- Biomedical Research (2005) Volume 16, Issue 1
L-NAME prevents EEG and behavioral alterations induced by Morphine and Deltorphin II in the rabbit
Anna Capasso* and Federica CavalloDipartimento di Scienze Farmaceutiche, Università di Salerno, Via Ponte Don Melillo (84084) Fisciano, Salerno, Italia
- *Corresponding Author:
- Anna Capasso
Dipartimento di Scienze Farmaceutiche
Università di Salerno Via Ponte Don Melillo (84084)
Fisciano Salerno, Italy e-mail: annacap ( at ) unisa.it
Accepted December 10 2004
Abstract
The present study investigated the possible role of nitric oxide (NO) in the development of morphine- and Deltorphin II-induced elec-troencephalographic (EEG) seizures in rabbits. Central administration of morphine and Deltorphin II (100 μg/icv/toto) produces EEG seizure activity in rabbits, associated with wet-dog shakes, myoclonic twitches and convulsive activity. L-NG-nitro arginine methyl ester (L-NAME) (300 μg/i.c.v./toto) did not induce significant EEG or behavioral changes whereas when injected 15 min before i.c.v. morphine or Deltorphin II (100 μg/icv/toto) dose dependently prevented the EEG ictal episodes, the spiking activity and the synchronized EEG pattern induced by morphine or Deltorphin II. The inhibitory effect of L-NAME on morphine or Deltorphin II seizures was dose-dependently reversed by L-arginine (300 μg/icv/toto) but not by D-arginine. Finally, glyceryl trinitrate on its own (300 μg/icv/toto) significantly increased morphine or Deltorphin II seizures in the rabbit and it was also able to reverse the inhibition on morphine or Deltorphin II seizures operated by L-NAME . These results provide evidence that NO may play a significant role in the development of opioids-EEG seizures
Keywords
Opioids, EEG, seizures, Nitric oxide (NO)
Introduction
Central administration of morphine and Deltorphin II produces EEG seizure activity in rats and in rabbits, associated with wet-dog shakes, myoclonic twitches and convulsive activity [1]. These opioids-induced effects were antagonized in rats by pretreatment with naloxone. Opioid peptide naloxone-reversible EEG seizures are reported after i.c.v. peptide administration in rats and rabbits [1].
In rabbits, EEG seizures induced by opioid peptide were unaccompained by convulsive motor activity and wet-dog shakes were also present [1].
Recently, we have demonstrated that DEX prevents epileptiform activity induced by morphine both in the rabbit and mice through a protein synthesis-dependent mechanism [2,3].
In this respect, since prostaglandins have been reported to be involved in the development of brain excitability [4] and glucocorticoids control prostaglandins biosynthesis by inhibiting the release of their common precursor, arachidonic acid through PLA2 inhibitory proteins [5-11], we demonstrated that DEX reduces S&W of DBA/2J mice by blocking the release of the pros-taglandin precursor, arachidonic acid [12]. In fact, arachidonic acid is released by the enzyme PLA2 [13-17] and it is subsequently converted to prostaglandins by the enzyme cyclooxy-genase [8-10,13]. Therefore, our data confirm and extend the above papers indicating that arachidonic acid and its metabolites (prostaglandins) are involved in the development of S&W of mice [12].
Also, it has been reported that there is a strong link between NO and prostaglandins. In fact, the nitric oxide synthase inhibitor L-NG-nitro arginine methyl ester reduces in parallel both NO and prostaglandin generation: this effect is reversed by L-arginine, the precursor for the NO synthesis, but not by D-arginine [19]. Moreover, both sodium nitroprusside and glyceryl trinitrate en-hance the production of prostaglandins, suggesting that NO stim-ulates prostaglandin biosynthesis through a direct interaction with cyclooxygenase enzymes [19].
Therefore, we cannot exclude the possibility that prostaglandins involved in the development of brain excitability [4] may be related to a NO activity.
