Review Article - Journal of Clinical Ophthalmology (2018) Volume 2, Issue 2
Limbal stem cell transplants and amniotic membrane grafts in ocular surface disease: current perspectives.
Ashok Sharma* and Rajan Sharma
Cornea Services, Cornea Centre, Chandigarh, India
- *Corresponding Author:
- Ashok Sharma
Dr. Ashok Sharma’s Cornea Centre, Chandigarh- 160022; India
Phone number: 0172-5005982
Fax: 91-172-5005982
E-mail: asharmapgius@yahoo.com
Accepted on September 11, 2018
DOI: 10.35841/clinical-ophthalmology.2.2.70-79
Visit for more related articles at Journal of Clinical OphthalmologyAbstract
Limbal stem cells (LSC) play pivotal role in corneal epithelial cell homeostasis and corneal epithelial healing. In addition to corneal epithelial cell regeneration, LSC act as barrier, preventing conjunctival epithelial cells to grow on to the cornea. Limbal stem cell deficiency (LSCD) may occur following trauma, immune mediated diseases, repeated surgical procedures at limbus and genetic diseases. Chemical eye injury remains the most frequent cause of LSCD. LSCD leads to conjunctivilization, corneal vascularization, recurrent erosions and corneal opacification. LSCD is best addressed with surgical procedure, limbal stem cell transplantation (LSCT). Uniocular LSCD is treated with autologus LSCT and bilateral LSCD with live-related LSCT or cadaveric LSCT. Recently cultivated limbal stem cell transplants (CLSCT) have been used to rehabilitate damaged ocular surface. Cultivated oral mucosal epithelial cell have also been used successfully to reconstruct the ocular surface. More recently, simple limbal epithelial cell transplantation (SLET) has been introduced to treat LSCD. The technique is simple, inexpensive and does not require elaborate laboratory set up. Following LSCT dry eye, glaucoma, eyelid abnormalities and ocular surface inflammation should be addressed. Inflammation is detrimental to the survival of LSC. Thus ocular surface inflammation should be kept minimum using topical or systemic corticosteroid or immune suppressants. Results of LSCT in inflammatory disorders including Stevens-Johnson syndrome and ocular cicatricial pemphigoid are less favorable. AMG and LSCT have revolutionized the ocular surface disease management.
Keywords
Limbal stem cell, Amniotic membrane, Chemical eye injury, Steven’s Johnson syndrome (SJS), Ocular cicatricial pemphgoid (OCP), Cultivated limbal stem cell transplant (CLSCT), Simple limbal epithelial transplant (SLET), Amniotic membrane graft (AMG).
Introduction
Ocular surface disorders constitute a major cause of ocular morbidity and pose a challenge to the ophthalmologists. The options of management in the past were limited and outcome used to be less favorable. The management of ocular surface disorders has undergone a complete revolution in the recent years. Newer concepts on the role of limbal stem cells in the corneal epithelial cell homeostasis and ocular surface disease have been introduced. With the result newer surgical procedures including limbal stem cell transplants and amniotic membrane grafts have been added to the corneal specialist's armamentarium. In the current review article, the basic concepts of limbal stem cells, clinical features of limbal stem cell deficiency, classification of limbal stem cell disorders and finally the role of limbal stem cell transplants, amniotic membrane grafts, cultured corneal epithelial or stem cell transplants in these disorders are discussed. The authors also aim to highlight appropriate use of these therapeutic modalities in ocular surface disease.
Normal Ocular Surface
Corneal epithelial cell homeostasis
Normal tear film, corneal and conjunctival epithelium constitute healthy ocular surface. Corneal transparency is maintained by limbal stem cells residing in limbal palisades of Vogt. Normal corneal epithelial mass is maintained by continuous centripetal movement of peripheral corneal epithelium towards the visual axis. In Thoft’s XYZ hypotheses, X represents the proliferation of basal epithelial cells; Y is the proliferation and centripetal migration of the limbal cells and Z the epithelial cell loss from the surface. For a state of equilibrium to be maintained X+Y must equal Z [1]. It is estimated that the corneal epithelium is constantly renewed every 7 to 10 days. The half time of corneal epithelial replacement is nine weeks, while the time required for 95-99% replacement is 9-12 months, i.e. the rate of exfoliation of epithelial cells is consistent with their production from limbal cells. Limbal stem cells also act as barrier to prevent access of conjunctival epithelium onto the cornea.
