- Biomedical Research (2010) Volume 21, Issue 4
Low rectal anastomosis leakage, keep it or move it
Faramarz Karimian, Karim Darbanian, Ali Aminian* , Rasoul Mirsharifi , Farhad Mehrkhani , Farshad Gharaee
Department of General Surgery, Tehran University of Medical Sciences, *Mehr General Hospital, Tehran, Irana
- *Corresponding Author:
- Ali Aminian
Department of General Surgery
Imam Khomeini Hospital
End of Keshavarz Boulevard
Tehran, Iran
Fax: +98-21-66581657
Tel: +989122054635
E-mail: aaminian@tums.ac.ir
Accepted date: May 23 2010
Abstract
Leakage of low rectal anastomosis is a potentially life threatening complication. Conven-tionally, in those patients who can tolerate a major operation, resection of anastomosis with end stoma is attempted. This management leads to permanent stoma in many patients. We try to show that in a defined group of patients, with overtly symptomatic clinical leak man-dating surgical intervention, the primary anastomosis may be saved. One hundred and fifty seven patients who underwent low rectal anastomosis during 7 years were followed post-operatively for leak. Patients with low rectal anastomosis disruption of less than a quarter of circumference, estimated by digital rectal examination, were selected. Proximal loop diver-sion with complete on-table wash out of distal limb and temporary closure of efferent open-ing plus peritoneal irrigation and drainage was performed as salvage procedure. Fifteen patients (9.5%) with major leakage and small anastomotic disruption, 10 males (66.6%) and 5 females (33.3%) were enrolled. The indication of primary operation was low rectal cancer in 12 (80%) patients and ulcerative colitis in 3 (20%) patients. Management was successful in 12 (80%) patients leading to preservation of their low rectal anastomosis and control of sepsis. Salvage procedure failed in three (20%) patients leaving no option but discontinuing the pelvic anastomosis in favor of end colostomy. There was one in-hospital death (6.66%). Patients with small disruption at low rectal anastomosis may be managed without resection of primary anastomosis. Controlling peritoneal infection and inhibiting ongoing contamination by proximal diverting stoma will help small deep pelvic leaks heal.
Keywords
Anastomosis leak, low anterior resection, rectal cancer, fecal diversion.
Introduction
In management of rectal cancers, very low anterior resec-tion and deep pelvic colorectal anastomosis is practiced more commonly in recent years. Availability of stapling instruments, developed surgical skills and more effective neo-adjuvant therapy all play role in this trend [1]. Anas-tomotic leak occurs more as the level of anastomosis goes deeper into the pelvis [1]. Clinically significant leak oc-curs in 1.2-10.7% of extra peritoneal rectal anastomosis [2,6]. If it causes a single abscess it may be successfully cured by percutaneous drainage, however with extensive peritoneal infections (multiple abscesses and generalized peritonitis) it is associated with 40 to 60 percent mortality [7]. Gastrointestinal diversion plus local decontamination and infection control are the mainstays of management. This is generally achieved by discontinuing the anastomo-sis in favor of an ostomy plus blind distal loop at best, if perineal resection is not attempted. Following a major abdomino-pelvic surgery with a technically difficult anas-tomosis construction at pelvic floor, both the surgeon and the patient are reluctant to lose the result. The operation might have been the last chance of avoiding a permanent stoma. If the anastomosis is taken down in favor of an end colostomy, restoring continuity may no longer be possible due to anatomic limitations. Also, inflammatory reactions after leak causing contamination and infection may lead to fibrosis, adhesions and mesenteric shrinkage which impede future attempts at re-anastomosis. These findings suggest managing low rectal anastomosis leakage in every way but disrupting. Obviously this is not always possible.
Loop ileostomy ensures fecal diversion to protect an anas-tomosis or anatomic colorectal, anorectal or perineal damage [7]. Within pelvis even large leaks may heal spontaneously when stool passage is diminished by a proximal diverting enterostomy [8].
We tried to save low rectal anastomosis with leakage. Based on previous personal experiences, we defined a group of patients who benefit most from our management protocol. Consequently, we conducted a clinical trial to check this management protocol.
Materials and Methods
A prospective study was conducted at a university affili-ated teaching hospital during 7 years period (from April 2000 to March 2007). Consecutive patients who under-went low rectal anastomosis were followed postopera-tively for leak. Open drainage of pelvic floor (penrose or corrugate drain through lower lateral abdominal stab inci-sion) was used routinely in primary operation for all pa-tients.
