Research Article - Journal of Clinical Ophthalmology (2023) Volume 7, Issue 3
Long term outcome of therapeutic penetrating keratoplasty for severe fungal keratitis.
Sathiya Kengpunpanich*, Pinnita Prabhasawat, Chencho Gem, Chareenun Chirapapaisan, Wipawee Booranapong, Panotsom Ngowyutagon
Department of Ophthalmology, Mahidol University, Bangkok, Thailand
- Corresponding Author:
- Sathiya Kengpunpanich
Department of Ophthalmology,
Mahidol University,
Bangkok,
Thailand
E-mail: sathiya.ken99@gmail.com
Received: 02-Jan-2023, Manuscript No. AACOVS-23-85209; Editor assigned: 04-Jan-2023, AACOVS-23-85209 (PQ); Reviewed: 18-Jan-2023, QC No. AACOVS-23-85209; Revised: 09-Mar-2023, Manuscript No. AACOVS-23-85209 (R); Published: 17-Mar-2023, DOI:10.35841/aacovs.7.2.637-644
Citation:Kengpunpanich S, Prabhasawat P, Gem C, et al.. Long term outcome of therapeutic penetrating keratoplasty for severe fungal keratitis. J Clin Ophthalmol. 2023;7(2): 637-644.
Abstract
Purpose: To evaluate the outcomes of Therapeutic Penetrating Keratoplasty (TPK) performed for severe fungal keratitis.
Methods: Medical records of all patients who underwent TPK in Siriraj medical center between April 2010 and July 2020 were culled and those in which fungal pathogens were definitively identified were studied. Patient records with follow up less than three months were excluded. Patient demographic data, outcome measures and complications following TPK were recorded. The primary outcome was eradication of the fungal infection. Secondary outcomes were preservation of anatomical integrity, graft survival and achievement of Visual Acuity (VA) greater than or equal to 3/60. Results: Sixty patients met the study criteria and were included in the analysis. The mean patient age was 56 (range: 23-79) years and most patients were men (46, 77%). Fifteen eyes (25%) sustained corneal perforation before undergoing TPK. Graft survival was 30% at 1 year, 18% at 5 years and 11% at 10 years. The most common organism was Fusarium (23 patients, 38%). The median duration from presentation to surgery was 14 (8-21) days. Disease eradication was achieved in 44 patients (73%) and VA better than 3/60 was achieved in 14 (23%). Anatomical integrity was maintained in 46 (76%) eyes. Repeat PKP was performed in 15 patients (25%), most commonly for recurrent infection.
Conclusion: TPK offers a good chance of disease eradication and maintenance of anatomical globe integrity and is a reasonable therapeutic option in patients with severe fungal infection.
Keywords
Therapeutic Penetrating Keratoplasty (TPK), Fungal keratitis, Fungal ulcer, Fungal pathogens, Visual Acuity (VA)
Introduction
Infectious keratitis is a vision threatening condition and the leading cause of corneal blindness worldwide. Bacteria, fungi, viruses and protozoa are all well recognized pathogens and severe cases can progress to endophthalmitis or corneal perforation with devastating results. Fungal keratitis represents a particular treatment challenge due to its typically middling response to medical therapy.
In warm climates, filamentous fungal species, most notably Fusarium and Aspergillus are the most common types of fungal pathogens [1,2]. In cooler climates, yeast species predominate, typically Candida [3]. Most patients have a recognized history of corneal trauma or contact lens wear. In general, the first line management of fungal keratitis is with topical and systemic anti-fungal medications. The number of available and approved topical agents is small and most are azoles, the exception being natamycin, a polyene compound [4]. Periodic corneal debridement may improve drug penetration and is often performed as an adjunctive therapeutic procedure.
