- Biomedical Research (2015) Volume 26, Issue 4
Long-term assessment of bone formation in response to Gen Os and Gel 40 xenografts in an experimental rat model
Hakan Develioğlu1, Serpil Ünver Saraydın2, Zeliha Akkus3, Zeynep Deniz Sahin4, Olcay Bakar51,3,5Department of Periodontology,Faculty of Dentistry,Cumhuriyet University,Sivas,Turkey
2,4Department of Histology and Embryology, Faculty of Medicine, Cumhuriyet University,Sivas, Turkey
- *Corresponding Author:
- Hakan Develioglu
Cumhuriyet University, Faculty of Dentistry
Department of Periodontology
Sivas, 58140 Turkey
Accepted June 25 2015
Abstract
The study?s aim was to assess long-term bone healing associated with two different xenografts in an experimental model of parietal bone defects in rats. We surgically created two symmetrical, full-thickness, parietal bone defects 5 mm in diameter in each of 12 rats, which were then divided randomly into three groups (eight defects per group): group 1, defects filled were with Gen Os; group 2, defects were filled with Gel 40; and group 3 (control) defects were left empty. There was substantial bone formation in group 1, but no to minimal bone formation was seen in the other groups. Significant differences were observed between groups 1 and 2 and groups 1 and 3 (p < 0.05 for both). Gen Os and Gel 40 were both osteoconductive and biocompatible. Based on the long-term outcomes in our study, Gen-Os xenograft is more conducive to bone regeneration, but further studies are requiredKeywords
critical-sized defect, xenograft, bone regeneration, parietal bone
Introduction
Periodontal diseases are destructive by nature, and the basic purpose of periodontal treatment is to regenerate periodontal tissue that has been lost [1]. This requires new bone formation and cementum, together with newly inserted, functionally oriented fibers at a tooth site previously exposed to the oral environment [2]. Many graft materials are used therapeutically, including xenografts, which are materials derived from other species with their organic components totally removed. Removal of the organic components prevents immune reactions by the host. The remaining inorganic structure provides a natural matrix and a perfect calcium source [3]. Xenografts have many advantages [4]; however, the high price, timeconsuming production process, and ethical issues pertaining to animal slaughter are disadvantages [5]. Thus, production of optimal bone graft material is desirable [6].
One potential source of xenograft material is immature calf bone, produced via a process that includes treatment with chemical detergents, freezing, and desiccation. Although the product may be acceptable as graft material to heal minor bone defects, it is not an effective bone substitue [7-8].
A critical-sized bone defect is a defect that will not heal spontaneously without osteopromotive material during the lifetime of the animal [9-10]. It is not entirely understood why a small defect can be repaired, but a large defect cannot [11] , and there is little information regarding how bone formation ceases during the repair of a critical-sized defect. Some studies have investigated healing after application of various biomaterials (e.g., xenografts) in critical-sized defects in animals [12-16].
To the best of our knowledge, Gen Os® and Gel 40® have not been investigated previously as xenografts in rat cranial defects. Therefore, the goal of the present study was to assess the long-term effects of Gen Os and Gel 40 on bone regeneration in experimentally created parietal bone defects in rats.
Materıals and Methods
Animals
Twelve albino Wistar rats weighing 230–300 g were used in the study. Rats were housed in separate cages under standard laboratory conditions and fed a standard food. The investigation was approved by the Animal Ethics Committee of Cumhuriyet University.
Materials
Gen Os (Tecnoss, Giaveno, Italy) is a mixture of cancellous and cortical heterologous porcine bone. The particles range in size from 300 to 1000 μm. Gel 40 (Tecnoss) is a mixture of 60% cortical and cancellous heterologous equine bone (300 μm particle size) and 40% collagens I– III.