Basic concept of stem cells
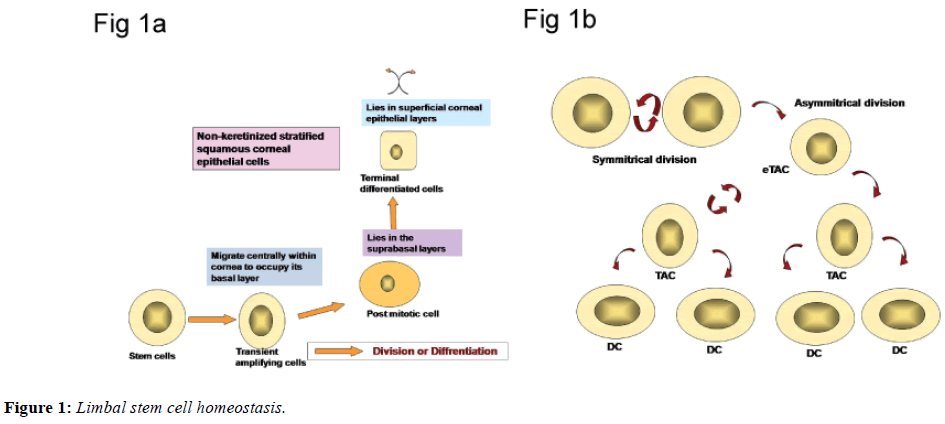
The existence of stem cells responsible for cellular maintenance has been identified for the number of other cells population including the limbus, epidermis and intestinal epithelium. Stem cells, by definition are present in all selfrenewing tissues located in limbal stem cell niches. The unique microenvironment, in the area stem cells reside contribute to maintain special features of stem cells. This microenvironment is constituted by cellular and extracellular components. The niche is responsible for regulation of limbal stem cells. The limbal stem cells are long lived, have great potential for clonogenic cell division and are ultimately responsible for cell replacement and tissue regeneration. The stem cells are least differentiated cells in the tissue and lack markers which indicate greater differentiation. In contrast to stem cells, transient amplifying cells (TAC), those are derived from stem cell mitosis, are characterized by a high mitotic rate and have capacity to proliferate [2]. All post mitotic cells are virtually non-proliferative and are committed to cellular differentiation. The ultimate expression of the functional aspect of the tissue is achieved by the terminally differentiated cells (Figures 1a and 1b). All cells except stem cells have limited life span and are destined to die.
Role of limbal stem cells in ocular surface healing
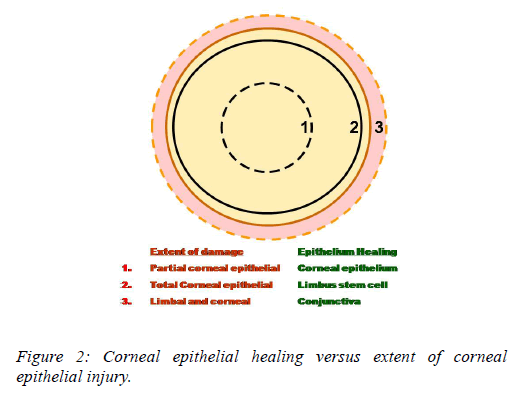
Following cornel epithelial injury recovery is dependent upon centripetal movement of the most proximal, viable epithelium. For small corneal epithelial defects, adjacent corneal epithelium fills the defect [3]. A complete corneal epithelial defect requires epithelium from the limbus whereas in extensive corneal and limbal injury, the surrounding conjunctival epithelium is the only source for epithelial regeneration. The rate of re-epithelialization and the ultimate functional competence of the ocular surface depend upon the source of regenerating epithelium. Following complete corneal and limbal epithelial damage, the surrounding conjunctival epithelium resurfaces the cornea also described as limbal stem cell deficiency (Figure 2). The damage to the limbal epithelium also disrupts the limbus barrier and facilitates the growth of conjunctival epithelial cells on to cornea.
Conjunctival transdifferentiation: not a reality
Conjunctivilization of corneal surface is a key feature of severe limbal stem cell deficiency (LSCD). Conjuctival epithelium is phenotypically different from corneal epithelium and shows goblet cells and no corneal epithelial basal cells. Earlier theory that on long term, with the continued avascularity and vitamin A deficiency, the conjunctival epithelium acquires characteristics of cornea epithelium has been proposed. The process had been described as conjunctival transdifferentiation.
This process is no longer believed to occur [4]. This probably occurred in cases with incomplete limbal stem cell deficiency. This process of transdifferentiation was exploited in the development of conjunctival autografts as a means of ocular surface reconstruction in the chemically injured eyes. Currently, the conjunctival autograft is not a favored modality in the treatment of non-healing corneal epithelial defects due to chemical injury.
Limbal Stem Cell Deficiency
Limbal stem cell disorders
Primary limbal stem cell deficiency occurring in aniridia is rare. Secondary limbal stem cell deficiency occurs in chemical injury, thermal injuries, radiation injury, cicatritial pemphigoid, Steven's Johnson syndrome, and pterygium. Iatrogenic limbal stem cell deficiency may occur following chronic medication, local application of mitomycin C and repeated surgical procedures at limbus [2,3].
Clinical presentation
Conjunctivalization of the corneal surface is suggestive of severe form of limbal stem cell deficiency. Conjunctival epithelium growth into the cornea vascularized pannus formation progress from the area of limbal stem cell deficiency and finally affects the vision. Associated dry eye is due to unstable tear film. Limbal stem cell deficiency can be total or partial. In partial limbal stem cell deficiency, limbal stem cell hypo function may result in non-healing epithelial defect. The various causes of limbal stem cell deficiency have been shown in Table 1.
| Limbal stem cell deficiency disorders: Classification |
|---|
| Primary limbal stem cell deficiency |
| Aniridia |
| Ectodermal dysplasia |
| Secondary limbal stem cell deficiency |
| Steven’s Johnson syndrome |
| Ocular cicatricial pemphigoid |
| Chemical eye injury |
| Thermal eye injury |
| Contact lens induced keratopathy |
| Pterygium |
| Intraepithelial neoplasms |
| Iatrogenic limbal stem cell deficiency |
| Antimetabolites (MMC) |
| Multiple ocular surgeries |
| Stem cell hypofunction in PK |
| Repeated peritectomies |
| Drug induced deficiency |
Table 1: Various causes of limbal stem cell deficiency.