Therapeutic Penetrating Keratoplasty (TPK) is commonly performed to treat very severe fungal keratitis, as it logically reduces the sheer bulk of infectious organisms and replaces lysed and lysing corneal tissue [5,6]. In general, the goal in such circumstances is saving the globe. While there seems to be a general consensus that this approach is effective, available data and details remain incomplete. We undertook this study to shed additional light on the existing repository of data regarding outcomes following TPK for severe fungal keratitis.
Materials and Methods
This was a retrospective observational study conducted at Siriraj hospital, a 2200 bed tertiary health care center in Bangkok, Thailand. The study was approved by the research committee, faculty of medicine, Siriraj hospital, Mahidol university (study number SI 412/2020) and was performed in accordance with the declaration of Helsinki. The requirement for informed consent was waived by the research committee.
Medical records were reviewed for patients who underwent TPK for a diagnosis of fungal keratitis between April 2010 and July 2020. Records were included for analysis if definitive identification of fungal organisms was achieved by Potassium Hydroxide (KOH) smear, fungal culture, In vivo Confocal Microscopy (IVCM) or histopathologic analysis of the cornea button resected at the time of TPK. The decision to proceed with TPK was made at the discretion of the attending physician based on rapid progression of the fungal infection and poor response to medical therapy. Additionally, TPK was performed when the cornea was perforated or when Pythium was identified as a pathogen. Patients were excluded from analysis if fungi were not identified or if follow-up duration following TPK was less than three months.
Preoperative clinical data were recorded including patient age, gender, underlying systemic disease, contact lens use, history of predisposing corneal trauma, characteristics of the corneal ulcer, causative pathogens, Best Corrected Visual Acuity (BCVA), in Log Mar format, Intraocular Pressure (IOP) and the presence of perforation. Corneal ulcers were categorized according to the size of the infiltrate in the longest dimension. Severe ulcers were >6 mm, moderate ulcers were 2 mm-6 mm and small ulcers were <2 mm. Postoperative data collection included BCVA, IOP and surgical complications.
Outcomes data included eradication of infection, graft survival as defined by no recurrence of fungal infection and no graft failure regardless of cause (including rejection or other reasons for endothelial failure), anatomical survival as defined by preservation of the globe intact without phthisis bulbi or the need for enucleation or evisceration and BCVA better than or equal to 3/60. Where TPK was repeated, data collection after the second procedure was limited to these outcomes.
Statistical analysis
Data were analyzed using SPSS (Statistical Package for Social Sciences) software. Version 18.0 (SPSS Inc. Chicago, IL, USA). Mean (± standard deviation) and median (along with Interquartile Range, IQR) values were used to describe the parametric and nonparametric data, respectively. Frequency and percentage were used to describe the data.
Fisher’s exact test and Mann-Whitney U test were used to analyze predictive factors associated with the success of an outcome, p-value<0.05 was considered statistically significant. Survival of the graft and anatomical survival of the globe were represented using Kaplan-Meier curves.
Results
Patient demographic data
During the study period, 60 patients underwent TPK for fungal keratitis at Siriraj hospital. 46 patients (77%) were male. The mean age at presentation was 56 years (range 23-79 years). Right eyes were affected in 32 patients, left eyes in 28. 40 keratitis (67%) were moderate in size and 18 (30%) were severe. 15 (25%) keratitis sustained corneal perforation before TPK was performed.
Predisposing factors
A history of ocular surface trauma was obtained in 47 patients (78%). Of these 47 patients, 21 (45%) recalled trauma with plant material, while eight (17%) gave a history of insect induced trauma and seven (15%) recalled soil contaminated trauma. 2 patients (3.3%) reported a history of contact lens wear just prior to developing corneal symptoms.
Causative pathogens
Fusarium was identified in 23 (38%) ulcers, Aspergillus was identified in 7 (12%) and Pythium in 7 (12%). 13 fungal pathogens (21%) were visualized by IVCM but could not be characterized. These results are shown in Table 1.
| Pathogen | Number (%) |
|---|---|
| Fusarium | 23 (38) |
| Aspergillus | 7 (12) |
| Pythium | 7 (12) |
| Candida | 3 (5) |
| Penicillium spp. | 3 (5) |
| Others (Exserohilium, Culvuria, Purpureocillium) | 4 (7) |
| Unidentified fungal pathogen | 13 (21) |
Note: % from total N=60.
