Research Article - Biomedical Research (2017) Volume 28, Issue 6
Mandibular dental implant placement using demineralized bone matrix (DBM)
Ramón Fuentes1*, Diego Saravia1,2, Alain Arias1, Ruth Prieto3 and Fernando Dias1
1Research Centre in Dental Sciences (CICO), Dental School, Universidad de La Frontera, Temuco, Chile
2Universidad Adventista de Chile, Chillán, Chile
3Department of Pediatrics, School of Medicine, Universidad de La Frontera, Temuco, Chile
- *Corresponding Author:
- Ramón Fuentes
Dental School, Universidad de La Frontera
01145 Avendia Fransisco Salazar, Temuco, Chile
E-mail: ramon.fuentes@ufrontera.cl
Accepted date: November 07, 2016
Abstract
Dental implant has proven to be a reliable long-term rehabilitation treatment showing considerable success rate and it is known that its stability depends on the bone quantity and quality present at the treated site. Different biomaterials, classified according to their origin, are used to obtain these bone features. The aim of this study was described the histological characteristics of alveolar bone that received demineralized bone matrix (DBM) in patients with atrophic mandibular ridge and adequate alveolar height; and briefly report the clinical features of the dental implants placement in these treated sites. Five mandibular sites were evaluated by clinical and histological analysis in two adult patients without systemic pathologies. Both patients received DBM 6 months before the placement of dental implants. The bone samples analyzed were obtained in the preparation phase for implant placement. A quantitative analysis of collagen type I and III density was also performed. The patients agreed to receive the proposed treatment, participate of this study and signed an informed consent. The dental implants showed no complications, no symptoms of pain or infection in clinical evaluations in the following weeks. Adequate bone height, none evidence of pathologies was observed radiographically. In the histological analysis were noted boundary lines suggesting binding of mature and newly formed bone tissue; the presence of mineralized tissue and increased osseous cell activity near DBM particles. The type I collagen fibers area was larger compared to the type III, it was noted tendencies of increase of type I and decrease of type III collagen near the DBM particles. The results suggest the DBM is a potential biomaterial when associated with dental implants in cases of atrophic mandible ridge.
Keywords
Bone graft, Dental implant, Demineralized bone matrix
Introduction
Endo-osseous dental implants have demonstrated to be a trustworthy and a long term rehabilitation therapy, showing more than 95% success rate in 5 years [1]. The treatment in this case is conditioned to the primary stability defined as the absence of implant mobility once insert in the bone tissue [2]. The primary stability also depends of the quantity and quality of the bone in the reception site [3] which may be drastically reduced after tooth loss [4]. In order to prevent or to recover the bone loss are used autologous bone and bone substitutes. These bone substitutes are classified, according to their origin, into allogenic grafts (from another individual of the same specie), xenogenic grafts (from other species) and alloplastic grafts (produced synthetically) [5,6]. In this case the autologous grafts are considered the gold standard for the reconstruction of atrophic alveolar ridges [6,7], however the alloplastic graft (allograft) is presented as an alternative with few limitations [7], which has the advantage of obtaining.
The demineralized bone matrix (DBM) is originated from human material, obtained when the cortical or corticalcancellous bone is processed in an acid solution following liofylization process to remove the bone mineral components [8]. The result is a material composed by collagen (type I principally), proteins no-collagen type and growth factors such as bone morphogenetic proteins (BMPs) [8,9]. The BMPs are able to promote the osseous tissue formation by inducing the differentiation of mesenchymal cells into osseous progenitors cells and finally into osteoblasts [10], giving an osseous-inductive feature to the DBM. The DBM effectiveness already was studied clinically, histologically and radiographically regarding to the alveolar preservation after dental extraction [11-13] and in the recuperation of the alveolar ridge thickness. Histologically has been observed mature, regular and mineralized bone in alveolus filled with DBM after 6 months [11,13]. This study aimed analyze the qualitative and quantitative histological features of alveolar bone that received demineralized bone matrix (DBM) of patients with atrophic mandibular ridge and adequate alveolar height; and also report the radiographic characteristics of the dental implants placement in these treated sites.
