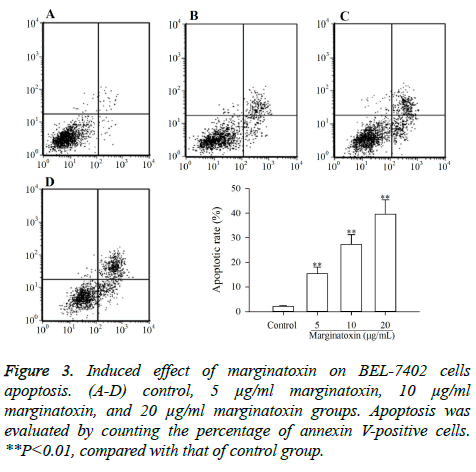
To investigate whether the cytotoxic effect of marginatoxin on BEL-7402 cells was caused by apoptosis, BEL-7402 cells treated with marginatoxin (10, 20 and 40 μg/ml) for 48 h were stained with FITC-conjugated annexin V and PI kits and analyzed by flow cytometry. As depicted in Figure 3, the proportion of stained cells were increased with the increase of marginatoxin dose, and the apoptotic cells were significantly increased (P<0.01), compared with those of control group, indicating that the cytotoxic effect of marginatoxin on BEL-7402 cells was related to apoptosis.
Marginatoxin induces activation of caspase-8, -10, and -3, and up-regulation of Fas, FasL and FADD
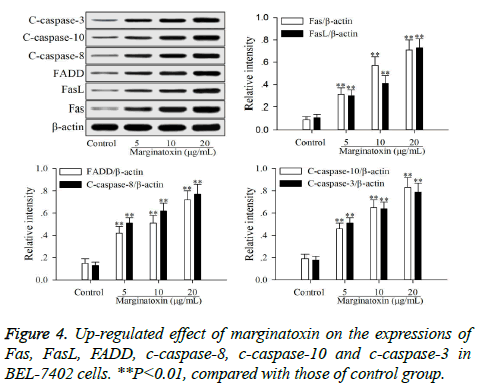
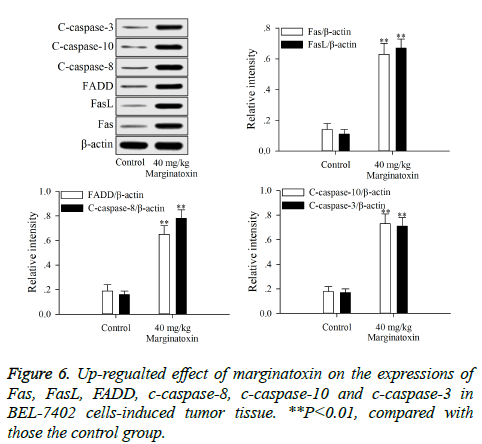
To investigate the effect of matginatoxin on Fas/FasL-mediated apoptotic pathway, the pro-apoptotic proteins (Fas, FasL, FADD, c-caspase-8 and c-caspase-10) and the executioner caspase (c-caspase-3) were investigated by western blot. After treatment with marginatoxin (10, 20 and 40 μg/ml) for 48 h, the expressions of Fas, FasL, FADD, c-caspase-8, c-caspase-10 and c-caspase-3 proteins were significantly (P<0.01) increased, compared with those of control group (Figure 4).
in vivo efficacy and mechanism of marginatoxin against BEL-7402 cells-induced tumor in nude mice
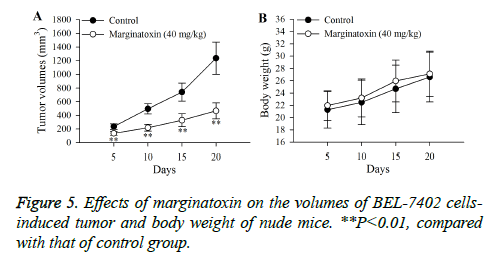
Xenograft assays was established to study the anti-tumor activity of marginatoxin on BEL-7402 cells-induced tumor in vivo. As shown in Figure 5, after administration of marginatoxin at 40 mg/kg for 20 days, the tumor volumes were significantly (P<0.01) decreased, compared with that of control group, and the body weight of nude mice was not affected by the administration of marginatoxin. As depicted in Figure 6, the western blot was conducted to investigate the mechanism of apoptotic pathway. The pro-apoptotic proteins (Fas, FasL, FADD, c-caspase-8 and c-caspase-10) and the executioner caspase (c-caspase-3) in the 40 mg/kg of marginatoxin group were significantly (P<0.01) increased, compared with those of control group.