Surgical procedure
Rats were anesthetized with a combination of ketamine (Ketalar®; Pfizer, Berlin, Germany) and xylazine HCL (Rompun®; Bayer, Leverkusen, Germany). The dorsal part of the cranium was shaved and then disinfected with povidone-iodine. A 3-cm midline linear skin incision was made on the dorsal part of the cranium. The skin and the periosteum were then dissected gently so that the parietal bones were visible. Two symmetrical, circular, 5-mm diameter, full-thickness bone defects were created with a trephine bur (Meisinger, Düsseldorf, Germany) under saline irrigation. Rats were randomly divided into three groups. In group 1 (n = 8 defects), the defects were filled with Gen Os. In group 2 (n = 8 defects), the defects were filled with Gel 40. In group 3 (n = 8 defects), the defects were left empty as controls. Extreme care was taken to avoid injury to the sinus and dura mater. After application of the products, soft tissues were repositioned and sutured with 3-0 silk suture material. Postoperatively, the appropriate antibiotics and analgesics were administered to the rats for infection and pain control. The sutures were removed 10 days after surgery. Healing was uneventful until the day that the rats were killed and the defects analyzed. No convulsions, inflammation, allergic reactions, or complications around the surgical area were observed.
The rats were killed by intravenous injection of sodium pentobarbital 9 months after the operation. Blocks were taken from the site on the cranium and included the defects and normal bone. Specimens were fixed in 10% buffered neutral formalin for 72 h and decalcified in Shandon TBD-1 rapid decalcifier (Thermo Scientific, West Palm Beach, FL, USA) for 48 h. After rinsing with tap water, specimens were dehydrated in increasing concentrations of ethanol and embedded in paraffin; 7-μm thick sections were made on the transverse plane and stained with Papanicalou’s solution 1a (Harris hematoxylin; Merck, Darmstadt, Germany) plus eosin Y 0.5% aqueous solution (Merck) (H&E) and van Gieson’s stain (MOS). Histological evaluations of the specimens were performed under a light microscope (Jenamed 2; Carl Zeiss, Jena, Germany), and bone healing was scored. New bone formation was classified according to a previously described semiquantitative classification system [17].
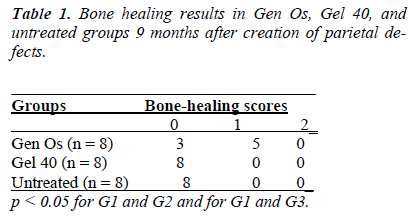
