Research Article - Biomedical Research (2017) Volume 28, Issue 2
Luteolin induces apoptosis in mouse liver cancer cells through ROS mediated pathway: A mechanistic investigation
Wei Wang1*, Fu-li Zhao1, Jing Zhang2, Dong-dong Gao11Department of Medical Oncology,Zhumadian City Centre Hospital, Zhumadian China
2Department of Nursing, Huanghuai University, Zhumadian, China
- *Corresponding Author:
- Wei Wang
Department of Medical Oncology
Zhumadian City Centre Hospital, Henan, PR China
Accepted on July 1, 2016
Abstract
Luteolin is a flavonoid and considered to be important for anti-cancer activity against many cancer cells. However its mechanism of action is poorly understood as well as systematics study on its action on different cancer cell line is still obscure. In this study we checked luteolin activity against number of cell line and according to our screen mouse liver cancer cells (HepG2) is found to be most susceptible to its action. We have also measured how different concentrations of luteolin effects on cell proliferation of HepG2 over 48 h of window. Next we studied the underlying mechanism of luteolin action, and found its inducing apoptosis of HepG2 cell, apoptosis was monitored based on propidium iodide internalization assay using flow cytometer (FACS). Next we measured ROS generation ability and lipid peroxidation effect induced by luteolin and measured using TBARS assay. Further investigation revealed that luteolin is causing extensive DNA degradation in HepG2 cells. Finally Quantitative real time PCR analysis results showed that luteolin significantly up-regulated the expression of both intrinsic and extrinsic caspases as well as executioner caspases. It is notable that intrinsic caspase (Caspase-9) and executioner caspase (Caspase-3) were highly expressed when compared with the extrinsic caspase (Caspase-8). Altogether our result clearly showed the details mechanistic pathway by which luteolin is inducing apoptosis in HepG2. The promising anticancer potential of luteolin can be effectively utilized in future for anticancer drug development.
Keywords
Luteolin, Anticancer potential, Hepatoma cells, ROS generation, Apoptosis, Caspase.
Introduction
Primary liver cancer, clinically termed as Hepatocellular Carcinoma (HCC) or malignant hepatoma, represents one of the most common neoplasms worldwide. It remains fifth and eighth largest in men and women respectively with major victims from developing countries of Asia and Africa [1]. In recent years, the survival of patients with HCC has significantly decreased in past three decades. Notably, a fiveyear survival rate of patients with HCC was reported below 9% which reveals its severity [2]. Majority of HCC cases are due to infections and inflammations caused by hepatitis B and C viruses, risk factors such as obesity, cirrhosis (both alcoholic and non-alcoholic) [3]. Dietary hepato-carcinogens such as mycotoxins (Aflatoxin, Fumonisin, and sterigmatocystin), Pyrrolizidine alkaloids from plants (Petasitenine, Senkirkine) and genotoxic agents (Nitrosoamines) induce mutations in DNA resulting in HCC [4].
Present treatment strategies involve surgical resection and liver transplantation. However, recurrence is the major cause of mortality after surgical treatment. Thus it is mandatory to find an effective natural therapeutic agent with anti-HCC activity.
Apoptosis is an immunological process which induces programmed death on its own cell to maintain a stable internal environment. It is an important mechanism which plays vital roles in various biological processes such as embryogenesis, cell differentiation, body development and pathological death [5]. Apoptosis, the key factor which stimulates programmed cell death in cancer cells has become the major focus to treat cancer tissues. Apoptosis is induced by two major signalling pathways namely extrinsic and intrinsic pathways [6]. Caspase proteins play a vital role in both extrinsic and intrinsic pathways. Caspase-8 initiates the extrinsic pathway whereas Caspase-9 is involved in activation of intrinsic pathway which subsequently activates the executioner caspases including caspase-3 [7].