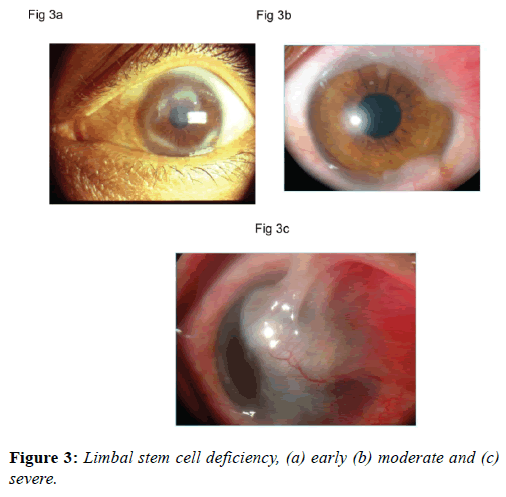
Diagnosis
Early diagnosis of limbal stem cell dysfunction is based on subtle changes in the epithelium texture, permeability, recurrent erosions and micro-epithelial defects. These changes may be detected on slit lamp biomicroscopy using fluorescein dye (Figure 3a). A disruption of perilimbal vascular arcade is also indicative of limbal stem cell damage. Conjunctivalization of the cornea, the hallmark of limbal stem cell deficiency, needs to be documented to make clinical diagnosis (Figure 3b). A severe form of conjunctivalization is usually obvious to the clinician (Figure 3c). Impression cytology of the cornea and histopathological examination of the excised fibro-vascular tissue may show the presence of goblet cells. Impression cytology can be performed by applying nitrocellulose filter paper onto corneal surface. All cytological specimens are processed and stained with periodic Acid-Schiff reagent and a modified Harris hematoxylin-eosin stain. Under a microscope with a 40X objective, the area with the highest cell density is counted at 5 different areas with each equivalent to 5625 μm2, while that of goblet cell density is measured in a similar manner but at an area equivalent to 10000 μm2. These data are then converted to number of cells per millimeter squared and are compared using the Student paired (match) t-test.
Treatment of Ocular Surface Disorders
In the seed, soil and rain theory ocular surface is equated to a beautiful flower. The seed represents limbal stem cells and soil is the microenvironment necessary for the survival of stem cells. Tear film resurfacing the clear cornea is equated to the rain necessary for the survival of the flowering plant. Amniotic membrane grafts improve micro environment for better survival of limbal stem cells in partial deficiency or in limbal stem cell transplants. Ocular surface diseases with focal or diffuse complete stem cell loss require limbal stem cell transplants.
Adjunct treatment
Medical measures in promoting corneal epithelialization include preservative free artificial tears, bandage contact lenses, autologous serum eye drops and topical retinoic acid. In severe cases we may have to do punctual occlusion may it be temporary or permanent fibronectin a glycoprotein present in the extra cellular matrix promotes cell-cell and cell-matrix adhesion but does not influence cell mitosis or migration[4]. Epidermal growth factor (EGF) a polypeptide that stimulates the uptake of DNA, RNA and protein processors by corneal epithelium and enhances the rate of epithelial migration by inducing hyperplasia [5]. Both fibronectin and EGF have been shown to be beneficial in promoting epithelialization in experimental alkali injuries.
Ocular surface reconstruction
Ocular surface transplantation techniques described earlier conjunctiva / Tendons advancement (Tenoplasty), glued on rigid gas permeable contact lenses, conjunctival and mucous membrane transplants [6-9]. These surgical procedures have been based on the principle of conjunctival transdifferentiation. The phenomenon is no longer believed to occur and surface epithelial changes are ascribed to the functional recovery of limbal stem cells. Keratoepithelioplasty was originally introduced by Thoft as a procedure for ocular surface rehabilitation in the bilateral chemical eye injuries [10]. In the recent studies there is renewed interest in Tenonplasty and the procedure has been reported effective in ocular surface healing.
Newer surgical procedures
Contrary to the earlier surgical procedures, newer procedures are based on the
Concept of limbal stem cell transplants and correcting microenvironment [11,12]. The current surgical procedures used in ocular surface reconstruction have been shown in Table 2.
| Limbal stem cell deficiency disorders: Treatment |
|---|
| The limbal stem cell transplants |
| Autolimbal |
| Live related |
| Allolimbal |
| Amniotic membrane transplants (AMT) |
| Combined limbal stem cell and AMT |
| Cultured corneal epithelial cells |
| Cultured limbal stem cells transplants (CLSCT) |
| Simple limbal epithelial transplant (SLET) |
Table 2: Surgical procedures used in ocular surface reconstruction.
Rationale of newer surgical options
For those ocular surface disorders characterized by the absence and aplasia of limbal stem cells transplants of limbal stem cells are required [13,14]. This is particularly feasible for those disorders, which have unilateral involvement. When the limbus is focally involved in one eye, an autograft can be obtained from the ipsilateral eye [15]. In pterygium for example, excision of the lesion together with anti-metabolites or beta irradiation gives recurrence rates ranging from 4.3 to 50% [16]. The use of an autologous source of bulbar conjunctiva for transplantation has been reported to yield better success with a recurrence rate of 5.3 to 6% [17]. The use of an autograft including limbus has been reported to decrease the recurrence rate to 3% [18]. Current studies as well as our experience show that it is not necessary to include limbal stem cells in performing routine pterygium surgery.