According to this classification system, no or minimal bone healing with fibrous tissue interposition was graded as 0, partial bone healing with occasional fibrous tissue ingrowth was graded as 1, and complete bone healing that bridged the defect was graded as 2.
The nonparametric Kruskal–Wallis and Mann–Whitney U tests were used for statistical analysis of the data. Statistical significance was set to p < 0.05. Statistical tests were performed with SPSS version 14.0 (SPSS Inc., Chicago, IL, USA)
Results
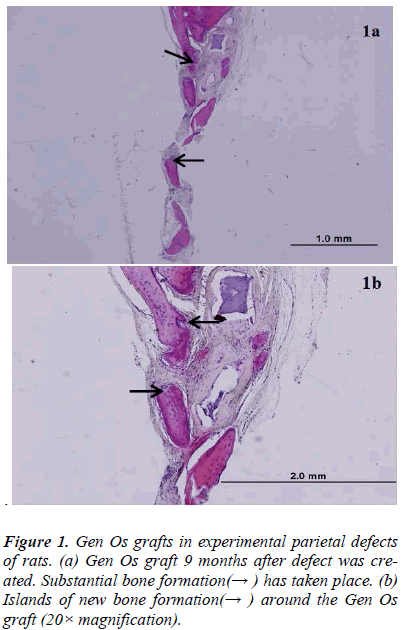
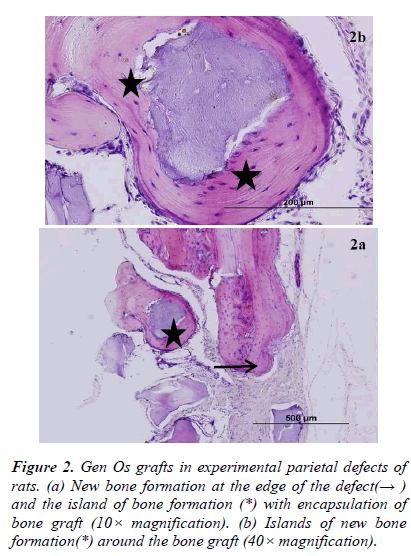
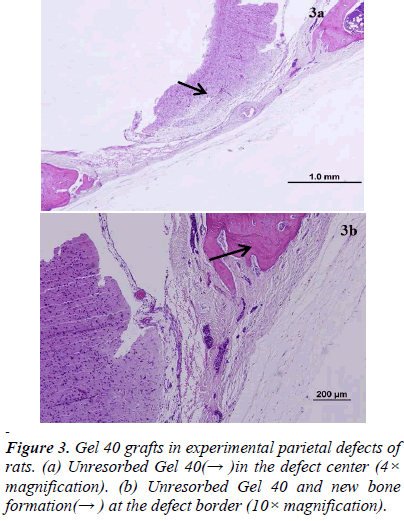
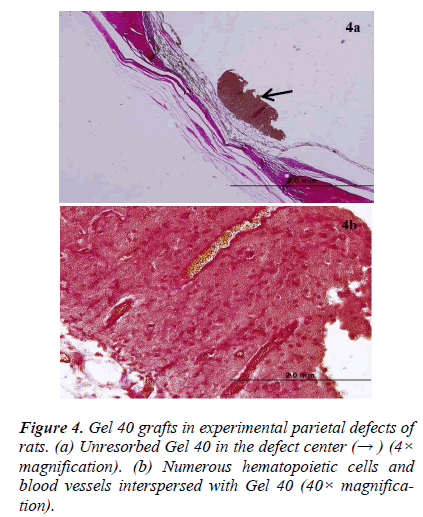
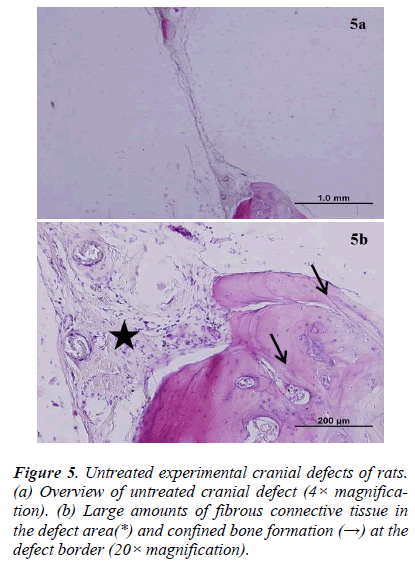
There was new bone formation at the center of the defects in group 1 rats. The Gen Os material was surrounded by fibrous connective and bone tissue in some areas (Figures 1, 2). Only minimal amounts of bone formation were detected at the borders of the defects in group 2. In this group, Gel 40 was not resorbed (Figures 3, 4). Many hematopoietic cells and blood vessels were seen in different areas interspersed with Gel 40 (Figure 4b). In the control group, there was no new bone formation in the defect center, although minimal new bone formation was seen at the edges of the residual bone (Figure 5). New bone formation was detected at the defect border in all of the groups. We did not observe any necrosis in the form of cytoplasmic or nuclear abnormalities or tumor formation in the area around the defect. In addition, no inflammation was observed. Differences in the amount of bone healing that had occurred were statistically significant between groups 1 and 3 and between groups 1 and 2 (p < 0.05 for both), but not between groups 2 and 3 (Table 1).
Discussıon
In the present study, we evaluated the bone-healing effects of two different types of xenograft, Gen Os and Gel 40, in experimentally created critical-sized defects in the parietal bone of rats. Nine months after the defects were created, we analyzed the results histologically and found that Gen Os, but not Gel 40, appeared to promote bone healing.
In one of the first attempts to investigate healing of the bones of the cranium, little scientific information was yielded, because the defects that were created did not reach the critical size [18]. It is now known that there is a size threshold beyond which a defect cannot heal spontanously without the presence of osteopromotive material. [9-10].The size of critical-sized defects can vary in rats, but in the present study, we selected 5 mm, since this size met our requirements in previous studies [14,19].