Flavonoids have been reported to act as anti-carcinogenic agents by inducing apoptosis in different cancer cells. Luteolin shown in Figure 1 is one of the important dietary flavonoids which are commonly present in various fruits and vegetables. Luteolin has been identified from various Chinese plants such as Lonicera japonica, Elsholtzia rugulosa, Brassica oleracea and Chrysanthemum morifolium [8]. Luteolin exhibits various pharmacological properties such as anti-inflammatory, anti allergic and anti-proliferative properties [9-11]. The anticancer effect of Luteolin was because of their DNA topoisomerase inhibiting activity. But their role on inducing caspase mediated apoptosis in liver cancer cells remains unclear.
In this study, we investigated the anticancer potential of luteolin against a number of different cell line and found its activity is highest against HepG2. Further we have found that it induces apoptosis in mouse HepG2 cells through ROS mediated pathway. The degree of apoptosis was determined by flow cytometric analysis. Detail mechanistic investigation reveals luteolin induced extensive DNA fragmentation. The pathways that Luteolin induce in hepatoma cells were not determined. Especially there are no reports available regarding caspase activation in those cells. This made us to focus on determining the caspase inducing activity of Luteolin in HepG2 cells. Caspase are classified into initiator and effector based on their function. Initiator caspases are further classified on the basis of the pathway they involved such as intrinsic and extrinsic pathways. To analyse the role of Luteolin in inducing apoptosis pathways, we analysed the temporal expressions of initiator caspases, caspase-9 (extrinsic) and caspase-8 (intrinsic) and also the executioner caspase (Caspase-3). This is the first study showing how luteolin-induction causes change in expression pattern of initiator and executioner caspase. Finally we tested efficacy of luteolin against in-vivo tumour model induced in nude mice and observed that in luteolin treated mice tumour volume reduces significantly compared to controls. Altogether all our studies indicate that luteolin has good potential to emerge as an alternative therapeutic option for hepatic carcinoma treatment in future.
Materials and Methods
Cell culture and reagents
Human hepatoma HepG2 cells and other cancer cells used in this study were grown in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, USA) containing 10% heat inactivated foetal bovine serum, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 2 mM L-glutamine. The cells were plated at a density of 2 × 104/cm2 into tissue-culture dishes and grown in 5% CO2 humidified atmosphere in Dulbecco’s modified Eagle’s medium at 37ºC.
Luteolin preparation
Luteolin was purchased from Sigma Aldrich (USA). One microgram Luteolin was dissolved in 100 μl of DMSO and stored at -20ºC. This was maintained as stock solution. To determine the effect of Luteolin on HepG2 cells, we plated the cells with DMEM with 10% FBS. The cells were then incubated in serum-free medium with 10, 50, 100 and 200 μM/L of Luteolin for 24, 48 and 72 hours. Cells incubated with DMSO were considered as untreated cells.
MTT assay
Cell proliferation assay was performed to determine the cell proliferation inhibitory activity of Luteolin. HepG2 cells (2 × 104 cells/mL) were seeded in 96-well microtiter plates. After exposure to various concentrations of Luteolin (10, 50, 100, 200 μM) for 48 hours, 20 μl MTT solution (5 mg/ml in PBS) was added to each well and the plates were incubated for additional 4 h at 37ºC. Cells incubated with DMSO were considered as untreated cells. 200 μl DMSO was added to each well, and then the optical density (OD) at 570 nm was determined by a spectrophotometer plate reader. The inhibitory percentage of Luteolin was determined using the formula (1- (A570 of experimented cells/A570 of untreated cells) × 100). All the assays were performed in triplicates and the results represent the mean value of the three individual assays. Morphological changes in HepG2 were determined using a standard fluorescence microscope.