Recently, we have demonstrated that L-NAME was able to reduce and GTN to increase S&W of DBA/2J mice [20] thus confirming the above hypothesis.3
Therefore, in the present study, in order to further investigate the possible involvement of NO in the control of brain excitability, the authors considered the possible role of nitric oxide (NO) on the morphine- and Deltorphin II-induced EEG seizure in rabbits.
Materials and Methods
Surgery. Male New Zealand white rabbits weighing 2.3 to 3.0 kg were used. All the procedures were approved by the Animal Care Committee of Istituto Superiore di Sanità. The animals were implanted. with permanent monopolar electrodes on the sensorimotor cortex with bipolar electrodes in the right dorsal hippocampus, and a cannula was inserted into the left cerebral ventricle for i.c.v. drug administration.
Surgery was performed under ether and local lidocaine anaesthesia, and post-mortem histological examination confirmed the location of the electrodes and cannula. A week after surgery, each animal was placed in a lighted and shielded recording chamber. After a period of acclimation, EEG tracing from the cortex and hippocampus was recorded on the paper and on magnetic tape continuously for 2 h in basal conditions and at least for 3 h after morphine or Deltorphin II or distiìled water i.c.v. administration. Each animal was used for only one experi-ment. During the EEG recording sessions, animal behavior was continuously observed, and the appearance of the exoph-thalmos and respiratory depression as well as the response to corneal reflex evocation was reported on the paper at 15-min intervals. EEG. Following parameters were evaluated:
1) ictal epileptiform episodes
2) postictal spiking activity
3) EEG background pattern
1. Ictal epileptiform episodes in the EEG were assessed in terms of appearance. Latency, duration wave frequency and amplitude. Statistical analysis among groups was performed by using the two-way ANOVA followed by orthogonal comparisons and also by using the Fisher exact test.
2. For the evaluation of postictal spiking activity, spikes were defined as isolated, high-voltage waves (at least two times the background hippocampal EEG voltage) showing a characteristic morphology (spike or spike-and-wave complex). Spikes were evaluated by their mean number per minute of EEG from the 30th to the l80th min after morphine administration. Statistical analysis was performed using the Student’s paired t test.
3. For each animal, the time spent in high-amplitude, slow-frequency waves in the cortical and hippocampal leads during the 30th to the l80th min after morphine or Deltorphin II or distiiled water i.c.v. administration was calculated as a time percentage of the whole 150-min test. For each group, the duration of EEG high-voltage, slow pattern was compared by means of the two-way ANOVA.
Results
I.c.v. administered (20 μl/toto) distilled water did not induce EEG or behavioral alterations. Also, power spectral EEG recorded from cortex and hippocampus were similar to those observed in nontreated animals.
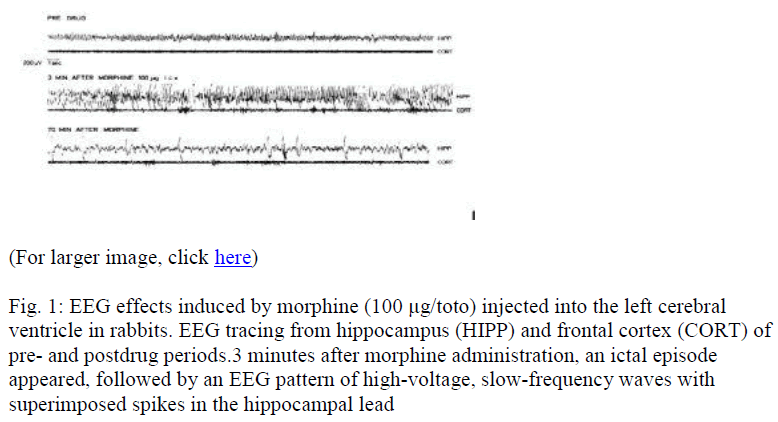
Morphine administered i.c.v. (100 μg/toto) showed ictal EEG episodes limited to the hippocampus and consisting of continuous high-voltage spikes (Fig. 1). The same results were obtained for Deltorphin II (data not shown).