Bilateral severe limbal stem cell deficiency due to chemical injuries, cicatricial pemphigoid and Steven's Johnson syndrome need limbal stem cell transplants [19,20]. In bilateral cases limbal stem cell transplants from either live related or donor eyes are performed. Adequate immune suppression is required in case cadaver eyes are used as source of limbal stem cell transplants [21]. The procedure may be combined with amniotic membrane transplants. In few reports combined limbal stem cell grafts with penetrating keratoplasty have shown encouraging results. Several authors prefer single stage surgery as the stem cell population may get depleted in twostaged procedure.
In acute chemical injuries of eye extent of limbal ischemia corresponds to stem cell loss. In persistent epithelial defect without associated limbal stem loss usually response to measures promoting epithelial healing is seen [22]. In persistent epithelial defects without limbal stem cell deficiency, epithelial healing has been achieved with the use of cultured corneal epithelial cells. In recent studies limbal stem cell cultures have been used to promote repopulation of limbal stem cells. Amniotic membrane grafts and cultured epithelial and limbal stem cells are newer surgical options. Amniotic membrane provides various growth factors, reduces inflammation and decreases symblepharon formation [23]. Amniotic membrane transplants (AMT) may be used in persistent epithelial defects not responding to conventional therapy, patients with partial limbal stem cell deficiency may also improve with amniotic membrane transplants [24,25]. Amniotic membrane transplants have also been recently found useful in shield ulcers, pterygium surgery and ocular surface reconstructive procedures, such as symblepharon release, excision of squamous cell carcinoma.
Routine use of limbal stem cell transplants and AMT have improved the prognosis of penetrating grafts in patients with severe limbal stem cell deficiency [26,27]. In partial limbal stem cell deficiency patients, vision may improve even without penetrating keratoplasty. With the use of cultured limbal stem cell on AMT in unilateral cases the problem of immune suppression required for allo limbal stem cell transplants may be avoided.
Unilateral limbal stem cell transplants (Auto) may be performed by taking graft from the fellow eye. A minimum of 50% of limbal stem cells need to be preserved to prevent limbal stem cell deficiency in the donor eye. Patients with total limbal stem cell deficiency need limbal stem cell transplant. Limbal stem cell transplantation is a modification of the original conjuntival transplantation technique by Thoft. It restores the normal corneal epithelial phenotype after injury and is the only currently available technique to reestablish a normal corneal phenotype. The technique involves harvesting two crescents of peripheral corneal limbal epithelium with a corresponding section of conjunctiva from the limbus of the patients uninjured or less injured contralateral eye (autograft), or from a close relative (allograft). The technique has been especially of help in chemical injuries. In grade IV injuries, proper extension of an appropriate vascular supply to the limbal region by tenonplasty, either before or at the same time as limbal stem cell transplantation is mandatory to ensure graft survival
In cases of bilateral chemical injuries, limbal allograft transplantation or the use of cultured limbal stem cells may be an alternative. The survival of donor cells is presumed by the stabilization of ocular surface and regression of the features of limbal insufficiency.
Surgical technique
Limbal stem cell transplants: Surgery may be performed under local or general anesthesia. All donor surgeries in live related donors are performed under peribulbar anesthesia. Donor and recipient surgeries are scheduled simultaneously in adjacent operating theaters. Donor limbal tissue is harvested from the superior and inferior limbus of the non-dominant eye of the donor or from the fellow eye in auto limbal transplants. Each graft is 2 to 3 clock hours in length and extended 2 mm on the conjunctival surface and 1 mm on the comeal epithelium [28]. The grafts are transferred to two bowls of sterile Ringer lactate solution, taking care to identify the superior and inferior graft. The donor sites are left unsutured. After pannus excision in the recipient cornea, the grafts are anchored with one interrupted 10-0 nylon suture at each circumferential end. The conjunctival portion of the graft is anchored to the underlying episcleral tissues with a 10-0 nylon mattress suture. The corneal edge of the graft is left unsutured [29,30].
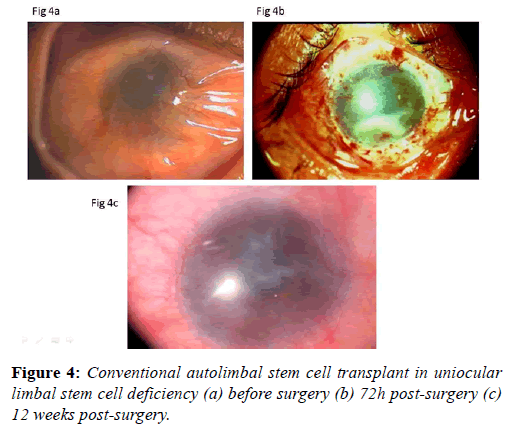
After surgery, the recipient eye is treated with betamethasone eye drops one drop every 2 hours, methylcellulose eye drops one drop every 3 h and ciprofloxacin eye drops twice daily. Topical steroids are discontinued after 4 to 6 months, and tear substitutes are continued for 3 months. Oral prednisolone (1 mg/kg body weight) is used in the first week after surgery and reduced gradually in the next 4 weeks. Limbal stem cell transplant procedure improves the ocular surface remarkably (Figures 4a-4c).