Assessment of intracellular ROS
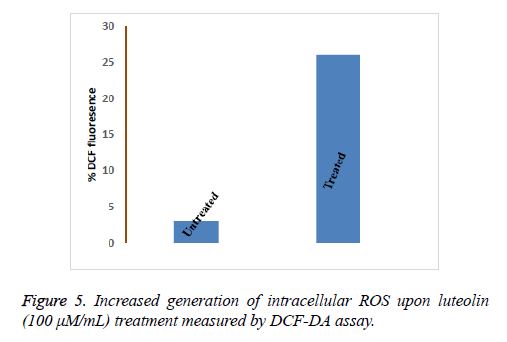
To determine ROS, treated and untreated cells were incubated with 10 μM of Dichlorofluorescindiacetate (DCF-DA, Sigma) for 20 min at 37ºC in the dark. DCF fluorescence was monitored using a plate-reader. DCF-DA is colourless and nonfluorescent until both of the acetate groups are hydrolysed and the products are subsequently oxidized to fluorescein derivatives (H2DCFDA). Fluorescence was measured at an excitation wavelength of 485 nm and emission wavelength of 528 nm using a fluorescence spectrometer (Perkin Elmer).
DNA fragmentation assay
The cells after incubation for 48 h at a fixed temperature of 30ºC with different concentrations of Luteolin (10, 50, 100, 200 μM) were harvested by scraping and brief centrifugation (5000 RPM for 10 minutes). Cells treated with DMSO were maintained as control. The cells were then washed with sterile PBS and DNA was collected from the cells using TRIzol (Invitrogen) as per manufacturer’s instructions. The quality and purity of isolated DNA was determined by UV spectrophotometer (Shimadzu, Japan). Equal amount of DNA from cells treated with different concentrations of Luteolin after different various incubation periods was electrophoresed in 1.5% agarose gel and visualized under UV light.
Flow cytometric assessment of apoptosis
The apoptotic effect of Luteolin on the HepG2 cells was investigated by assessing the internalization of DNA intercalating dye Propidium Iodide (PI). Briefly, HepG2 cells (2 × 105 cells/mL) were treated with various concentrations of Luteolin (50, 100, 200 μM) cultured at 37ºC 5% CO2 atmosphere for 48 h. The cells were washed using PBS and centrifuged. Washed cells were stained with Propidium Iodide (PI) solutions at a concentration of 5 μg/mL (Sigma-Aldrich) and incubated for 10 minutes at room temperature. After incubation, 20 000 events were acquired ungated in a FACSort flow cytometer (BD Biosciences) system. The results were analysed using CELLQUEST software (BD Biosciences). Within this gate, the percentage of PI-positive HepG2 cells was evaluated on a red-fluorescence histogram.
RNA extraction, cDNA synthesis and gene expression analysis
The effect of luteolin on apoptosis genes were estimated by quantitative real time PCR. The apoptosis genes included in this study are I) Casp 8, extrinsic pro-apoptotic Caspase; II) Casp 9, intrinsic pro-apoptotic caspase and III) Casp 3, effector caspase. The cells after incubation with 200 μM of Luteolin were harvested by brief centrifugation. Total RNA was extracted from HepG2 cells using High Pure RNA Tissue kit (Roche Diagnostics GmbH, Germany) and the purity and quantity of RNA was determined by spectrophotometry (Nano Drop, Thermo Scientific, US). cDNA was synthesized from 200 ng total RNA template using Omniscript RT Kit (Qiagen, Santa Clarita, CA, USA). Luteolin-induced modulation of Caspase mRNA transcripts was analysed by Light Cycler 96 Real Time PCR system with Fast SYBR® Green Master Mix (Roche Diagnostics GmbH, Germany). Primers used for the analysis was tabulated in Table 1. GAPDH gene was maintained as internal control. Reaction cycle was performed as 95ºC for 30 s, 40 cycles of 95ºC for 5 s and 58ºC for 60 s using a 20 μl qRT-PCR mixture which consisted of 2 μl cDNA sample, 10 μl Fast SYBR® Green Master Mix, 0.5 μl PCR forward/reverse primers (10 mM) and 7 μl molecular grade water. CT values were calculated by the Light Cycler 96 software and relative gene expression levels of caspases were expressed as the difference in CT values of Caspase genes and the internal control gene (GAPDH). The results were analysed and calculated using 2-ΔΔCT method [12].