To the best of our knowledge, the present study was the first to perform a long-term assessment of Gen Os and Gel 40 xenograft materials in rat critical-sized parietal bone defects. Gen Os and Gel 40 are in the form of dried granules and gel, respectively, which were easy to apply to the defects. Based on our histological analysis, Gen Os promoted new bone growth. In addition, it is likely that the multinucleated giant cells that we observed were reponsible for resorption of the granular material, as previously described [20] . On the other hand, there was minimal to no new bone formation in the Gel 40 group. It is possible that the gel xenograft was still present after 9 months due to the location of the defect and the physical conditions in the adjacent areas. In some of the Gel 40– treated defects, there were cells that likely originated in the bone marrow interspersed among the remaining gel particles. The control defects were filled with fibrovascular tissue and only minimal or no bone formation was observed. Explanations of why critical-sized defects cannot heal spontaneously have been published previously [21,22].
There have been few investigations in which commercially available xenograft materials were tested in experimental models of cranial defects [14-16,23,24]. Generally, these materials are used for clinical purposes [4,25,26] and there have been some experiments in which they were used in experimentally created defects in parts of the body other than the cranium [27-29].
The xenogenic graft material Bio-Oss® has been investigated by many researchers. It is reported to be biocompatible and osteoconductive and is replaced by newly formed bone [4,26]. However, there have been conflicting findings regarding the resorption characteristics of Bio- Oss. Some researchers have shown that it is resorbed [30] , while others have claimed that the resorption rate of Bio- Oss is very slow [31]. In general, good clinical results have been achieved with Bio-Oss for sinus floor augmentation [32] and to fill the sockets of extracted teeth [4,25]. Good results have also been yielded by studies that employed rabbit mandibles [33] and calvaria [34]. Based on our findings, Gen Os is biocompatible and osteoconductive and thus has properties similar to those of Bio-Oss.
In a study similar to the present one, Develioglu et al. [15,16] investigated the effects of Unilab Surgibone® xenografts on bone healing after short- and long-term implantation in rat parietal critical-sized bone defects. According to their results, this material was not resorbed either in the short or long term. Moreover, the xenograft particles were surrounded by a fibrous tissue layer at the implantation site. Osteoclast-like cells were also observed. Unilab Surgibone appeared to be osteoconductive and biocompatible, but it did not have a significant effect on bone regeneration and there was very little resorption. Another experimental study with Unilab Surgibone confirmed that this material does not have cytotoxic effects [34]. By contrast, in the present study, Gen Os was associated with bone healing; thus, this material may significantly promote bone regeneration without adverse effects. In a recent case report in which Unilab Surgibone was used to treat furcation defects in a patient, good results were seen both clinically and radiographically, suggesting that it would be suitable for routine clinical use [35]. By contrast, poor results were reported from a case series [36] in which Unilab Surgibone was used in revision hip surgery. Festa et al [37] used Gen Os to treat extraction sockets in humans and showed that this material, when combined with a membrane, can reduce hard tissue resorption. This is in line with our results for critical-sized defects treated with Gen Os. Moreover, in another study, a mixed bovine bone xenograft was tested in calvarial critical-sized defects in rats. Bone growth was examined 1, 3, 6, and 9 months after the defect was created. According to the results, this xenograft material did not strongly stimulate bone regeneration in the defects [23].
Gel 40 was tested previously in an experimental study of rabbit maxilla defects [38].The authors found that it had good effects on bone regeneration and underwent resorption after 8 wk of observation. This in in contrast with our finding that Gel 40 remained unresorbed. It is possible that these differing results were due to the different locations of the defects and how the gel was applied. An experimental study of calvarial defects in a rat model with a different gel biomaterial yielded positive findings similar to those of the rabbit maxilla study [39]. Moreover, the different features of maxilla bone also should be considered. Future studies of Gel 40 at different anotomical locations may clarify the resorption process and the effects of the gel on bone healing.