Table 1. Etiology of fungal keratitis.
Preoperative management
Preoperatively, most patients were treated with combine 2 topical antifungal medications; topical amphotericin B and topical fluconazole every 1 hour around the clock, although this regimen varied somewhat during a few years of the study period and topical natamycin 5% suspension and topical voriconazole 2% hourly were used in the later part of the study. The intrastromal and intracameral injection with fluconazole or voriconazole also considered in the case of deep keratitis or fungal ball in anterior chamber. Systemic oral itraconazole or oral voriconazole daily were used according to the culture result. Corticosteroids by any route were not used in any patients, preoperatively. The average duration between admission and TPK was 14 (8-21) days.
Surgical procedures
All TPK procedures were performed under general anesthesia. Intraoperatively, care was taken to size the graft 0.5 mm-1 mm greater than the widest dimension of the infiltrate. Following trephination and resection of the infected portion of host cornea, the anterior chamber and iris surface were irrigated with amphotericin B or voriconazole to remove exudates and hypopyon and the graft margin was inspected carefully to ensure that no necrotic tissue remained. The average graft size was nine 8 mm-10 mm. Each resected cornea button was divided into two roughly equal parts, one of which was further subdivided for culture on blood agar, chocolate agar, Sabouraud’s dextrose agar and thioglycollate broth agar. The second part was sent in formalin 1% for histopathological analysis.
58 (97%) eyes underwent TPK alone, 2 (3%) eyes underwent TPK combined with extracapsular cataract extraction, no patients sustained expulsive choroidal hemorrhage or other serious intraoperative complications.
Postoperative management
Postoperatively, patients were treated with topical natamycin 5% suspension and topical voriconazole 2% every hour, combined with oral itraconazole or voriconazole. The dose was adjusted depending on clinical response, assessments being made daily with attention to signs of recurrent infection. Topical and oral antiglaucoma medications were added as necessary in patients with IOP elevation. Topical corticosteroids may be used after 1 month if there was no sign of any fungal recurrence.
Postoperative findings and outcomes
During the first two postoperative weeks (acute phase), suture related complications were noted in 35 (58%) eyes and recurrent fungal infection was noted in 19 (32%). Subsequently (late phase), graft failure was noted in 25 (42%) eyes, glaucoma in 16 (27%) and cataract in 4 (7%). These results are shown in Table 2.
| Phase | Complications | Number* (%) |
|---|---|---|
| Acute (<2 weeks) | Suture related | 35 (58) |
| Recurrence of infection | 19 (32) | |
| PED | 9 (15) | |
| Endophthalmitis | 2 (3) | |
| Chronic (>2 weeks) | Graft failure | 25 (42) |
| Glaucoma | 16 (27) | |
| Graft rejection | 10 (17) | |
| Cataract | 4 (7) | |
| Phthisis | 1 (2) |
Note: PED: Persistent Epithelial Defect; *some patient may have more than one complication; % from total N=60.
Table 2. Complications of TPK.