Patients with major leakage from deep pelvic anastomo-sis, that mandated surgical intervention, were selected for this clinical trial. Major leakage was defined as feculent discharge from pelvic drain(s) and/or development of acute abdominal condition, associated with systemic in-flammatory response and imaging findings suggesting leak. Fever (temperature > 38.5 ̊C) and/or leukocytosis (white blood cell counts > 15000 cells/mm3) were taken as systemic response. All patients had an ultrasound ex-amination to check for the presence of free fluid and col-lection leading to CT scan in equivocal cases. Upon diag-nosis of leakage, digital rectal exam was performed to determine the circumferential length of anastomotic dis-ruption. Those with a disruption larger than a quarter of circumference were scheduled for emergent take down of anastomosis, end colostomy and blind rectal pouch (Hartmann's procedure). This group of patients was ex-cluded from the study. For patients with anastomotic dis-ruption less than a quarter of circumference management consisted of emergent laparotomy, profuse abdominal and pelvic irrigation, proximal loop ostomy (colostomy or ileostomy), complete on-table wash out of distal limb with thin betadine solution, open drainage of pelvis through bilateral lower abdominal stab incisions by pen-rose or corrugate drains, mucosa to mucosa closure of distal opening of loop colostomy by non-absorbable su-tures (silk), and empiric wide spectrum parenteral antibi-otics to cover enteric gram negative and anaerobic spe-cies, pending culture result. No drain was brought out through posterior anal stab incision as it might facilitate enterocutaneous fistula formation in these patients. No patient had anastomosis discontinued at first salvage operation.
This management was explained thoroughly with details to all patients, in their own words as far as possible, dis-cussing the risks and benefits compared to conventional approach and they made the choice between conventional or proposed management. The following variables were assessed: gender, age, indication of primary operation, type of primary operation, technique of anastomosis, dis-tance of anastomosis from anal verge, presence of protec-tive stoma, drain type, drain site, use of steroids within previous 6 months, history of radiation to pelvis, history of chemotherapy, presentation of leakage, time of presen-tation after primary operation, site of disruption in anas-tomosis circumference, length of disruption, operative findings at laparotomy for leakage, repeat operation for peritoneal wash out, development of dehiscence after sal-vage operations, length of hospital stay(days), and death.
Results
Low anterior resections with deep pelvic anastomosis construction were performed in 157 patients during seven years. Seventeen (10.8%) patients developed major clini-cal leakage. Two male patients had disruptions larger than a quarter of circumference of anastomosis ring. They were scheduled for anastomosis take down and end co-lostmy. Data of these two patients are not presented in this report.
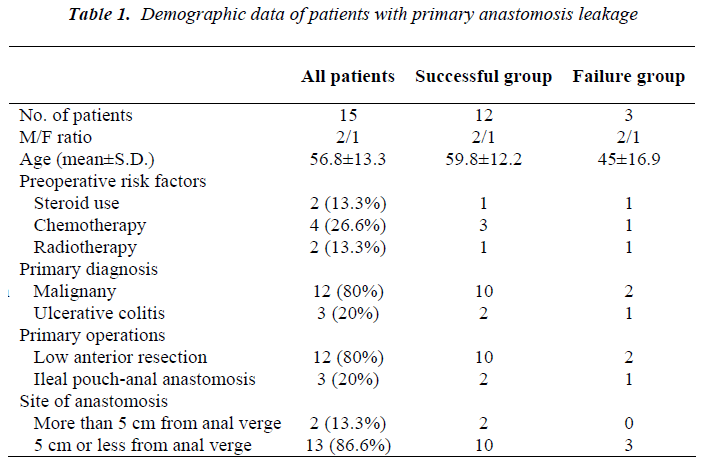
Type of operation was low anterior resection with total mesorectal excision in rectal cancers and restorative total procto-colectomy with ileal pouch (J shape-20 cm limb) construction and protective loop ileostomy in ulcerative colitis. All anastomoses were stapled: end to end colo-anal configuration for rectal cancer and J-pouches with 3 consecutive 75 mm linear shots and circular anastomoses with 29 or 31 mm circular staplers for ulcerative colitis. All staple lines were tested for fluid and air leak after construction. Demographic data of patients with primary anastomosis leakage are shown in table 1 including pre-disposing risk factors, primary diagnosis and operation, and level of anastomosis.