In summary, the present study revealed that Gen Os xenografts promoted bone regeneration compared to Gel 40 and untreated defects. However, further large studies are needed to understand whether either of these materials are suitable for the treatment of periodontal and periimplant defects.
References
- Zander HA, Polson AM, Heijl LC. Goals of periodontaltherapy.J Periodontol 1976; 47: 261-266.
- Moon HJ, Kim KN, Kim KM, Choi SH, Kim CK, LeGeros RZ, Lee YK. Effect of calcium phosphate glass on bone formation in calvarial defects of Sprague- Dawley rats.J Mater Sci Mater Med. 2006; 17: 807- 813.
- Doherty MJ, Schlag G, Schwarz N, Mollan RA, Nolan PC, Wilson DJ. Biocompatibility of xenogeneic bone, commercially available coral, a bioceramic and tissue sealant for human osteoblasts.Biomaterials. 1994; 15:601-608.
- Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003; 14: 137-143.
- Amirfeyz R, Stanley D. Allograft-prosthesis composite reconstruction for the management of failed elbow replacement with massive structural bone loss: a medium- term follow-up. J Bone Joint Surg Br; 93: 1382-1388.
- Hoexter DL. Bone regeneration graft materials.J Oral Implantol.2002; 28: 290-294.
- Ellis E 3rd, Sinn DP. Use of homologous bone in maxillofacial surgery. J Oral Maxillofac Surg. 1993 ; 51:1181-1193.
- Rissolo AR, Bennett J. Bone grafting and its essential role in implant dentistry. Dent Clin North Am. 1998 ;42: 91-116.
- Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacialnonunions.ClinOrthopRelat Res 1986; 205: 299-308.
- Develioglu H. Critical sized and non critical sized defects. CumhuriyetUniv Dis HekFakDerg 2003; 1: 60-63.
- Honma T, Itagaki T, Nakamura M, Kamakura S, Takahashi I, EchigoS,Sasano Y. Bone formation in rat calvaria ceases within a limited period regardless of completion of defect repair. Oral Dis 2008 ; 14: 457-464.
- Kamakura S, Sasano Y, Homma H, Suzuki O, KagayamaM, Motegi K. Implantation of octacalcium phosphate nucleates isolated bone formation in rat skull defects. Oral Dis; 2001;7: 259-265.
- Dupoirieux L, Pourquier D, Picot MC, Neves M. The effect of pentosanpolysulphate on bone healing of rat cranial defects. J CraniomaxillofacSurg 1999; 27: 314- 320.
- Develioglu H, Saraydın SU, Bolayır G, Dupoirieux L. Assessment of the effect of a biphasic ceramic on bone response in a rat calvarial defect model. J Biomed Mater Res A 2006; 77: 627-631.
- Develioglu H, UnverSaraydin S, Kartal U. The bonehealingeffect of a xenograft in a rat calvarial defect model. Dent Mater J 2009; 28: 396-400.
- Develioglu H, Saraydın S, Kartal U, Taner L. Evaluation of the long-term results of rat cranial bone repair using a particular xenograft. J Oral Implantol2010; 36: 167-173.
- Bosch C, Melsen B, Vargervik K. Guided bone regeneration in calvarial bone defects using polytetrafluoroethylenemembranes. Cleft Palate Craniofac J 1995; 32:311-317.
- Turnbull RS, Freeman E. Use of wounds in the parietalbone of the rat for evaluating bone marrow for grafting into periodontal defects.J Periodontal Res 1974; 9: 39- 43.
- Bosch C, Melsen B,Vargervik K. Importance of the critical-size bone defect in testing bone-regenerating materials. J CraniofacSurg 1998; 9: 310-316.
- Anderson JM. Multinucleated giant cells.CurrOpinHematol 2000; 7: 40-47.
- Najjar TA, Kahn D. Comparative study of healing and remodeling in various bones.J Oral Surg 1977; 35: 375-379.
- Dersot JM, Septier D, Llorens A, Saffar JL. Multinucleated giant cells-a time-related quantitative study in a rat skull defect. Cells Mater 1993; 3: 395-405.