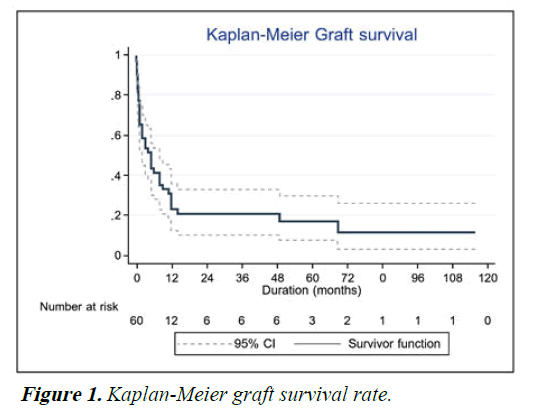
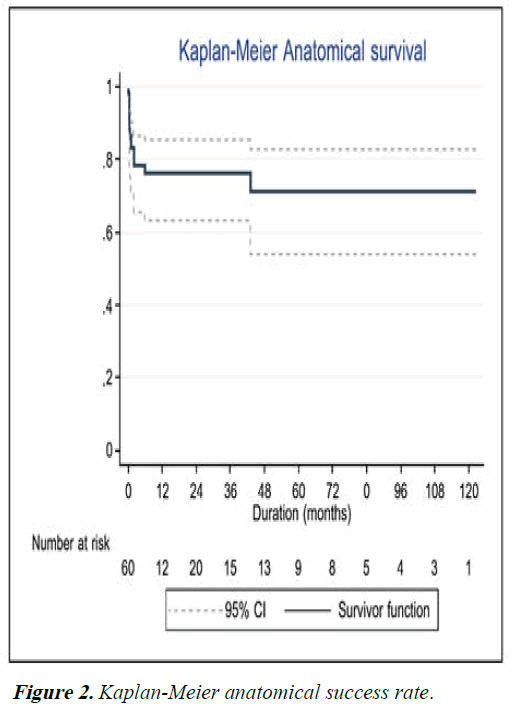
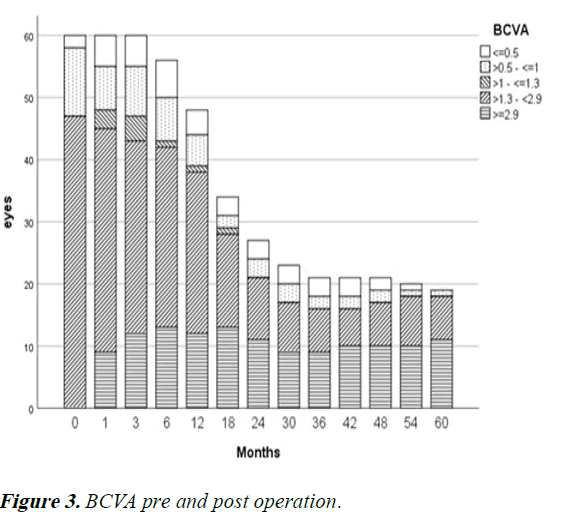
Recurrence of infection was noted in 19 (32%) eyes following TPK. 8 (14%) eyes underwent repeat TPK for this indication, while nine patients (15%) ultimately underwent enucleation and four (7%) ultimately underwent evisceration. Graft survival at one year following TPK was 30%, decreasing to 18% at 5 years and 11% at 10 years. These results are shown using Kaplan-Meier curve in Figure 1. Anatomical survival was achieved in 76% of eyes at one year, decreasing to 70% at 5 years and remaining at that level for 10 years as shown in Figure 2. Overall, 14 eyes (23%) achieved BCVA >3/60. Visual results are shown in Figure 3. Of the 15 eyes that sustained corneal perforation prior to TPK, graft survival was observed in 7 (47%) eyes and anatomical survival was observed 11 (73%) eyes at five years.
Repeat TPK was performed in 15 (25%) patients. Of this number, the indications for repeat TPK were fungal infection in eight (56%), graft failure in four (25%), graft rejection in 2 (13%) and 1 (6%) graft melting. Three of the repeat TPK procedures were in eyes that had sustained corneal perforation before the first TPK; one of these repeat TPKs was for recurrent infection and two were for graft failure (Table 3).