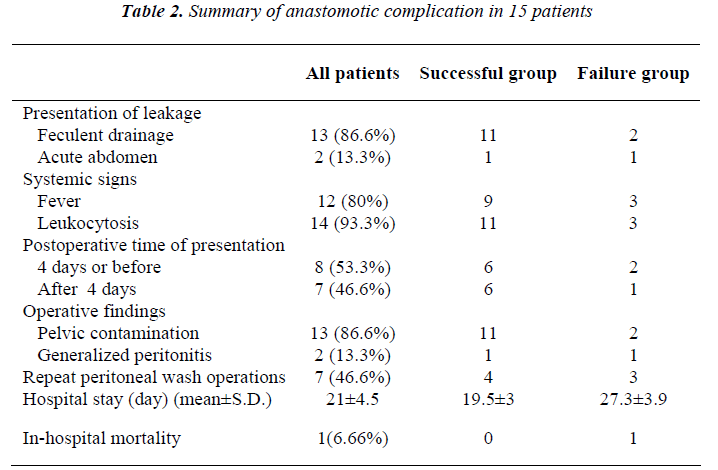
Successful group of patients were cured completely and resumed intestinal continuity after the initial management with proximal diversion and surgical drainage. Failure group of patients had persistent anastomosis leakage after operative management with proximal diversion and surgi-cal drainage. Data concerning anastomotic complications including mode of clinical presentation of leakage, time of leak presentation, and findings at operation; as well as other related variables are inserted in table 2.
Site of disruption, as determined by digital rectal exami-nation, was anterior in 2 patients, posterior in 9, posterior and right lateral in 1, right lateral in 2 and left lateral in 1 patient. Length of disruption was less than a quarter of circumference in 12 (80%) patients and near to a quarter of circumference in 3 (20%) patients, as evaluated by dig-ital examination. Operative management was the same for all patients and included aggressive abdominal and pelvic decontamination and wash out, on-table distal limb irriga-tion, open drain inserted in pelvis through lower lateral abdominal stab incisions and loop ileostomy with hand sewn closure of distal opening by silk sutures. Overall, 7(46.6%) patients needed a total number of 11 times re-peat peritoneal wash with a mean of 1.57 times (range: 1-3).
Management was successful in 12 (80%) patients leading to preservation of their low rectal anastomosis and control of sepsis. Three (20%) patients went on with poorly con-trolled sepsis leaving no option but discontinuing the pel-vic anastomosis in favor of end colostomy. Two (13.3%) patients developed dehiscence of abdominal closure. Overall mean hospital stay was 21±4.5 days
There was one in-hospital death (6.66%). All of the pa-tients with salvaged primary anastomosis had their stoma closed successfully within following 3 to 12 months. The long term functional result of this manage-ment, after closure of diversion, was the same as primary anastomosis without leak. There was no complication related to healing process at the leak site, which was our concern.
Based on the outcome of the initial salvage operation we divided the patients into two groups. Success group in-cluded 12 patients with preservation of anastomosis after salvage procedure. Failure group included 3 patients with uncontrolled sepsis associated with systemic manifesta-tions after our salvage procedure, who had their anasto-mosis taken down finally. In failure group, one patient had history of taking steroid and one patient had received neo-adjuvant therapy. In the success group 4 (33.3%) pa-tients needed repeat operation for peritoneal wash with a mean of 1.25 times, but all of patients in the failure group needed repeat operation for peritoneal wash with a mean of 2 times. One patient in the failure group died after 31 days from multiple organ failure as a consequence of un-controlled sepsis. The small sample size precluded any meaningful statistical comparison between two groups.
Discussion
The incidence of anastomosis leakage in colorectal sur-gery varies from 1% to 12%; perhaps more frequent with those constructed in extra peritoneal deep pelvic space [8-19].Those patients who are re-operated generally have their anastomosis discontinued with an end colostomy created. Many of these stomas are never reversed [4]. However fecal stream diversion reports indicate anasto-motic healing ranging from 77-92%.[7,20,21]
Leakage of intra-peritoneal anastomosis usually contami-nates peritoneal cavity very soon. It may rarely be con-fined to a specific anatomic region for more than a few hours. However leakage from deep pelvic extra-peritoneal anastomosis may not spread into abdominal cavity for a while. This is due to its dependent position and wall off effect contributed by small bowel and omentum, and the lower volume of its content. This relative advantage pro-vides an opportunity for the surgeon to design a different management plan for this group of patients. Anastomotic leakage, when comes to surgery, is usually managed by discontinuing the anastomosis and exteriorization of both or the proximal limb. This approach is followed for both intra-peritoneal and extra-peritoneal anastomosis leakage. However extra-peritoneal leakage may be successfully managed by diversion [12].