| Primer name | Primer sequence (5’-3’) |
|---|---|
| GAPDH F1 | AAGGTGAAGGTCGGAGTCAAC |
| GAPDH R2 | GGGGTCATTGATGGCAACAATA |
| Casp-3 F3 | GGTGGGATCAAAGCTTAGTG |
| Casp-3 R4 | TCTTCCTAGGACTGCAGACT |
| Casp-8 F5 | AGAGTCTGTGCCCAAATCAAC |
| Casp-8 R6 | GCTGCTTCTCTCTTTGCTGAA |
| Casp-9 F7 | AGCCAGATGCTGTCCCATAC |
| Casp-9 R8 | CAGGAGACAAAACCTGGGAA |
Table 1. Forward and reverse primers of each caspase gene used for quantitative real-time PCR analysis.
Animal experiments
Twenty four male nude mice, aged between 3-6 weeks (~25 g weight), were randomly distributed into two groups. Mice were intraperitoneally administered control (n=12) luteolin (2 mg/Kg bodyweight) (n=12), every day for 12 days before induction of tumour. After induction of tumour also intraperitoneal administration of luteolin was continued for next 30 days. Finally the tumour volume was calculated using the formula: V=π/6 × larger diameter × (smaller diameter)2.
Statistical analysis
All data are analysed using SPSS software (Version 11.5) and presented as a mean value with its standard deviation indicated (Mean ± SD). P<0.05 was considered significantly different. For reproducibility experiments were carried out at least three (n=3) times.
Results
Inhibitory activity of luteolin on HepG2 cell proliferation
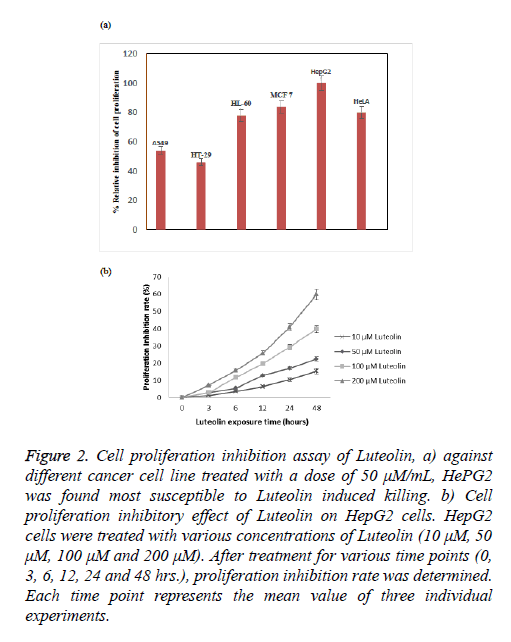
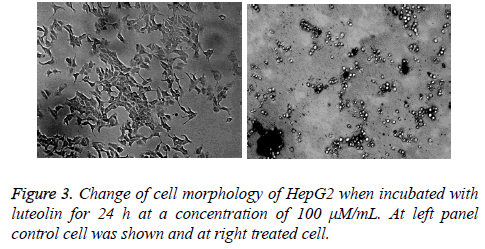
Luteolin is known for its anticancer action; however there is lack of systematic study against its activity against different cancer cell line. To understand its effect on different cell line we took number of cell line including lung (A549), colon (HT29), leukaemia (HT60), breast (MCF-7), cervical (HeLa) and hepatocarcinoma (HePG2). We found highest activity of luteolin against HePG2 followed by MCF-7, HeLa and HL-60 shown in Figure 2a. In case of other 2 cell lines the effect is more reduced. At this moment we are not sure why we observed such a difference in activity, study is undergoing in our lab to understand this phenomenon. As we have found from our screen that HePG2 is most susceptible to luteolin treatment we further carried out all our experiment against this cell line only. Further the inhibition by luteolin in the human hepatoma cell HepG2 cell line was assessed after 3, 6, 12, 24, 48 h of exposure. Though there was no significant inhibitory activity at low concentration (10 μM) of luteolin, maximum inhibitory activity was observed at increased concentrations (50, 100 μM). Results revealed that the inhibitory activity is time and concentration dependent which increased with increasing concentration of luteolin shown in Figure 2b. According to the results, Luteolin at a concentration of 200 μM after 48 hours of incubation were able to inhibit 59% of proliferation of HepG2 cells. At 100 μM dosage after 48 hours, 40% inhibition was observed and 50 μM Luteolin induced 22% inhibition after 48 hours of inhibition. These results clearly depicted the significant inhibitory activity of HepG2 proliferation. We also monitored the change in cell morphology in presence of luteolin; from their normal cellular structure we found abrupt change on luteolin treatment cells become roundish which is early sign of apoptosis shown in Figure 3.