Human amniotic membrane transplants: Human amniotic membrane grafts have been used in variety of ocular surface disorders. Human amniotic membrane consists of epithelial cell layer, basement membrane and stroma. The basement membrane of amnion is thickest. The stroma has compact layer, firm layer and spongy layer. The stroma traps the inflammatory cell, thus reduces inflammatory mediators and ocular surface inflammation. Amniotic membrane has antiinflammatory, anti angiogenic, anti-fibrotic and anti-infective properties. In addition it promotes the epithelialization of the ocular surface. Human amniotic membrane also improves the microenvironment / stem cell niche and prevents limbal stem cell damage.
Human amniotic membrane may be applied as inlay graft or overlay graft. The membrane can also be applied with epithelial cells facing down (the recipient stroma) or facing up. The epithelial or stromal surface of amniotic membrane can be recognized by touching the membrane with cellulose sponge. In case stromal surface is touched and cellulose sponge is lifted thin strands attaches the sponge and lifts the membrane. No such strand appears on touching the epithelial surface.
Human amniotic membrane can easily be prepared for clinical use in the hospital settings. Placenta is collected under aseptic measures from fully screened patients undergoing Caesarean section. Pre prepared amniotic membrane can be stored at -800°C and can be used when needed. Currently super dry amniotic membrane is commercially available. Dry amniotic membrane has advantage of easy storage at room temperature and ease of transportation. Dry amniotic membrane is prepared by cleaning, drying and sterilizing the allografts. A water mark put on the dry amniotic membrane helps clinician to identify the basement membrane and the stromal surface.
Preparation of preserved human amniotic membrane: Human amniotic membrane is prepared and preserved using following method [31,32]. The human placenta is obtained shortly after elective cesarean delivery when human immunodeficiency virus, human hepatitis type B and C, and syphilis had been excluded by serological tests. Under a lamellar-flow hood, the placenta is cleaned of blood clots with sterile Earle's Balanced Salt Solution (Life Technologies Inc., Gaithersburg, MD) containing 50 μg/mL penicillin, 50 μg/mL streptomycin, l00 μg/mL neomycin, and 2.5 μg/mL amphotericin B (Life Technologies Inc.) [33]. The amnion is separated from the rest of the chorion by blunt dissection through the potential spaces situated between these 2 tissues, and flattened onto a nitrocellulose paper with a pore size of 0.45 μg (Bio-Rad, Gainesville, Fla), with the epithelium/ basement membrane surface up. The paper with the adherent amniotic membrane is then cut into 3 × 4 cm disks and stored before transplantation at -800°C in a sterile vial containing Dulbecco's Modified Eagle Medium (Life Technologies Inc) and glycerol (Baxter Healthcare Corporation, Stone Mountain, Ga) at the ratio of 1:1 (V/V) [34,35].
Amniotic membrane transplantations: All surgeries in adults are performed under local anesthesia. Following the thorough removal of the conjunctival, limbal lesion and/or corneal pannus, the amniotic membrane is removed from the storage medium, peeled off the nitrocellulose filter paper, transferred to the recipient eye, and fitted to cover the defect by trimming off the excess edges. In the patients with limbal deficiency, the amniotic membrane covered the entire corneal surface and the perilimbal area extending 5 to 7 mm from the limbus. This fashioned membrane is then secured to the corneal edge of the defect by interrupted 10-0 nylon sutures if the covered limbal circumference is less than 2 clock h, or by a purse-string running suture at the limbal area if it is more than 2 clock hours, and to the surrounding conjunctival edge with interrupted 9-0 Vicryl sutures with episcleral bites. This is followed by topical application of ciprofloxacin 0.3%, and betamethasone drops. After surgery, all patients receive prednisolone acetate eye drops every 2 hours while awake and ciprofloxacin twice daily. Sutures are removed at 3 weeks [36,37].
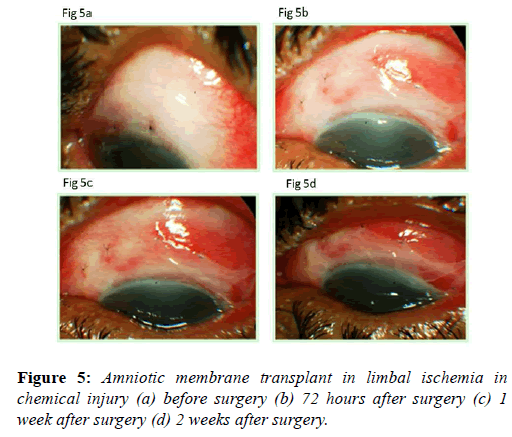
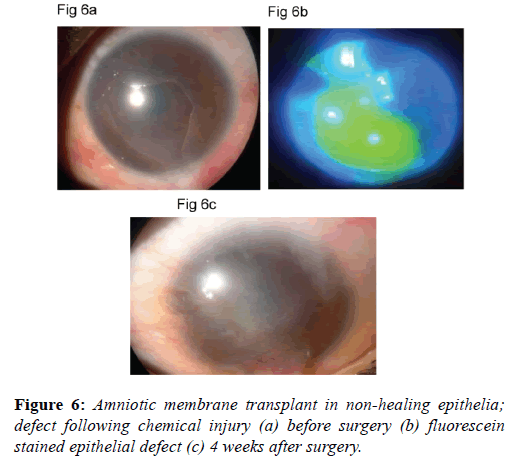
Amniotic membrane transplants clinical uses: Amnion promotes corneal epithelialization and improves limbal ischemia (Figures 5a-5d). As the extent of limbal ischemia equates to the limbal stem cell damage, indirectly amnion preserves limbal stem cells [38]. Amniotic membrane grafts are also helpful in healing ocular surface in acute chemical injuries (Figures 6a-6c). Amniotic membrane grafts reduces ocular surface inflammation and provides limbal stem cell friendly microenvironment. Thus it limits the further damage to the limbal stem cells.