Figure 1: EEG effects induced by morphine (100 μg/toto) injected into the left cerebral ventricle in rabbits. EEG tracing from hippocampus (HIPP) and frontal cortex (CORT) of pre- and postdrug periods.3 minutes after morphine administration, an ictal episode appeared, followed by an EEG pattern of high-voltage, slow-frequency waves with superimposed spikes in the hippocampal lead
During ictal episodes, rabbit maintained frozen posture and neither wet-dog shakes nor other behavioral alterations or clonic or tonic convulsions were observed. After these ictal episodes, hippocampal EEG voltage showed a progressive slowing in fre-quency and displayed typical morphine EEG synchronized activ-ity, consisting of high-voltage slow waves with superimposed spikes. This EEG pattern appears 5 to 10 min after morphine and lasts from 30 min to 180 min after morphine administration (Fig. 1). The same results were observed for Deltorphin II (data not shown).
During these tardive EEG alterations, animals remained mo-tionless and exhibited after respiratory depression with loss of corneal reflex and exophthalmos.
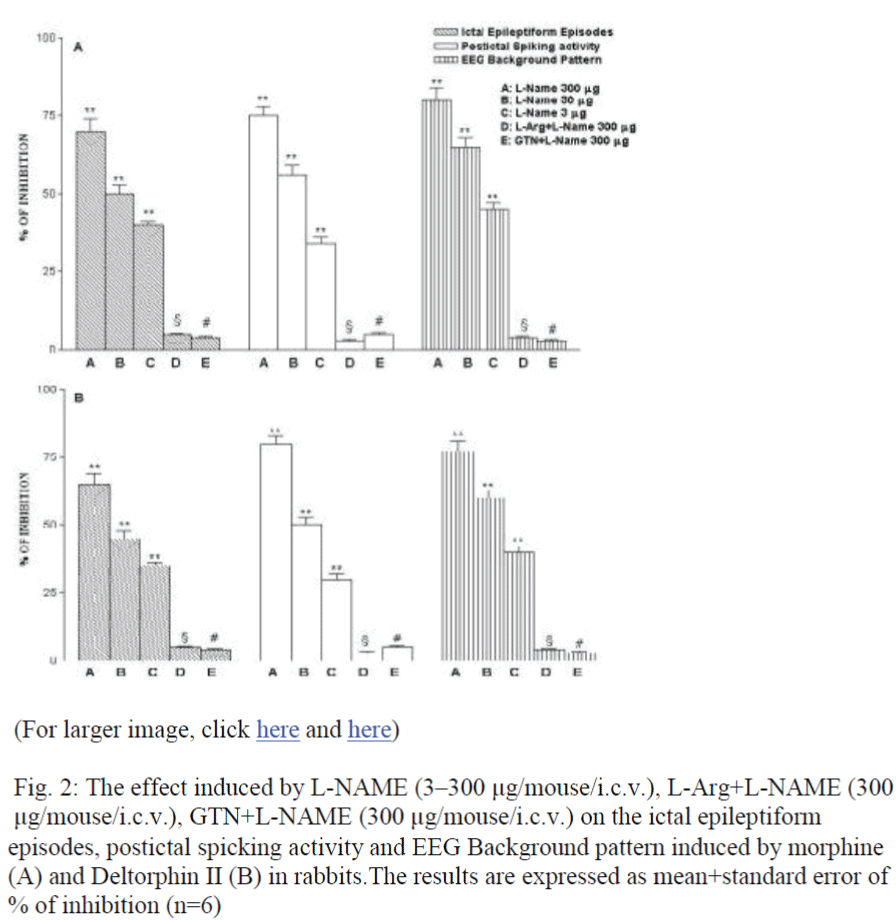
L-NAME (3-300 μg/icv/toto) did not induce significant EEG or behavioral changes whereas when injected 30 min before i.c.v. morphine or Deltorphin II (100 μg/toto) prevented the EEG ictal episodes, the spiking activity and the synchronized EEG pattern induced by morphine or Deltorphin II (Fig. 2 and B). Behavioral alterations as well as the frozen posture, the appearance of ex-ophthalmos, loss of the corneal reflex and the respiratory de-pression induced by morphine or Deltorphin II were also pre-vented (data not shown).