Figure 2: Cell proliferation inhibition assay of Luteolin, a) against different cancer cell line treated with a dose of 50 μM/mL, HePG2 was found most susceptible to Luteolin induced killing. b) Cell proliferation inhibitory effect of Luteolin on HepG2 cells. HepG2 cells were treated with various concentrations of Luteolin (10 μM, 50 μM, 100 μM and 200 μM). After treatment for various time points (0, 3, 6, 12, 24 and 48 hrs.), proliferation inhibition rate was determined. Each time point represents the mean value of three individual experiments.
Luteolin induces apoptosis in HepG2 Cells
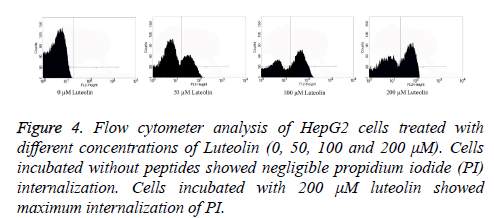
Flow cytometry was performed to analyse the number of live and dead cells by counting the number of cells stained by Propidium Iodide (PI). Propidium iodide is a membrane impermeant dye that is generally excluded from viable cells but can penetrate cell membranes of dying or dead cells. Accordingly, the cells undergoing apoptosis were counted by incubating the luteolin-exposed cells with PI for 10 minutes. As shown in Figure 4, the total apoptosis rate for Luteolin treated HepG2 cells reached to 61% (200 μM for 48 hours) and 52% (100 μM for 48 hours) which are significantly different from untreated cells (1%). At lower concentration of Luteolin (50 μM for 48 hours) relatively low percentage (31%) of PI positive HepG2 cells were observed. This explained the dose-dependent apoptotic activity of Luteolin on HepG2 hepatoma cells. Next we tried to understand how luteolin is inducing apoptosis in HepG2. It was reported for many other flavonoids that they cause apoptosis by generating ROS so we first checked the intracellular ROS level by DCF fluorescence of luteolin treated cells compared to untreated cell and we have found a marked increase in ROS level as shown in Figure 5 on treatment. Which confirms our doubt that luteolin is inducing apoptosis in HepG2 through ROS mediated way.
DNA Fragmenting ability of Luteolin in HepG2 cells
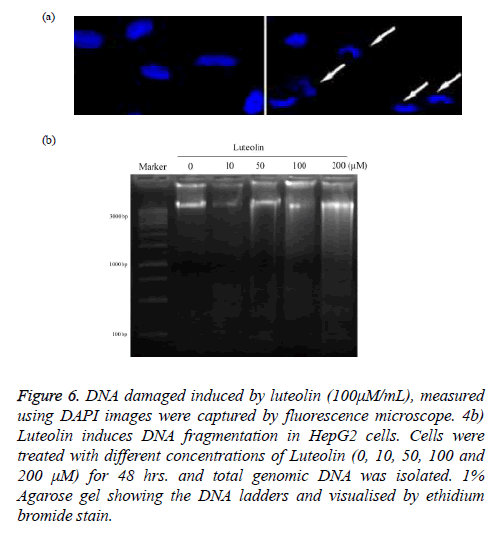
DNA damage by luteolin was fist validated by imaging of treated cancer cell with 4', 6-diamidino-2-phenylindole (DAPI) as in Figure 6a. The image clearly showed that normal nuclear morphology get altered on treatment of luteolin. Further we isolate the DNA from nucleus of HepG2 and treated with different concentrations of luteolin (10, 50, 100, 200 μM) for 24 hours and finally run in agarose gel electrophoresis system. Our result clearly revealed a ladder-like pattern of DNA fragmentation. At low concentration of Luteolin (10 μM), no fragments were observed which are shown in Figure 6b.