Discussion
The present study demonstrates that marginatoxin, can not only induce the apoptosis of human hepatoma BEL-7402 cells in vitro, but also inhibit the BEL-7402 cells-induced tumor growth of nude mice in vivo. The mechanism of this action was related to the activation of the Fas/FasL-mediated pathway. The CCK-8 assay is used to verify the anti-proliferation effect of anticancer agents, and the flow cytometery is used to investigate whether the anti-proliferation effect is related to apoptosis and can also tell the percentage of apoptotic cells [17,18]. In this study, the results of CCK-8 assay indicated that marginatoxin showed significantly cytotoxic activity against BEL-7402 cells (Figure 2). Further, the results of flow cytometery showed that the cytotoxic activity was related to apoptosis and the percentage of apoptotic cells was increased with the increase of the marginatoxin dose (Figure 3).
Stimulation of Fas has been exhibited highly effective in the inhibition of tumor cells [19]. At the cell surface, Fas receptor binding to its ligand FasL induces receptor oligomerization and the formation of the death signaling complex, which contains the adaptor protein FADD [20]. The N terminal of FADD forms the death effector domain (DED), and the DED-DED inter-action leads to the auto-cleavage and activation of caspase-8 and -10 [21,22]. A large amount of active caspase-8 and -10 in turn activates downstream caspase-3, causing apoptosis [23]. The present study showed that the protein expressions of Fas, FasL and FADD were up-regulated, and caspase-8 and -10, -3 were activated with marginatoxin treatment (Figure 4). These results suggest that a Fas/FasLmediated apoptotic pathway might play a critical role in marginatoxin-induced apoptosis in BEL -7402 cells.
Xenograft, an accepted model, is used to study the effects of anticancer agents on the cancer cells-induced tumor and possible mechanisms in vivo [24,25]. The results of xenograft assay (Figures 5 and 6) indicated that maginatoxin significantly inhibited the growth of BEL-7402 cells-induced tumor without effect on the body weight of nude mouse. The mechanism of this inhibitory effect was investigated by western blot. The results of western blot showed that the mechanism of this action was related to the activation of the Fas/FasL-mediated apoptotic pathway. The protein expressions of Fas, FasL and FADD were up-regulated, and the caspase-8 and -10, -3 were activated with maginatoxin treatment. These results suggest that a Fas/FasL-mediated apoptotic pathway might play a critical role in marginatoxin-induced apoptosis in BEL-7402 cells-induced tumor in vivo.
Conclusion
In the study, we concluded that the molecular mechanism during marginatoxin-mediated growth inhibition of human hepatoma BEL-7402 cells was related to induction of apoptosis and initiation of Fas/FasL-mediated apoptotic pathway. Furthermore, the in vivo assay suggested that maginatoxin could significantly inhibit the growth of BEL-7402 cellsinduced tumor without effect on the body weight of nude mouse by activating Fas/FasL-mediated apoptotic pathway. All these findings demonstrated that marginatoxin may be a promising chemopreventive agent for treating HCC. Moreover, there is a need to further investigate marginatoxin in this regard.
References
- Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N A 2015; 24: 1-17.
- Llovet MJ, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015; 12: 408-424.
- Kern MA, Breuhahn K, Schirmacher P. Molecular pathogenesis of human hepatocellular carcinoma. Adv Cancer Res 2002; 86: 67-112.
- Hsu YL, Kuo YC, Kuo PL, Ng LT, Kuo YH, Lin CC. Apoptotic effects of extract from Antrodia camphorata fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett 2005; 221: 77-89.