Figure 2: The effect induced by L-NAME (3–300 μg/mouse/i.c.v.), L-Arg+L-NAME (300 μg/mouse/i.c.v.), GTN+L-NAME (300 μg/mouse/i.c.v.) on the ictal epileptiform episodes, postictal spicking activity and EEG Background pattern induced by morphine (A) and Deltorphin II (B) in rabbits.The results are expressed as mean+standard error of % of inhibition (n=6)
The inhibitory effect of L-NAME on morphine and Deltorphin II seizures was reversed by L-arginine (300 μg/icv/toto) (Fig. 2A and B) but not by D-arginine (Data not shown).
Finally, glyceryl trinitrate on its own (300 μg/icv/toto) significantly increased morphine and Deltorphin II seizures in the rab-bit (data not shown). Also, it was also able to reverse the inhibi-tion on morphine or Deltorphin II seizures operated by L-NAME (Fig. 2A and B).
Discussion
The results of our experiments provide a strong evidence that NO may be involved in the control of brain excitability thus confirming our previous paper performed with DBA/2J mice [20]. In fact, the ability of L-NG-nitro arginine methyl ester to reduce and of glyceryl trinitrate to increase EEG pattern suggest that during epilepsy NO may be released after nitric oxide synthase activation. This relationship between NO and opioid epilepsy is further sup-ported by data showing that nitric oxide synthase inhibitors abol-ish some aspects of the brain excitability [21-23].
This paper evaluated the role of NO in the expression of opiate epilepsy and the results of our experiments indicate that both μ and δ opioid receptors are involved in the NO effect during opioid epilepsy since L-NG-nitro arginine methyl ester reduced and glyceryl trinitrate increased both μ and δ opioid epilepsy.
It has been reported that there is a strong link between NO and prostaglandins. In fact, the nitric oxide synthase inhibitor L-NG-nitro arginine methyl ester reduces in parallel both NO and pros-taglandin generation: this effect is reversed by L-arginine, the precursor for the NO synthesis, but not by D-arginine [19]. Moreover, both sodium nitroprusside and glyceryl trinitrate enhance the production of prostaglandins, suggesting that NO stim-ulates prostaglandin biosynthesis through a direct interaction with cyclooxygenase enzymes [19].
Recently, we have demonstrated that arachidonic acid metabo-lites are involved in the expression of brain excitability since both cyclooxygenase and lipooxygenase inhibitors reduced brain excitability [12]. Taken together, our data support the possibility that the reduction of opiate epilepsy induced by L-NG-nitro arginine methyl ester is related to its ability to inhibit cyclooxygenase activity whereas the ability of glyceryl trinitrate to increase opiate epilepsy is related to its ability to stimulate cyclooxygenase activity. This hypothesis may be supported by our previous papers indicating that both dexamethasone and indomethacin are able to reduce brain excitability [2,3,12].
In conclusion, the results of the present study indicate the involvement of NO in the expression of opiate epilepsy and suggest that there is a link between NO, prostaglandins and opiate epilepsy.
The physio-pathological significance of nitric oxide in brain excitability may be relevant considering the several diseases related to brain excitability. Further studies are needed to establish whether nitric oxide is a direct modulator of the properties of excitable membranes or if the loss of nitric oxide is a signaling mechanism.
References
- Frenk H. Pro and anticonvulsant actions of morphine on the endogenous opioids: involvement and interactions of multiple opiate and non-opiate system. Brain Res Rev 1983; 6: 197-210.
- Pieretti S, Di Giannuario A, Loizzo A, Sagratella S, Scotti de Carolis A, Capasso A, Sorrentino L. Dexamethasone prevents epileptiform activity induced by morphine in in vivo and in vitro experiments. J Pharm Exp Ther 1992; 263: 830- 839.