Figure 6: DNA damaged induced by luteolin (100μM/mL), measured using DAPI images were captured by fluorescence microscope. 4b) Luteolin induces DNA fragmentation in HepG2 cells. Cells were treated with different concentrations of Luteolin (0, 10, 50, 100 and 200 μM) for 48 hrs. and total genomic DNA was isolated. 1% Agarose gel showing the DNA ladders and visualised by ethidium bromide stain.
However, early DNA fragmentation was observed in the form of shearing on agarose gel in HepG2 cells exposed to 50 μM Luteolin. Clear ladder of DNA fragments was observed in cells exposed to higher concentration of Luteolin (100, 200 μM).
Luteolin induces activation of caspases-3, 8 and -9
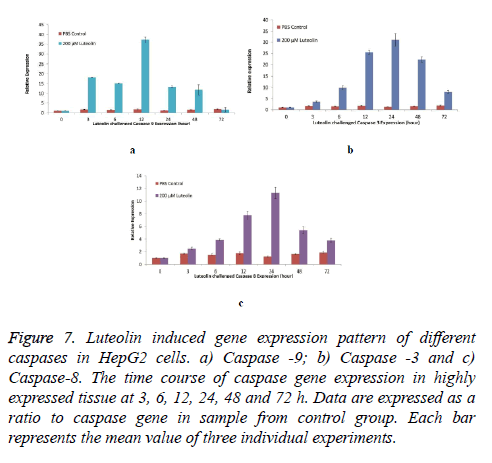
To further establish the luteolin-induced apoptosis, activation of caspases-3, -8 and -9 expression was analysed in cells treated with 200 μM of Luteolin. Exposure of HepG2 cells to different concentrations of Luteolin increased all the three Caspases. Highest induction was observed in Caspase-9 as shown in Figure 7a transcripts which was maximum expressed after 12 hours of Luteolin expression (37 Folds more than untreated cells) however, increase in expression was observed immediately after exposure of Luteolin (18 Folds in 3 hours). Second highest induction was in Caspase-3 shown in Figure 7b transcripts. 60 μM Luteolin induced 31 folds of expression after 24 hours of exposure though significant elevation in expression was observed after 12 hours of exposure.
Figure 7: Luteolin induced gene expression pattern of different caspases in HepG2 cells. a) Caspase -9; b) Caspase -3 and c) Caspase-8. The time course of caspase gene expression in highly expressed tissue at 3, 6, 12, 24, 48 and 72 h. Data are expressed as a ratio to caspase gene in sample from control group. Each bar represents the mean value of three individual experiments.
+Luteolin was also able to induce the expression of Caspase-8, however the folds of expression (11 folds after 24 hours of 60 μM Luteolin) was lesser when compared to other two caspases detailed in Figure 7c.
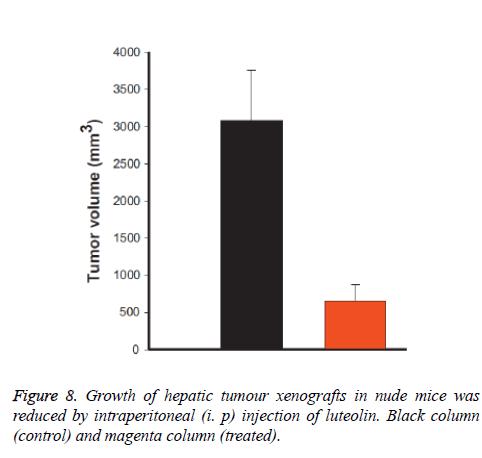
Luteolin reduces tumour volume mice
We also checked the effect of luteolin in mice (in-vivo) and we have observed that luteolin treated mice are much healthier compare to normal control mice. We measured the tumour volume and found that it was reduced almost 95% by volume when treated with luteolin as shown in Figure 8. We have also found that during experiments that few control mice were died due to massive cancer progression, however not a single luteolin treated mice were died, and their body weight also remain constant during treatment progresses.