| Factors | Success (%) | Failure (%) | p-value | |
|---|---|---|---|---|
| Gender | Male | 34 | 12 | 1.00 |
| Female | 10 | 4 | ||
| Contact lens use | No | 43 | 15 | 0.47 |
| Daily | 0 | 1 | ||
| Monthly | 1 | 0 | ||
| Trauma details | No | 8 | 5 | 0.95 |
| Contaminated water | 4 | 1 | ||
| Agriculture products | 16 | 5 | ||
| Soil | 5 | 2 | ||
| Insects | 6 | 2 | ||
| Others | 5 | 1 | ||
| Perforation | No | 33 | 12 | 1.00 |
| Yes | 11 | 4 | ||
| Pathogen | Fusarium | 19 | 4 | 0.15 |
| Aspergillus | 3 | 4 | ||
| Pythium | 3 | 4 | ||
| Candida | 2 | 1 | ||
| Purpureocillium lilacinum | 1 | 0 | ||
| Exserohilium spp. | 1 | 0 | ||
| Penicillium spp. | 3 | 0 | ||
| Culvuria | 1 | 1 | ||
| Unidentified | 11 | 2 | ||
| Size of ulcer | Grade 1 (<2 mm) | 2 | 0 | 0.75 |
| Grade 2 (2 mm-6 mm) | 30 | 10 | ||
| Grade 3 (>6 mm) | 12 | 6 | ||
| Duration admission to surgery (days) | 15 | 8 | 0.056 | |
| Graft size | ≤ 8 mm | 9 | 9 | 0.42 |
| >8 mm | 28 | 14 | ||
Note: spp.=several species.
Table 3. Factors association with eradication of the disease.
Factor association with the functional outcome
Graft size less than 8 mm and preoperative IOP below 21 mmHg were statistically correlated with improved functional outcome. Moreover, we found that Candida, Purpureocillium lilacinum and Penicillium spp. were associated with better functional outcome. No other clinical findings were statistically significantly associated with disease eradication. These results are shown in Table 4.
| Factors | Success (%) | Failure (%) | p-value | |
|---|---|---|---|---|
| Gender | Male | 11 | 35 | 1.000 |
| Female | 3 | 11 | ||
| Contact lens use | No | 14 | 44 | 1.000 |
| Daily | 0 | 1 | ||
| Monthly | 0 | 1 | ||
| Trauma details | No | 3 | 10 | 0.780 |
| Contaminated water | 1 | 4 | ||
| Agriculture products | 4 | 17 | ||
| Soil | 3 | 4 | ||
| Insects | 1 | 7 | ||
| Others | 2 | 4 | ||
| Perforation | No | 9 | 36 | 0.300 |
| Yes | 5 | 10 | ||
| Pathogen | Fusarium | 2 | 21 | 0.015* |
| Aspergillus | 0 | 7 | ||
| Pythium | 2 | 5 | ||
| Candida | 2 | 1 | ||
| Purpureocillium lilacinum | 1 | 0 | ||
| Exserohilium spp. | 0 | 1 | ||
| Penicillium spp. | 2 | 1 | ||
| Culvuria | 1 | 1 | ||
| Unidentified | 4 | 9 | ||
| Duration admission to surgery(days) | 14 | 46 | 0.220 | |
| Pre-operative IOP | Normal <21 mmHg | 4 | 2 | 0.031* |
| High ≥ 21 mmHg | 10 | 44 | ||
| Graft size | ≤ 8 mm. | 9 | 9 | 0.003* |
| >8 mm | 5 | 37 | ||
Note: spp.=several species; statistically significant difference when *p<0.05.
Table 4. Factors association with the functional outcome.
Discussion
In this retrospective 10 years study of 60 patients with fungal keratitis, a single TPK procedure resulted in eradication of the infection in 73% of patients. Fusarium was the most identified pathogen, followed by Aspergillus and Pythium species. Ultimately anatomical preservation of the globe was achieved in 76%, while graft survival was 30% at 1 year. BCVA was 3/60 or better in 23% of affected eyes. Corneal perforation had occurred prior to TPK in 25% of cases and anatomic survival was achieved in nearly three fourths of these.