Amniotic membrane grafts is also helpful in rehabilitating the ocular surface. Patients with cojunctivilzation and fibrous tissue growth onto the cornea may be taken up for superficial keratectomy with amniotic membrane transplant to improve the ocular surface. Limbal stem cell transplant may be performed as subsequent surgery. Amniotic membrane transplant is also helpful in performing surgical procedure to release the symblepharon.
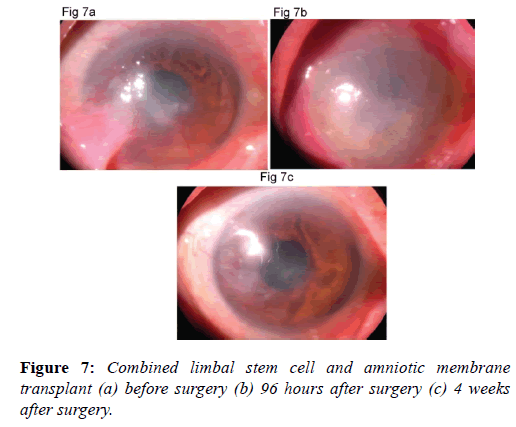
Combined use of limbal stem cells and amniotic membrane transplants: Combined use of limbal stem cells and amniotic membrane transplants has vastly improved the prognosis of penetrating keratoplasty in ocular surface disorders with severe limbal stem cell deficiency [39]. Recent literature describes successful management of patients suffering from end stage bilateral chemical eye burns, Steven Johnson’s syndrome and ocular cicatricial pemphigoid [40]. One should carefully select cases for limbal stem cell and amniotic membrane transplants to get satisfactory results (Figures 7a-7c). However these patients require not only multiple surgical procedures but also aggressive management for associated tear film and adnexal dysfunctions. With the introduction of cultured corneal epithelial and limbal stem cell transplantation, using amniotic membrane as carrier, there appears to be a new ray of hope at the horizon for ocular surface disease.
Corneal and conjunctival epithelial cell transplants: Healing of ocular surface epithelial defect may be promoted by several methods. Preservative free artificial tear drops, bandage contact lenses, amniotic membrane transplants are some of the options available. Autologous serum drops, cord serum drops and platelet rich plasma have been found effective in promoting epithelization. Corneal epithelial and conjunctival epithelial cell cultures have been proved effective in both clinical and experimental studies. In recent preliminary studies human conjunctival epithelial cell cultures have been shown effective in treating patients with total limbal stem cell deficiency [41]. The study showed that cultured conjunctival epithelial cells have microscopic features resembling human corneal epithelial cells. Clinical outcomes in terms of transparency, smoothness and absence of epithelial defects was similar in patients
Cultured limbal stem cell transplant: In uniocular chemical eye injuries autologus limbal stem cell transplant have been successfully performed. Autologus limbal stem cell transplant requires large area of limbal stem cells to be harvested. Although harvesting large area of limbal stem cell transplants have not produced iatrogenic limbal stem cell deficiency. Patients feel insecure when explained that a small piece of limbal tissue would be taken from the healthy eye. Cultured limbal stem cell transplants require a small tissue and there is no risk for the healthy eye [42].
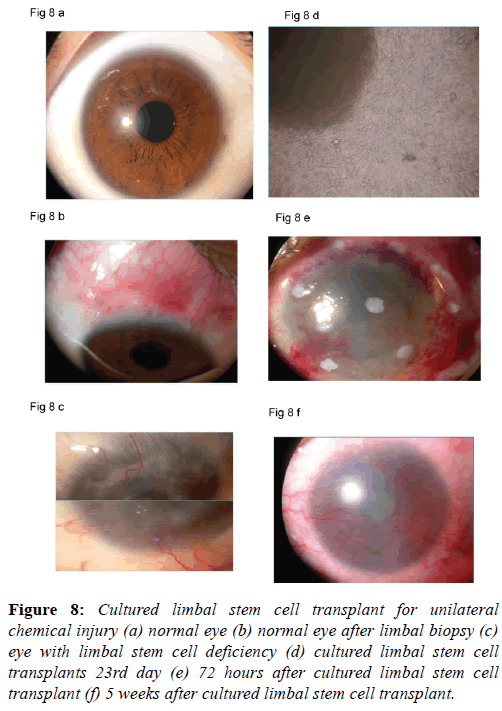
The surgical procedure is similar to the autolimbal stem cell transplant except that the procedure needs to be performed in two stages. In the first stage the limbal biopsy is performed and a small tissue from the limbus containing stem cells in the pallisades of Vogt is harvested. The limbal biopsy is taken to the laboratory and limbal stem cells are grown on the amniotic membrane. Cell cultures are performed under strict aseptic measures. The growth of the limbal stem cells is closely monitored. It takes around two weeks for the limbal stem cell to grow. Once the growth of the cells is complete the corneal surgeon is informed to make arrangement for limbal stem cell transplant. Once the cultured limbal stem cells are released from the laboratory the stem cell transplant should be performed within 24 hours. Superficial keratectomy is performed. An exact plane of dissection is identified and fibrovascular tissues are removed. Fibrovascular tissue from the cornea and 2 mm from the limbus is removed. Complete hemostasis is secured. The surface of the cornea is smoothened. Amniotic membrane supporting cultured limbal stem cells is placed over the entire corneal and limbus. Amniotic membrane is secured using vicryl 8 ‘O’ on the limbus and 100 monofilament nylon sutures (Alcon) on the cornea. A bandage contact lens is placed on the amniotic membrane. In the post-operative period topical antibiotic, steroid, cycloplegic and preservative free artificial tear drops are prescribed. Patients are monitored for epithelial healing. Bandage contact lens is continued for six weeks (Figures 8a-8f). The results of cultured limbal stem cell transplant have been reported satisfactory on long term follow-up [43,44]. We have gratifying results with cultured limbal stem cell transplants. Improvement and stabilization of ocular surface in all patients and improvement in 25% in whom the stroma happened to be clear.