Discussion
Carcinoma can be effectively suppressed by proliferation inhibition and apoptotic induction of tumour cells. Though there are numerous anti-tumour agents with potent antitumor activities, their mechanism of action remains unclear. One of such agent is Luteolin whose antitumor activity is well established. Elangovan et al. [13] reported that luteolin affects inhibits the propagation of cancer. It has also been reported that luteolin enhances the detoxification protein activity in cancer cells. Luteolin was able to induce anti-carcinogenic effects in various cells such human myeloid leukaemia cells [14], human HepG2 cells [15]. Lee et al. [15] reported that apoptosis of luteolin in human hepatoma HepG2 cells involve mitochondria translocation of Bax/Bak and activation of JNK, still, the caspase mediated apoptosis-inducing mechanism of Luteolin at different dosages and time points remain unclear. Present investigations demonstrate that Luteolin inhibited the proliferation of mouse HepG2 cells by inducing caspasemediated apoptosis. In addition, we identified that Luteolin induced cell death in HepG2 cells by generating ROS and breaking down the DNA.
Our results demonstrated that Luteolin inhibit cancer progression against many cancer cell line though it’s most effective against hepato-carcinoma cell line (HepG2). Luteolin significantly inhibited the cell proliferation in a time and concentration dependent way and we have also confirmed that Luteolin induced DNA fragmentation which is in agreement with the previous studies [16]. Flow cytometry analysis clearly explained that Luteolin induces apoptosis in a dose dependent manner in HepG2 cells. Kim et al. [17] also reported that luteolin induce apoptosis in a time- and dose-dependent manner. Similar reports have been reported by several others such as Yee et al. [18] and Hwang et al. [19] state the cell proliferation inhibition and apoptosis inducing activity of Luteolin. Further we have found that it accelerates intracellular ROS generation heavily in treated cell, which in turns triggers apoptosis.
So far, various contrary reports have been published regarding the role of luteolin in inducing caspase mediated apoptosis. Hara et al. [20] reported that mitochondrial apoptosis pathway is the major targets of anti-cancer drugs. Thus, it state that luteolin could follow caspase-mediated apoptosis pathway. But, Hwang et al. [16] reported that luteolin induces caspaseindependent apoptosis pathway. Thus, the pathway involved in luteolin-induced apoptosis remains as a debate. In this study, the caspase gene expression pattern demonstrated that luteolin activates caspase-mediated apoptosis specifically in HepG2 cancer cells. The temporal expression clearly demonstrated that all the three caspases; extrinsic initiator caspase (Caspase-9), intrinsic initiator caspase (caspase-8) and effector caspase (caspase-3) were stimulated and their expression level increased by Luteolin. It is notable that caspase-9 and caspase-3 are highly expressed. Also, the extrinsic caspase (caspase-8) is comparatively least expressed, however, its expression is induced. Ding et al. [21] reported that luteolin induced caspase-3 expressions significantly in liver cancer cell line SMMC-7721, BEL-7402 whereas luteolin did not affect caspase expression in normal liver cells HL-7702. This confirmed that Luteolin induces caspase mediated apoptosis in mouse hepatoma cells. However, the exact mechanism of caspase activation is not clear. One chance is the disruption or modifications in the pathways upstream of caspase activation which could be a reason for the cancer-cell specific luteolin activity. This has to be elucidated in detail by performing further studies. Finally our in-vivo data clearly showed that it’s very effective in reducing hepatic tumour in nude mice, which further authenticated our in-vitro observations.