Material and Method
The present case series study was performed in two patients without systemic pathologies attending to dental private practice in Temuco, Chile. From them were obtained five mandibular sites which were evaluated through clinical examination and histological analysis. Demographic data and characteristics of the histological sample sites are shown in Graph 1. Both patients required bone substitute material before the placement of endo-osseous dental implants in the sites mentioned. In the five mandibular sites of both volunteers was observed alveolar ridge thickness of 3.0 mm or less. The patients received a detailed explanation of the proposed treatment, agreed and authorized the histological analysis of the bone samples and publication of the results through an informed consent.
The application of DBM followed these steps: a mucoperiosteal flap was performed in the area and moved towards vestibular. Small holes were made in the alveolar bone with a round carbide bur (012) to activate blood flow and to place the bone substitute material. Finally, periosteum was cut in the deepest internal area of the flap to increase length and then sutured. The bone substitute used in this study was a demineralized human cortical bone present in putty DBM (DynaGraft·D™ putty, Keystone Dental, Burlington, U.S.A; Graph 1). After 6 months (Graph 1) of the bone substitute application the dental implants were placed. The bone samples histologically analyzed in this study were obtained in the alveolar preparation phase for implant placement with trephine drill (3 mm, external diameter), thus was not caused additional damage to the patient.
The bone samples were fixed in buffered 4% formalin and stored at 4°C for ~4 weeks. Subsequently, samples were rinsed with saline solution (NaOH, 0.9%) and decalcified with 300 ml of EDTA solution (10%, pH 7, 22-24°C); this EDTA solution was renewed every 3 days for a period of 2 months. The samples were cut longitudinally with a Leica® RM2255 microtome with 3 μm thickness. The hematoxylin-eosin (HE) and Sirius red stainings were performed, then the stained microscope slides were analyzed with an optical microscope Leica® DM750 and camera Leica® ICC50 HD, at a final magnification of X100 and X500.
In the samples stained with Sirius red was performed a quantitative analysis of collagen (types I and III) densities, which were analyzed under polarized light and pixel-counting method performed in digital images, following the method previously described [14]. Thus, using the ImageJ software (1.48s, Free, NIH - USA) the calibrated photomicrographs stained with Sirius red by conventional light (Figure 1a) were transformed into binary images using the “Make binary” tool (Figure 1b), the black area was calculated using the “area calculator” tool serving as the base evaluated area (total bone area) in each figure.
Figure 1: Quantitative analysis of collagen type I and III areas. A) Bone microscopy stained with Sirius red; B) Binary (black and white) colored bone structure with Sirius red; C) Stained bone structure with Sirius red under polarized light, showing two types of collagen; D) Photomicrograph prepared in ImageJ to show only red color (type I collagen); E) Photomicrography prepared in ImageJ to only show the color green (collagen type III); F) Binary image of collagen type III portion.
Subsequently the calibrated images in polarized light (Figure 1c) had the red (collagen type I, Figure 1d) and green (collagen type III, Figure 1e) channels separated, using “color>split channels” tool and also converted into binary images (Figure 1f, make binary tool) for analysis area (area calculator) of each type of collagen. Once obtained the areas of the two types of collagens were calculated their average densities using the collagen type I area/total bone area and collagen type III area/ total bone area ratios. The means and standard deviations from the collagen data were obtained and compared between the types of collagens and the near and distant areas of the bone substitute material (DBM), by ANOVA (p=0.05) and Bonferoni post-test.
Results
Clinical characteristics
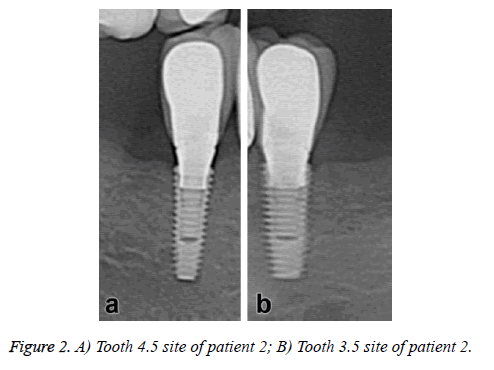
The dental implants showed no immediate postoperative complications. No symptoms of pain or sign of infection were observed during the clinical evaluation in the following weeks; however some dental plaque was observed in the periodontal probe use. In the radiographic evaluation reveals an adequate bone height in all dental implants sites and no evidence of absorption or other associated pathologies (Figure 2).