Figure 8: Cultured limbal stem cell transplant for unilateral chemical injury (a) normal eye (b) normal eye after limbal biopsy (c) eye with limbal stem cell deficiency (d) cultured limbal stem cell transplants 23rd day (e) 72 hours after cultured limbal stem cell transplant (f) 5 weeks after cultured limbal stem cell transplant.
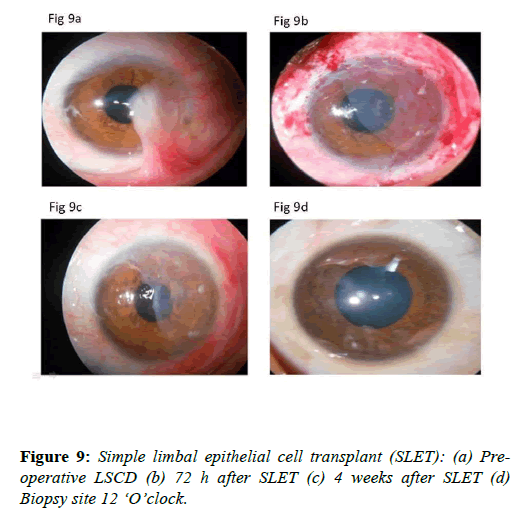
Simple limbal epithelial cell transplant (SLET): Sangwan et al. described simple limbal epithelial cell transplant (SLET) for the treatment of limbal stem cell deficiency disorders [44]. In this technique excision of the fibrous tissue 2 mm beyond limbus is done and the ocular surface is smoothened. After that amniotic membrane is placed on the cornea, limbus and conjunctiva. Limbal biopsy taken from healthy limbus is cut into small pieces. Fibrin glue is applied on the amniotic membrane. Then the seedlings prepared from limbal biopsy tissue are placed on the amniotic membrane. A bandage contact lens is applied. The ocular surface heals very well. The results of this surgical procedure have been reported equivalent to cultutred limbal stem cell technique (Figures 9a-9d).
This technique offers an advantage that no complex cell culture techniques are required [45]. In addition the surgical procedure is simple and cost effective. Encouraged by the results of this technique authors reported a mini-SLET for pterygium surgery. In pterygium there is localized limbal stem cell deficiency [45]. In patient’s not suitable candidates for conjunctival autograft, SLET may be used. Authors hypothesized that the stem cells contained in the mini-SLET pieces could reduce recurrence rates may improve the cosmesis. Vazirani et al treated early recurrence of limbal stem cell deficiency following SLET procedure, by directly placing the small pieces of limbal tissue on the stroma fixed with fibrin glue. These were covered with amniotic membrane. Authors named this procedure as customized SLET [46]. SLET procedure has also been used to treat bilateral limbal stem cell deficiency. Instead of limbal biopsy from the other eye, limbal tissue from cadaver donor eyes has been used. However, the patients undergoing allo SLET require long term immunesupression [47].
D Prognosis and outcome: For optimum results from limbal stem cell transplant, tear film, ocular surface and eyelids need to be in proper health. In symblepharon is present, it should be operated first, before performing LSCT any deformity of eyelids, eye lashes and tear drainage system should be operated before considering limbal stem cell transplant IOP should be monitored and controlled with anti-glaucoma medication. In case IOP is not controlled with medicine filtering surgery with AGV should be performed. Ocular surface inflammation should also be controlled before attempting limbal stem cell transplants. One may use topical steroid drops, topical cyclosporine or tacrolimus.