Management of patient with leak should aim at achieving three goals, control of sepsis, local decontamination, and salvage of anastomosis. The most important factor in con-trol of sepsis is inhibiting the ongoing local contamination and infection, the origin of sepsis. This is best achieved by diversion of fecal stream. Studies show that proximal diversion is just as effective as anastomosis take down [20]. Complete proximal diversion may be accomplished by a loop enterostomy and closure of distal opening, ei-ther sutured or stapled. Sutures can be easily removed later when healing has occurred, however staples don’t provide this option. Diversion may not be enough if the colon proximal to leak site contains bacterial load. Al-though colon preparation is performed for patients before colorectal surgery, bacterial re-colonization of gut lumen takes place soon after the chemical preparation is washed out by mucus stream. Therefore, to help control local con-tamination, complete on table wash out of distal limb of loop diversion is preferred. All the primary anastomoses were stapled, therefore the role of anastomosis technique is omitted. All of our patients had open drainage of pelvis during the primary operation which helped detect leakage at early stage. Unexpectedly, secretions drain effectively cephalad through lower abdominal drains.
This study was conducted to evaluate a clinical manage-ment in patients with major leakage from low rectal anas-tomosis aimed at saving the anastomosis in place while providing conditions that help disruption seal. According to our experience we selected those patients who would benefit more, mainly those with small disruption. Data indicate that post operative fistulas originating from large disruptions (>30% of circumference) rarely close with conservative management [21]. Accordingly, we selected cases with disruption of less than a quarter of circumfer-ence (< 25% of circumference) for conservative manage-ment. Patients with malignancy and inflammatory bowel disease, as their primary pathology, were both included as it was presumed that their anastomoses were done at healthy tissue, free from disease, so healing will proceed normally. Although 2 of 3 patients with ulcerative colitis had taken steroids recently but they were younger and the proximal side of their anastomoses was ileal pouch with a generally better perfusion than a left colon in aged pa-tients with malignancy.
There are several risk factors for anastomoses leakage. History of taking steroids, radiation to pelvis and chemo-therapy for cancer are known important factors [10,22,23]. Other risk factors include malnutrition, weight loss, use of alcohol, intra-operative contamination, long operation time, and multiple blood transfusions [24]. Rate of leakage from rectal anastomosis has been inversely related to the distance of anastomosis from anal verge [8,10,22]. In failure group of our study, one patient had history of taking steroid and one patient had received neo-adjuvant therapy. Thirteen patients had anastomosis site < 5cm from anal verge: 10 patients (83.3%) in the success group, and 3 patients (100%) in the failure group. Mortal-ity after anastomotic leaks is reported to range from 0.5% to 36 percent [21,25,26]. In our series, the mortality rate was 6.6%.
We were able to save anastomosis in 80% of patients with major clinical leak. 86.6% of patients had primary anas-tomosis made at a distance less than 5 cm from anal verge. Comparatively, we may state that our management leads to a high number of preserved primary anastomoses after an anastomotic leak [7,20]. Given the small number of patients, this study cannot definitely conclude that the suggested management is the preferred method of han-dling a small very low rectal anastomosis leak. However this study shows that 80% of patients with clinically sig-nificant anastomotic leaks due to small disruptions (less than a quarter of circumference) may proceed to complete healing with fecal diversion, peritoneal toilet and sepsis control. Furthermore, morbidity and mortality rates may be lowered in this way compared to anastomotic resec-tion.
In conclusion, patients with very low rectal anastomosis that develop major leakage may be exempted anastomosis take down, if disruption length is small and complete di-version plus local and systemic infection control is ac-complished. However, we don’t consider this manage-ment appropriate for minor, subclinical, leaks that are better managed more conservatively and with less inter-vention.