- Qi F, Inagaki Y, Gao B, Cui X, Xu H, Kokudo N, Li A, Wei Tang. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci 2011; 102: 951-958.
- Aravalli RN, Steer CJ, Cressman ENK. Molecular mechanisms of hepatocellular carcinoma. Hematology 2008; 48: 2047-2063.
- Liu L, Cao YC, Chen C, Zhang XM, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006; 66: 11851-11858.
- Lovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveria AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med 2008; 359: 378-390.
- Tsuchiya M, Kono H, Matsuda M, Fujii H, Rusyn I. Protective effect of Juzen-taiho-to hepatocarcinogenesis is mediated through the inhibition of kupffer cell-induced oxidative stress. Int J Cancer 2008; 123: 2503-2511.
- Ashour ML, Wink M. Genus Bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol 2011; 63: 305-321.
- Ashour ML, EI-Readi MZ, Hamoud R, Eid SY, Ahmady SHE, Nibret E, Herrmann F, Youns M, Tarani A, Kaufmann D, Wink M. Anti-infective and cytotoxic properties of Bupleurum marginatum. Chin Med 2014; 9: 4.
- Ashour ML, EI-Readi MZ, Tahrani A, Eid SY, Wink M. A novel cytotoxic aryltetraline lactone form Bupleurum marginatum (Apiaceae). Phytochem Lett 2012; 5: 387-392.
- Jiang YB, Zhong M, Peng W. Qualitative analysis of plant-derived samples by liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry. Trop J Pharm Res 2015; 14: 925-930.
- Lifshitz Y, Levi L, Eyal I, Cohen T, Tessler S. Sub-chronic (13-week) oral toxicity study, preceded by an in utero exposer phase and genotoxicity studies with fish source phosphatidylserine in rats. Food Chem Toxicol 2015; 86: 234-244.
- Mou H, Zheng Y, Zhao P, Bao H, Fang W, Xu N. Celastrol induces apoptosis in non-small-cell A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol in Vitro 2011; 25: 1027-1032.
- The National research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals: Eight Edition. Washington, D.C.: The National Academies Press; 2010.
- Rahman MA, Dhar DK, Masunaga R, Yamanoi A, Kohno H, Nagasue N. Sulindac and exisulind exhibit a significant antiproliferative effect and induce apoptosis in human hepatocellular carcinoma cell lines. Cancer Res 2000; 60: 2085-2089.
- Wu JT, Lv SM, Lu CH, Gong J, An JB. Effect of 3,3’-biisofraxidin on apoptosis of human gastric cancer BGC-823 cells. Trop J Pharm Res 2015; 14: 1803-1811.
- Goto M. Evaluation of soluble Fas (APO-1, CD95) ligand in natural aging and Werner syndrome. Biosci Trends 2008; 2: 124-127.
- Nagata S, Golstein P. The Fas death factor. Science 1995; 267: 1449-1456.
- Wajant H. The Fas signaling pathway: more than a paradigm. Science 2002; 196: 1635-1636.
- Abd El-Ghany RM, Sharaf NM, Kassem LA, Mahran LG, Heikal OA. Thymoquinone triggers anti-apoptotic signaling targeting death ligand and apoptotic regulators in a model of hepatic ischemia reperfusion injury. Toxicol Lett 2009; 3: 296-306.
- Milhas D, Cuviller O, Therville N, Clavé P, Thomsmen M, Levade M, Benoist H, Ségui B. Caspase-10 triggers Bid cleavage and caspase cascade activation in FasL-induced apoptosis. J Biol Chem 2006; 280: 19836-19842.
- Liu H, Zhu Y, Zhang T, Zhao Z, Zhao Y, Cheng P, Li H, Gao H, Su X. Anti-tumor effects of atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules 2013; 18: 13357-13368.
- Zhu H, Zheng Z, Zhang J, Liu X, Liu Y, Yang W, Liu Y, Zhang T, Zhao Y, Liu Y, Su X, Gu X. Anticancer effect of 2, 7-dihydroxy-3-methylanthraquinone on human gastric cancer SGC-7901 cells in vitro and in vivo. Pharm Biol 2016; 54: 285-292.