Histological characteristics
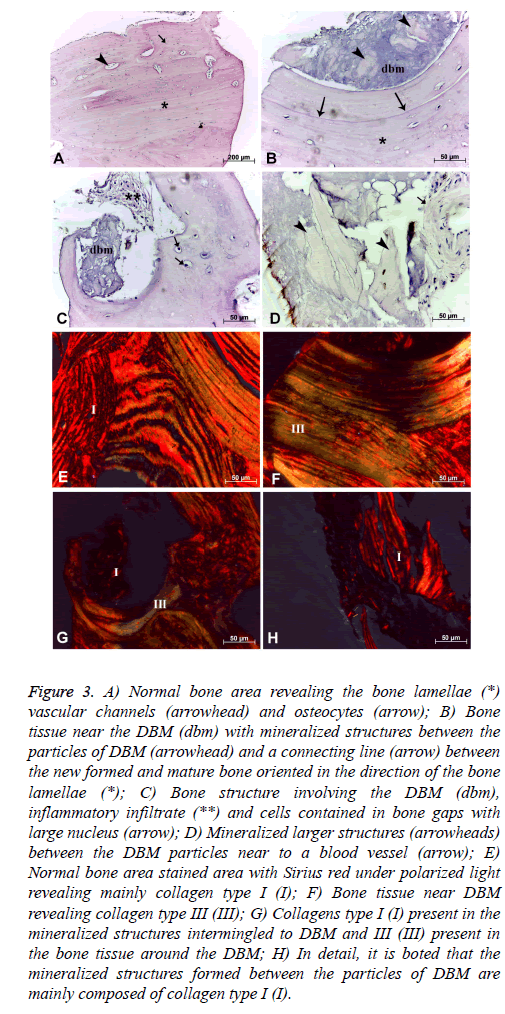
General bone tissue: The histological samples of bone tissue filling with DBM revealed several areas with mature lamellar bone structure and organized in different directions composing the “Havers’ system”, preferably following the major axis of the bone samples. Some vascular spaces corresponding to the "Havers" and "Volkmann" channels with regular distribution in mature bone tissue was noted. In this region was also observed cellular lacunas containing cells which reveal reduced cytoplasm and nucleus size, suggesting the presence of osteocytes, whose metabolic activity was reduced (Figure 3A).
Figure 3: A) Normal bone area revealing the bone lamellae (*) vascular channels (arrowhead) and osteocytes (arrow); B) Bone tissue near the DBM (dbm) with mineralized structures between the particles of DBM (arrowhead) and a connecting line (arrow) between the new formed and mature bone oriented in the direction of the bone lamellae (*); C) Bone structure involving the DBM (dbm), inflammatory infiltrate (**) and cells contained in bone gaps with large nucleus (arrow); D) Mineralized larger structures (arrowheads) between the DBM particles near to a blood vessel (arrow); E) Normal bone area stained area with Sirius red under polarized light revealing mainly collagen type I (I); F) Bone tissue near DBM revealing collagen type III (III); G) Collagens type I (I) present in the mineralized structures intermingled to DBM and III (III) present in the bone tissue around the DBM; H) In detail, it is boted that the mineralized structures formed between the particles of DBM are mainly composed of collagen type I (I).