Our epidemiologic findings were quite similar to those of prior studies of fungal keratitis [7-12]. The mean patient age was 56 years and the majority were males. Trauma was the most common predisposing risk factor (78%), especially plant induced trauma (45%), quite as with other studies in Asian developing countries [13-15]. Fusarium has consistently ranked as the most common fungal keratitis pathogen in warm climates and our results followed this pattern.
No fungal pathogen could be isolated or observed on histopathologic analysis in 13 eyes (21%) despite our strong clinical impression along these lines and observation of fungal elements on IVCM. We assume this diagnostic shortfall is attributable to fungal organisms being present insufficient quantities to enable detection by culture or histopathologic analysis, the deeper of the lesion than corneal scraping or the medications that the patients received before coming to our tertiary center. In any case, the sensitivity of fungal culture is limited, often cited at approximately 44% so efforts at microbiologic detection and characterization are often disappointing [16-20].
The rate of pre-TPK corneal perforation in our patients was 25% (15 eyes) which is less than other studies of this therapeutic modality. Studies in Thailand, India and Nepal showed rates of 51.9%, 56% and 71%, respectively [21,22]. We assume this represents the degree to which patients have access to tertiary care facilities. Anatomical success was ultimately achieved in 73% of perforated eyes. Corresponding with previous studies, anatomical success in perforated eyes was 61.9% in Thailand and 82.5% in China [23]. TPK effected eradication of the fungal infection in 73% of eyes, maintenance of globe integrity in 76% at one year and 70% at 10 years follow-up. These results suggested that TPK had high beneficial advantage to eradicate and protect the globe in the fungal medication including the perforated eyes. Functional success was judged to have been achieved in 23% of patients.
These rates approximate or in the case of functional success fall a bit short of those in studies by Raj and Bajracharya. We suspect our lower functional success reflects differences in three important factors: 1) Patient population most obviously that bacterial keratitis was not included in the current study, 2) Precisely how functional success was determined and 3) The quality of donor corneas. Thailand has a more or less chronic shortage of donor corneas and those used in salvage procedures like TPK had generally lower endothelial cell counts than grafts reserved for more promising cases.
Functional outcomes in this study were significantly associated with three factors: 1) The identified pathogen; 2) Preoperative IOP and 3) Graft size. Candida, Purpureocillium lilacinum and Penicillium spp. were associated with better functional outcomes. Whether this observation is incidental or reflects actual lower virulence of these organisms remains unclear. Preoperative IOP<21 mmHg predicted a better functional outcome in our patients, a finding that suggests that elevated IOP reflects more advanced corneal infections, as has been previously suggested [24]. Larger graft sizes are well recognized to be more likely to sustain rejection in the other hand, the larger graft reflects the more severe of the keratitis. In the current study 8 mm was the cutoff, above which functional outcome was less likely. Our median graft size was 9 mm, which presumably affected our patients’ graft survival rate [25]. Taken together, these factors imply that the TKP since in the earlier stage of the disease may give the better the functional survival rate. However, regrafting in the recurrence fungal infection or failed graft still gives the benefit for saving the globe.
This study showed that our patients’ most common postoperative complication was suture related (58%) and fungal recurrence of infection (35%) in the first two weeks postoperatively [26]. Recurrence rates in similar studies have ranged from 26% to nearly 53%. Late phase complications were most commonly graft failure (42%) and glaucoma (27%). This rate of postoperative glaucoma was considerably higher than that colleagues have reported, a finding we suspect may be related to severity of fungal corneal ulcer case and postoperative PAS induced by large graft size and post-operative inflammation [27-29].
The most problematic pathogen in our patients was Pythium; one-fourths affected patients underwent evisceration or enucleation. Nevertheless, TPK effected anatomic success in 9 of these patients and only 2 required regrafting, a relatively good outcomes rate that suggests that early TPK may be in patients’ interest when this Pythium is detected.