- Capasso A, Sorrentino L, Di Giannuario A, Palazzesi S, Pieretti S, Loizzo A. Dexamethasone and hormones related to the hypothalamic-pituitary-adrenal axis modulate inherited neo-cortical spindling in DBA/2J mice. Neuropsychobiology, 1994; 29: 143-151.
- Bazan NG, Birkle DL, Tang W, Reddy TS. The accumulation of free arachidonic acid, diacylglycerols, prostaglandins and lipoxygenase reaction products in the brain during experi-mental epilepsy. Advances in Neurology, edited by A.V. Delgado-Escueta, AA. Ward Jr., DM Woodbury, RJ Porter, Raven Press, New York, 1986; 44: 879-902.
- Gryglewski RJ. Steroid hormones, anti-inflammatory steroids and prostaglandins.Pharmacol Res Commun 1976; 8: 337-348.
- Di Rosa M, Persico P. Mechanism of inhibition of prostaglandin biosynthesis by hydrocortisone in rat leucocytes. Br J Pharmacol 1979; 66: 161-163.
- Blackwell GJ, Carnuccio R, Di Rosa M, Flower RJ, Parente L, Persico P. Macrocortin: A polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature 1980; 287: 147-149.
- Dennis EA. Regulation of eicosanoids production: Role of phospholipase and inhibitors. Bio/Technology 1987; 5: 1294-1300.
- Shimizu T, Wolfe SL. Arachidonic acid cascade and signal transduction. J Neurochem 1990; 55: 1-15.
- Glaser KB, Mobilio D, Chang JY, Senko N. Phospholipase A2 enzymes: regulation and inhibition. Trends Pharmacol Sci 1993; 14: 92-98.
- Barnes JP, Adcock I. Antiinflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci 1993; 14: 436-441.
- Capasso A, Loizzo A. Arachidonic acid and its metabolites are involved in the expression of neocortical spike-and-wave spindling episodes in DBA/2J mice. J Pharm Pharmacol 2001; 53: 883-888.
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature 1971; 231: 232-235.
- Hirata F, Shiffman E, Venkatasubramanian K, Salomon D, Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutriphils induced by glucocorticoids. Proc Natl Acad 1980; 7: 2533-2536.
- Carnuccio R, Di Rosa M, Flower RJ, Pinto A. The inhibition by hydrocortisone of prostaglandins biosynthesis in the rat peritoneal leucytes is correlated with intracellular macrocortin levels. Br J Pharmacol 1981; 74: 322-324.
- Flower RJ, Blackwell GJ. Antiinflammatory steroids induce biosynthesis of phospholipase A2 inhibitor which prevents prostaglandins generation. Nature 1979; 278: 456-459.
- Rothhut F, Russo-Marie F, Wood J, Di Rosa M, Flower RJ. Further characterization of the glucocorticoid induced anti-phoapholipase protein “renocortin”. Biochem Biophys Res Commun 1983; 117: 878-884.
- Murphy RC, Hammarstrom S, Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci USA 1979; 76: 4275-4279.
- Di Rosa M, Ialenti A, Ianaro A, Sautebin L. Interaction between nitric oxide and cyclooxygenase pathways. In: Prostaglandins, Leukotrienes and Essential Fatty Acids 1996; 54: 229-238.
- Capasso A, Bianchi A, Loizzo A. Nitric oxide is involved in the expression of neocortical spike-and-wave spindling episodes in DBA/2J mice. J Pharm Pharmacol 2003; 55: 1115-1119.
- Khavandgar S, Homayoun H, Dehpour AR. The role of nitric oxide in the proconvulsant effect of Delta-opioid agonist SNC80 in mice. Neurosci Lett 2002; 329: 237-239.
- Homayoun H, Khavandgar S, Namiranian K, Gaskari SA, Dehpour AR. The role of nitric oxide in anticonvulsant and proconvulsant effects of morphine in mice. Epilepsy Res 2002; 48: 33-41.
- Homayoun H, Khavandgar S, Dehpour AR. The involvement of endogenous opioids and nitricoxidergic pathway in the anticonvulsant effects of foot-shock stress in mice. Epilepsy Res 2002; 49: 131-142.