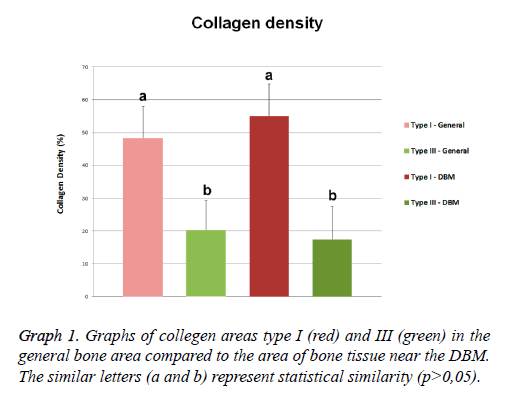
The analysis of the composition of the collagen fibers, stained with Sirius red under polarized light, revealed presence of collagen types I (red) and III (green) with wide and different distribution in the mature bone area. Quantitative analysis of the bone samples of this region revealed the presence of 48.42 ± 9.58% area of type I collagen fibers, and the type III collagen fibers showed an average area of 20.26 ± 9.05% (Figure 3E).
Bone tissue near DBM: In the region near the bone defect filled with DBM lamellar formations were observed in this case follow the orientation of the bone defect with the presence of a well-demarcated boundary line that suggests the union of mature and newly formed bone tissue around the DBM deposited (Figure 3B). Among the DBM particles were noted numerous structures which suggest mineralized tissue (Figure 3D) as well as bone formation bulkier interspersed with DBM. However in the same area where also observed inflammatory infiltration (Figure 3C). The cells present in the region showed a higher metabolic by the presence of cells with osteoblast characteristics, that showed core with greater volume within the bone lacunas (Figure 3C). Finally, in this region were also noted fewer vascular channels in comparison to the mature bone region (Figures 3B and 3C).
The collagen composition of the area through the Sirius red staining also showed the presence of collagen types I and III fibers (Figures 3F and 3G). The area corresponding to type I collagen represented 55.06 ± 9.67% of the total area of this region, and collagen type III presented an mean area of 17.38 ± 10.02% in the same region. The mineralized structures among the DBM particles were formed mainly of collagen type I (Figure 3H).
Discussion
The present study evaluated the morphological features and quantitative characteristics of collagen in the bone tissue of clinical cases where was applied the demineralized bone matrix (DBM), in addition provide a briefly clinical description of implant placement sites treated with this biomaterial. In general, the DBM showed adequate response in the treatment of bone defects, therefore may be considered as a promising therapy in bone regeneration. The bone near DBM area showed increased bone cell activity compared to mature normal bone area with cells inside lacunae with bulkier nuclei, suggesting the presence of osteoblastic cells. The bone area near the DBM revealed fewer vascular channels in comparison with the areas of mature bone. Previously, has been reported that others type of allogenic graft bone particles may not be completely replaced and remained encapsulated within connective tissue [15,16], it was also observed in this study with DBM. It was noted the presence of mineralized structures around the DBM particles, these are composed mainly of collagen type I, corroborating the observations of previous studies [8,9].
Other studies that have also used DBM in patients reported that graft residual particles are surrounded by neo-formed bone tissue and active osteocytes [12,17], this particles were clearly defined, however in some locations was not possible to notice where the residual graft ends and the neo-form bone begins [17]. Its characteristic has been also reported in animal study employing rabbit model, in this case, the DBM was able to promote bone consolidation after distraction osteogenesis [18]. The results presented in this study also showed residual particles surrounded by new bone formation, as previously described in other studies [12,17]. The histological result of our study also reveals this characteristic by the presence of a connecting line between the newly formed bone tissue in contact with DBM particles and the mature bone tissue (Figure 3B) suggesting bone consolidation.
In our histological results was observed the presence of collagen type I and III fibers over all bone tissue, near or not to the DBM particles. The distribution of collagen types revealed that collagen type I showed a higher area compared to collagen type III. The quantitative analysis of collagen types were confirmed by the morphological observations. The type I collagen presented higher density compared to type III collagen in both bone region and the surrounding areas of the bone injury filled with DBM. Both types of collagens showed no differences in bone or nearby regions of DBM. Although, it is observed a reduction of collagen type III and an increased in type I, these tendencies were not statistically confirmed. The variation of these densities was similar between the two areas analyzed and also for the two types of collagen.