The limitations of the study include the retrospective study. Comparisons between corneal ulcer studies are always complicated by interobserver variability: Clinical assessments are largely subjective, a fact which naturally limits the degree to which findings, including ours, can be generalized. Prevailing circumstances also impacted our study in that donor cornea availability was limited, meaning the timing of TPK was not entirely a question of surgeon preference and donor tissue was often suboptimal and we strongly suspect this impacted our graft survival and functional outcomes [30].
Conclusion
In conclusion, TPK is a reasonable option in severe fungal keratitis which is refractory to medical treatment, resulting in preservation of the globe when such an outcome seems otherwise unlikely. This study effort benefits from a relatively long follow-up period and contributes to the rather limited fund of data on TPK in the setting of fungal keratitis.
Acknowledgment
We thank Dr. Paul A. Gaudio for proofreading our manuscript.
Disclosure
The authors report no conflicts of interest in this work.
Conflict of Interest
No conflicting relationship exists for any author.
References
- Prajna VN, Prajna L, Muthiah S. Fungal keratitis: The Aravind experience. Ind J Ophthalmol. 2017;65(10):912.
[Crossref] [Google Scholar] [PubMed]
- Nath R, Baruah S, Saikia L, et al. Mycotic corneal ulcers in upper Assam. Ind J Ophthalmol. 2011;59(5):367-71.
[Crossref] [Google Scholar] [PubMed]
- Ting DS, Galal M, Kulkarni B, et al. Clinical characteristics and outcomes of fungal keratitis in the United Kingdom 2011-2020: A 10-year study. J Fungi. 2021;7(11):966.
[Crossref] [Google Scholar] [PubMed]
- Lakhani P, Patil A, Majumdar S. Challenges in the polyene and azole based pharmacotherapy of ocular fungal infections. J Ocul Pharmacol Ther. 2019;35(1):6-22.
[Crossref] [Google Scholar] [PubMed]
- Bajracharya L, Gurung R. Outcome of therapeutic penetrating keratoplasty in a tertiary eye care center in Nepal. Clin Ophthalmol. 2015;9:2299-304.
[Crossref] [Google Scholar] [PubMed]
- Prakash A, Singh P, Shah RP, et al. Therapeutic penetrating keratoplasty a retrospective analysis in rural population of central India. Del J Ophthalmol. 2012;23(1):23-6.
- Selver OB, Egrilmez S, Palamar M, et al. Therapeutic corneal transplant for fungal keratitis refractory to medical therapy. Exp Clin Transplant. 2015;13(4):355-9.
[Crossref] [Google Scholar] [PubMed]
- Tangpagasit W, Reanpinyawat T. Outcome of urgent penetrating keratoplasty for corneal ulcer at Thammasat university hospital. J Med Assoc Thai. 2016;99(1):71-6.
[Google Scholar] [PubMed]
- Yao YF, Zhang YM, Zhou P, et al. Therapeutic penetrating keratoplasty in severe fungal keratitis using cryopreserved donor corneas. Br J Ophthalmol. 2003;87(5):543-7.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhao GQ, Che CY, et al. Effect of corneal graft diameter on therapeutic penetrating keratoplasty for fungal keratitis. Int J Ophthalmol. 2012;5(6):698-703.
[Crossref] [Google Scholar] [PubMed]
- Ho JW, Fernandez MM, Rebong RA, et al. Microbiological profiles of fungal keratitis: A 10 years study at a tertiary referral center. J Ophthalmic Inflamm Infect. 2016;6(1):5.
[Crossref] [Google Scholar] [PubMed]
- Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85(9):1070-4.
[Crossref] [Google Scholar] [PubMed]
- Tananuvat N, Punyakhum O, Ausayakhun S, et al. Etiology and clinical outcomes of microbial keratitis at a tertiary eye care center in northern Thailand. J Med Assoc Thai. 2012;95:S8-17.