Conclusively, these results suggest that Luteolin inhibits the cell proliferation in mouse hepatoma cells and they induce apoptosis in HepG2 cells by means of caspase-mediated apoptosis pathway. This study provides a clear insight into the molecular mechanism of luteolin mediated apoptosis which could be used for cancer therapy in future.
References
- Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis2010; 31: 100-110.
- Yeh MM, Yeung RS, Apisarnthanarax S. Multidisciplinary perspective of hepatocellular carcinoma: A Pacific Northwest experience. World J Hepatol 2015; 7: 1460-1483.
- Constantin CV, Streba CT, Rogoveanu I, Nita-Stefanescu L, Ionescu AG. Cirrhosis and Chronic Viral Hepatitis as Risk Factors for Hepatocellular Carcinoma: Romanian Single-clinic Experience. Maedica2010; 5: 265-270.
- Sugimura T. Nutrition and dietary carcinogens. Carcinogen 2000; 21: 387-395.
- Elmore S. Apoptosis-A Review of Programmed Cell Death. Toxicolpathol 2007; 35: 495-516.
- Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. IntegrBiolCamb 2011; 3: 279-296.
- McIlwain DR, Berger T, Mak TW. Caspase Functions in Cell Death and Disease. CSH Perspect.Biol2013; 5: 8656.
- Lopez-Lazaro M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev Med Chem 2009; 9: 31-59.
- Jiang D, Li D, Wu W. Inhibitory Effects and Mechanisms of Luteolin on Proliferation and Migration of Vascular Smooth Muscle Cells. Nutrients 2013; 5: 1648-1659.
- Kim H, Cheon K, Kim BS, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW264.7 and their structure-activity relationships. BiochemPharmacol1999; 58: 759-765.
- Kimata M, Inagaki N, Naga H. Effects of luteolin and other flavonoids on IgE-mediated allergic reactions. Planta Med 2000; 66: 25-29.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Meth 2001; 25: 402-408.
- Elangovan V, Sekar N, Govindasamy S. Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Cancer Lett1994; 87: 107-113.
- Ko WG, Kang TH, Lee SJ, Kim YC, Lee BH. Effects of luteolin on the inhibition of proliferation and induction of apoptosis in human myeloid leukaemia cells. Phytother Res2002; 16: 295-298.
- Lee HJ, Wang CJ, Kuo HC, Chou FP, Jean LF, Tseng TH. Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. ToxicolApplPharmacol 2005;203: 124-131.
- Hwang YJ, Lee EJ, Kim HR, Hwang KA. Molecular mechanisms of luteolin-7-O-glucoside-induced growth inhibition on human liver cancer cells: G2/M cell cycle arrest and caspase-independent apoptotic signaling pathways. BMB Reports2013; 46: 611-616.
- Kim MJ, Woo JS, Kwon CH, Kim JH, Kim YK, Kim KH. Luteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell lines. Cell BiolInt 2012; 36: 339-344.
- Yee SB, Choi HJ, Chung SW, Park DH, Sung B, Chung HY, Kim ND. Growth inhibition of luteolin on HepG2 cells is induced via p53 and Fas/Fas-ligand besides the TGF-β pathway. Int J Oncol 2015; 47:747-54.
- Hwang JT, Park OJ, Lee YK, Sung MJ, Hur HJ, Kim MS, Ha JH, Kwon DY. Anti-tumor effect of luteolin is accompanied by AMP-activated protein kinase and nuclear factor-κB modulation in HepG2 hepatocarcinoma cells. Int J Mol Med 2011; 28:25-31.
- Hara K, Kasahara E, Takahashi N, Konishi M, Inoue J, Jikumaru M, Kubo S, Okamura H, Sato E, Inoue M. Mitochondria determine the efficacy of anticancer agents that interact with DNA but not the cytoskeleton. JPharmacolExpTher 2011; 337: 838-845.
- Ding S, Hu A, Hu Y, Ma J, Weng P, Dai J. Anti-hepatoma cells function of luteolin through inducing apoptosis and cell cycle arrest. Tumour Biol2014;35: 3053-3060.