The collagen fibers type I and III are considered classic, having been the first kind described. These two types of collagens are commonly present concomitantly in connective tissue, wherein collagen type preferably is composed by thicker fibers than collagen type III [19]. Early studies in animal models also reported collagen fibers present in bone tissue associated with the use of DBM [20,21]. However these studies have not commented on the differences among collagen types, as assessed in our study, in addition to the clear difference has been made in animals. Thus it is also worth noting that our results corroborate these previously observations. The larger amount of collagen type I, present in bone tissue suggests greater resistance due to the presence of thicker fibers [19]. We would like to emphasize that the bone formed among the DBM particles were mainly composed of this type of collagen.
The results of this study revealed bone formation in dental alveoli region with irregular alveolar ridge obtained with demineralized bone matrix (DBM) with osseointegration and the presence of collagen fibers mainly of type I. These characteristics show proper bone formation in these cases reflected in the clinical success of its use. Thus, allowing conclude that DBM is a promising material when associated with endo-osseous dental implants.
References
- Jung RE, Pjetursson BE, Glauser R, Zembic A, Zwahlen M, Lang NP. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res 2008; 19: 119-130.
- Javed F, Romanos GE. The role of primary stability for successful immediate loading of dental implants. A literature review. J Dentistry 2010; 38: 612-620.
- Marquezan M, Osório A, Sant'Anna E, Souza MM, Maia L. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin Oral Implants Res 2012; 23: 767-774.
- Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int Periodontics Restorative Dent 2003; 23: 313-323.
- Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res 2014; 93: 950-958.
- Papageorgiou S, Papageorgiou P, Deschner J, Götz W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: Systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J Dent 2016; 48: 1-8.
- Araújo PP, Oliveira KP, Montenegro SC, Carreiro AF, Silva JS, Germano AR. Block allograft for reconstruction of alveolar bone ridge in implantology: a systematic review. Implant Dent 2013; 22: 304-308.
- Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 2012; 64: 1063-1077.
- Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to Autogenous Bonem Graft: Efficacy and Indications. J Am Acad Orthop Surg 1995; 3: 1-8.
- Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res 1971; 50: 392-406.
- El-Chaar ES. Demineralized bone matrix in extraction sockets: a clinical and histologic case series. Implant Dent 2013; 22: 120-126.
- Brownfield LA, Weltman RL. Ridge preservation with or without an osteoinductive allograft: a clinical, radiographic, micro-computed tomography, and histologic study evaluating dimensional changes and new bone formation of the alveolar ridge. J Periodontol 2012; 83: 581-589.
- Fuentes R, Issa JPM, Iyomasa MM, Oporto G, Prieto R, Borie E. The behavior of demineralized bone matrix (DBM) in post-extraction sockets. Int J Morphol 2012; 30: 394-398.
- Cury DP, Dias FJ, Miglino MA, Watanabe IS Structural and Ultrastructural Characteristics of Bone-Tendon Junction of the Calcaneal Tendon of Adult and Elderly Wistar Rats. PLoS One 2016; 11: e0153568.
- Listgarten MA, Rosenberg MM. Histological study of repair following new attachment procedures in human periodontal lesions. J Periodontol 1979; 50: 333-344.
- Stahl SS, Froum SJ, Kushner L. Healing responses of human intraosseous lesions following the use of debridement, grafting and citric acid root treatment. II. Clinical and histologic observations: one year postsurgery. J Periodontol 1983; 54: 325-338.
- Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol 2012; 83: 329-336.
- Ni M, Tang P, Wang Y, Li G. Experimental study on promoting bone consolidation by using platelet-rich plasma and decalcified bone matrix during distraction osteogenesis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2011; 25: 661-667.
- Maynes, Richard. Structure and function of collagen types. Elsevier, 2012.
- Liang X, Yang Z, Xiang Z, Li X, Huang G, Zhi W. Experimental studies on biocompatibility of heterogeneous demineralized bone matrix particles. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009; 23: 76-81.
- Kadkhodazadeh M, Mollaverdi F, Abdolsamadi HR, Azar R, Ghasemian Pour M, Ahmadpour S. Histologic effects of demineralized bone matrix on regeneration of alveolar socket in diabetic rats. Iran Endod J 2009; 4: 20-24.