[Google Scholar] [PubMed]
- Tewari A, Sood N, Vegad MM, et al. Epidemiological and microbiological profile of infective keratitis in Ahmedabad. Indian J Ophthalmol. 2012;60(4):267-72.
[Crossref] [Google Scholar] [PubMed]
- Dhakhwa K, Sharma MK, Bajimaya S, et al. Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nepal J Ophthalmol. 2012;4(1):119-27.
[Crossref] [Google Scholar] [PubMed]
- Khor WB, Prajna VN, Garg P, et al. The Asia cornea society infectious keratitis study: A prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol. 2018; 195:161-70.
[Crossref] [Google Scholar] [PubMed]
- Das S, Sharma S, Kar S, et al. Is inclusion of Sabouraud dextrose agar essential for the laboratory diagnosis of fungal keratitis? Indian J Ophthalmol. 2010;58(4):281-6.
[Crossref] [Google Scholar] [PubMed]
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: A pooled analysis. Dermatol Res Pract. 2010.
[Crossref] [Google Scholar] [PubMed]
- Badiee P, Nejabat M, Alborzi A, et al. Comparative study of gram stain, potassium hydroxide smear, culture and nested PCR in the diagnosis of fungal keratitis. Ophthalmic Res. 2010;44(4):251-6.
[Crossref] [Google Scholar] [PubMed]
- Zhao G, Zhai H, Yuan Q, et al. Rapid and sensitive diagnosis of fungal keratitis with direct PCR without template DNA extraction. Clin Microbiol Infect. 2014;20(10):776-82.
[Crossref] [Google Scholar] [PubMed]
- Raj A, Bahadur H, Dhasmana R. Outcome of therapeutic penetrating keratoplasty in advanced infectious keratitis. J Curr Ophthalmol. 2018;30(4):315-20.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Zhao M, Xu M, et al. Outcomes of therapeutic keratoplasty for severe infectious keratitis in Chongqing, a 16 year experience. Infect Drug Resist. 2019;12:2487-93.
[Crossref] [Google Scholar] [PubMed]
- Sakiyalak D, Chattagoon Y. Incidence of and risk factors for secondary ocular hypertension in moderate to severe infectious ulcerative keratitis. Clin Ophthalmol. 2018;12:2121-8.
[Crossref] [Google Scholar] [PubMed]
- Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: Diagnosis and treatment. Curr Fungal Infect Rep. 2013;7(3):209-18.
[Google Scholar] [PubMed]
- Nurozler AB, Salvarli S, Budak K, et al. Results of therapeutic penetrating keratoplasty. Jpn J Ophthalmol. 2004;48(4):368-71.
[Crossref] [Google Scholar] [PubMed]
- Roozbahani M, Hammersmith KM, Nagra PK, et al. Therapeutic penetrating keratoplasty: A retrospective review. Eye Contact Lens. 2018;44 (Suppl 2):433-41.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Zhao M, Xu M, et al. Outcomes of therapeutic keratoplasty for severe infectious keratitis in Chongqing, a 16 years’ experience. Infect Drug Resist. 2019; 12:2487-93.
[Crossref] [Google Scholar] [PubMed]
- Mundra J, Dhakal R, Mohamed A, et al. Outcomes of therapeutic penetrating keratoplasty in 198 eyes with fungal keratitis. Indian J Ophthalmol. 2019;67(10):1599-1605.
[Crossref] [Google Scholar] [PubMed]
- Tew TB, Chu HS, Hou YC, et al. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 2001 to 2014. J Formos Med Assoc. 2020;119(6):1061-9.
[Crossref] [Google Scholar] [PubMed]
- Hasika R, Lalitha P, Radhakrishnan N, et al. Pythium keratitis in South India: Incidence, clinical profile, management and treatment recommendation. Indian J Ophthalmol. 2019;67(1):42-7.
[Crossref] [Google Scholar] [PubMed]