- Accorsi-Mendonça T, Zambuzzi WF, Bramante CM,CestariTM,TagaR,Sader M, de AlmeidasaaresGD, Granjeiro JM . Biological monitoring of a xenomaterialfor grafting: an evaluation in critical-size calvarial defects. J Mater Sci Mater Med. 2011; 22: 997- 1004.
- Beltrán V, EngelkeW, Dias FJ, Leiva C, Fuentes R, Borie E. Occlusive barriers in combination with particulate Bio-Oss® graft: a pilot study on rabbit calvaria. Int J ClinExp Med. 2014; 15: 1714-1720.
- Araújo MG, da Silva JC, de Mendonça AF, Lindhe J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial.Clin Oral Implants Res 2015; 26(4): 407-412.
- Sculean A, Chiantella GC, Windisch P, Gera I, Reich E. Clinical evaluation of an enamel matrix protein derivative (Emdogain) combined with a bovine-derived xenograft (Bio-Oss) for the treatment of intrabony periodontal defects in humans.Int J Periodontics Restorative Dent 2002; 22: 259-267.
- Donos N, Bosshardt D, Lang N,GrazianiF,TonettiM,Karring K, Kostopoulus L. Bone formation byenamel matrix proteins and xenografts: an experimental study in the rat ramus. Clin Oral Implants Res 2005; 16: 140-146.
- Zhang X, CaiQ, Liu H, Heng BC, Peng H, Song Y,Yang Z, Deng X . Osteoconductive effectiveness of bone graft derived from antler cancellous bone: an experimentalstudy in the rabbit mandible defect model. Int J Oral MaxillofacSurg 2012; 41: 1330-1337.
- Zambuzzi WF, Oliveira RC, Subitoni BL, Menezes R, Taga R, Granjeiro JM. Biological monitoring of a promissoryxenogenic pin for biomedical applications: a preliminary intraosseous study in rats. Clin Oral Implants Res 2012; 23: 367-372.
- Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation in rats by Bio-Oss. Scand J Dent Res. 1991; 99: 154-161.
- Klinge B, Alberius P, Isaksson S, JönssonJ . Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J Oral MaxillofacSurg 1992; 50: 241-249.
- Bassil J, Senni K, Changotade S, Baroukh B, Kassis C, Naaman N, Godeau G. Expression of MMP-2, 9 and 13 in newly formed bone after sinus augmentation using inorganic bovine bone in human. J Periodontal Res 2011; 46: 756-762.
- Veis A, Dabarakis N, Koutrogiannis C, Petsa E, RomanosG. Evaluation of vertical bone regeneration using block and particulate forms of Bio-Oss® bongraft. A histological study in rabbit mandible. J OralImplantol 2015; 41(3): 66-72.
- Saraydın SÜ, Develioğlu H. Evaluation of the bone repair capacity and the cytotoxic properties of a particular xenograft: An experimental study in rats. TurkiyeKlinikleri J Med Sci 2011; 31; 541-547.
- Develioglu H, Altintepe SS. A New Bone Substitute in the Definitive Management of Furcation Involvement: A Case Report. West Indian Med J 2014; 63: 201-204.
- Charalambides, C., Beer M , Cobb AG . Poor results after augmenting autograft with xenograft (Surgibone) in hip revision surgery: a report of 27 cases. ActaOrthop2005; 76: 544-549.
- Festa VM, AddabboF, Laino L, Femiano F, Rullo R. Porcine-derived xenograft combined with a soft cortical membrane versus extraction alone for implant site development: a clinical study in humans. Clin Implant Dent Relat Res 2013; 15: 707-713.
- Nannmark U, Azarmehr I. Short communication: collagenatedcortico-cancellous porcine bone grafts. A study in rabbit maxillary defects. Clin Implant Dent RelatRes 2010;12: 161-163.
- Stephan SJ, Tholpady SS, Gross B, Petrie-Aronin CE, BotchwayEA,Nair LS, Ogle RC, Park SS. Injectable Tissue-Engineered Bone Repair of a Rat Calvarial Defect.Laryngoscope 2010; 120: 895-901.