References
- Meade B, Moran B. Reducing the incidence and man-aging the consequences of anastomotic leakage after rectal resection. Acta Chir Iugosl 2004; 51: 19-23.
- van Geldere D, Fa-Si-Oen P, Noach LA, Rietra PJ, Peterse JL, Boom RP. Complications after colorectal surgery without mechanical bowel preparation. J Am Coll Surg 2002; 194: 40-47.
- Platell C, Barwood N, Dorfmann G, Makin G. The in-cidence of anastomotic leaks in patients undergoing co-lorectal surgery. Colorectal Dis 2007; 9: 71-79.
- Isbister WH. Anastomotic leak in colorectal surgery: a single surgeon’s experience. ANZ J Surg 2001; 71: 516-520.
- Tuson JR, Everett WG. A retrospective study of colos-tomies, leaks and strictures after colorectal anastomo-sis. Int J Colorectal Dis 1990; 5(1): 44-48.
- Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 2004; 6: 462-469.
- Petit T, Maurel J, Lebreton G, Javois C, Gignoux M, Segol P. Results and Indications of lateral ileostomy functionally terminated in colorectal surgery. Ann chir 1999; 53: 949-953.
- Willis S, Stumpf M. Leakages after surgery of the low-er gastrointestinal tract. Chirurg 2005; 76: 612-613.
- Testini M, Margari A, Amoruso M, Lissidini G, Bonomo GM. The dehiscence of colorectal anasto-moses the risk factors. Ann Ital Chir 2000; 71: 433-440.
- Lipska MA, Bissett IP, Parry BR, Merrie AE. Anasto-motic leakage after lower gastrointestinal anastomosis: men are at higher risk. ANZ J Surg 2006; 76: 579-585.
- Eckmann C, Kujath P, Schiedeck TH, Shekarriz H, Bruch HP. Anastomotic leakage following low anterior resection: results of a standardized diagnostic and the-rapeutic approach. Int J Colorectal Dis 2004; 19 (2): 128-133.
- Schmidt O, Merkel S, Hohenberger W. Anastomotic leakage after low rectal stapler anastomosis: signifi-cance of intraoperative anastomotic testing. Eur J Surg Oncol 2003; 29: 239-243.
- Kanellos I, Zacharakis E, Christoforidis E, De-metriades H, Betsis D. Low anterior resection without defunctioning stoma. Tech Coloproctol 2002; 6:153-156.
- Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: its later than you think. Ann Surg 2007; 245: 254-258.
- Penna Ch. Management of anastomotic fistula follow-ing excision of rectal cancer. J Chir 2003;140:149-155.
- Bellows CF, Webber LS, Albo D, Awad S, Berger DH. Early predictors of anastomotic leaks after colectomy. Tech Coloproctol 2009; 13 (1): 41-47.
- Kruschewski M, Rieger H, Pohlen U, Hotz HG, Buhr HJ. Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer. Int J Colorectal Dis 2007; 22 (8): 919-927.
- Chambers WH, Mortensen NJ. Postoperative leakage and abscess formation after colorectal surgery. Best Pract Res Clin Gastroenterol 2004; 18: 865-880.
- Leester B, Asztalos T, Polnyib C. Septic complications after low anterior rectal resection diverting stoma. Acta Chir Iugosl 2002: 49: 67-71.
- Damjanovich L, Bartha I, Balazs G, Lukacs G. The role of ileostomy in the prevention and treatment of compli-cations of deep rectal anastomoses. Magy Seb 2003; 56: 113-115.
- Hedrick TL, Sawyer RG, Foley EF, Friel CM. Anasto-motic leak and the loop ileostomy: friend or foe. Dis Colon Rectum 2006; 49: 1167-1176.
- Alberts JC, Parvaiz A, Moran BJ. Predicting risk and diminishing the consequence of anastomotic dehis-cence following rectal resection. Colorectal Dis 2003; 5: 478-482.
- Rodríguez-Ramírez SE, Uribe A, Ruiz-García EB, La-bastida S, Luna-Pérez P. Risk factors for anastomotic leakage after preoperative chemoradiation therapy and low anterior resection with total mesorectal excision for locally advanced rectal cancer. Rev Invest Clin 2006; 58: 204-210.
- Makela JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum 2003; 46: 653-660.
- Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum 2005; 48: 1021-1026.
- Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal sur-gery: a prospective monocentric study. Int J Colorectal Dis 2008; 23 (3): 265-270.