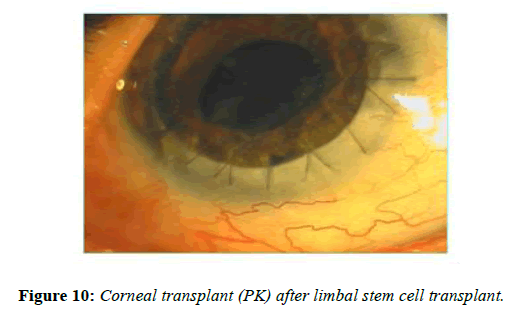
Visual acuity improvement in ocular surface disease depends upon various factors. In most cases the limbal stem cell transplants prepares the recipient’s corneal for corneal transplant surgery. In some cases the stroma may be transparent and limbal stem cell transplant alone may improve the visual acuity. More often, corneal stroma is opaque either partial thickness or full thickness. In case ocular surface has improved, tear film and eyelids are normal corneal transplant either deep anterior lamellar keratoplasty or penetrating keratoplasty can be considered. There are reports on successful penetrating keratoplasty following limbal stem cell transplants (Figure 10). In case ocular surface does not show improvement or ocular surface is too bad to consider limbal stem cell transplant one may consider keratoprosthesis
Conclusion
Limbal stem cell transplants and amniotic membrane transplant are valuable therapeutic modalities to retain visual potential and rehabilitate ocular surface. In partial LSCD, AMT may be performed. However in complete LSCD, LSC transplant is the procedure of choice. Cultured LSC transplants and cultured corneal/ conjunctival epithelial cell transplants have revolutionized the management of ocular surface disease. Simple limbal stem cell transplants are easy to perform and eliminate the need for complex cell culture techniques. This procedure has been reported to give results equivalent to cultured limbal stem cell transplants. With the introduction of cultured limbal stem transplants, there is a paradigm shift in the surgical management of limbal stem cell deficiency patients. It is possible that in future we start using cultivated sheets in the acute phase of limbal stem cell deficiency and if required the procedure may even be repeated.
References
- Schwab IR. Cutured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891-986.
- Tseng SCG, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 1998;116:431-41.
- Boudreau N, Sympson CJ, Werb Z, et al. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995;267:891-3.
- Tseng SCG, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol 1997:124:825-35.
- Kruse FE, Chen JJ, Tsai RJ, et al. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci.1990;31:1903-13.
- Sheardown H, Cheng YL. Mechanisms of corneal epithelial wound healing. Chem Eng Sci. 1996;51:4517-29.
- Meller D, Pires RT, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial progenitor cells by amniotic membrane. Br J Ophthalmol. 2002;86:463-71.
- Siegel R. Buccal mucous membrane grafts in treatment of bums of the eye. Arch Ophthalmol. 1944,32:104-8.
- Naumann GOH, Lang GK, Rummelt V, et al. Autologous nasal mucosa transplantation in severe bilateral conjunctival mucus deficiency syndrome. Ophthalmology. 1990;97:1011-7.
- Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci 1999;40:878-86.
- Tsubota K., Satake Y, Kaido M, et al. Treatment of severe ocular surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med 1999,340:1697-703.
- Lee SH, Teseng SCG. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 1997;123:303-12.
- Tseng SC, Prabhasawat P, Lee S-H. Amniotic membrane transplantation for conjuctival surface reconstruction. Am J Ophthalmol 1997;124:765-74.
- Kenyon KR, Tseng SCG. Limbal autorgraft transplantation for ocular surface disorders. Ophthalmology 1989:96:709-23.
- Tsubota K. Toda I, Saito H, et al. Reconstruction of the comeal epithelium by tenbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486-96.
- Sharma A, Gupta A, Ram J, et al. Low dose intra-operative mitomycin-c Versus conjunctival autograft in pterygium surgery: Long term follow up. Ophthalmic Surg Lasers. 2000;31:301-7.
- Jun Shimazaki, Naoshi Shinozaki, Kazuo Tsubota. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblephron. Br J Ophthalmol. 1998;82:235-40.
- Prabhasawat P, Barton K, Burkett G, et al. Comparison of conjunctival autografts, amniotic membrane grafts and primary closure for pterygium excision. Ophthalmology. 1997;104:974-85.
- Tsubota K, Satake Y, Ohyama M, et al. Surgical reconstruction of ocular surface in advanced ocular cicatricial pemphigoid and Steven-Johnson syndrome. Am J Ophthalmol.1996Jul;122:38-52.
- Kruse FE, Volcker HE. Transplantation of amniotic membrane for reconstruction of the eye surface. Ophthalmologe. 1998;98:114-9.
- Avila M, Espana M, Moreno C, et al. Reconstruction of ocular surface with heterologous limbal epithelium and amniotic membrane in rabbit model. Cornea. 2001;20:414-20.
- Gabric N, Mravicic T, Karaman Z. Human amniotic membrane in the reconstruction of ocular surface. Doc Ophthalmol. 1999;98:273-83.
- Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Eng J Med. 2000; 13;343(2):86-93.
- Rakowska E, Zagoriski Z. Application of amniotic membrane transplantation in severe corneal diseases. Klin Oczna. 1999;101:417-21.
- Rodriguez-Ares MT, Tourino R, Capeans C, et al. Repair of scleral perforation with preserved scleral and amniotic membrane in Marfan’s syndrome. Ophthalmic Surg Lasers. 1999;30:485-7.
- Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325-35.
- Kruse FE, Rohrschneider K, Volcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep comeal ulcers. Ophthalmology. 1999;106:1504-10.
- Taylor RJ, Wang MX. Rate of re-epithelialization following amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 1998;39:S1038.
- De Rotth A. Plastic repair of conjunctival defects with fetal membrane. Arch Ophthalmol. 1940;23:522-5.
- Sorsby A, H. M. Symons J. Amniotic membrane grafts in caustic soda bums. Br J Ophthalmol. 1946;30:337-45.
- Kim JC, Tseng SCG. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473-84.
- He YG, Alizadeh H, Kinoshita, Kayoko, et al. Experimental transplantation of cultured human limbal and amniotic epithelial cells onto the corneal surface. Cornea. 1999;18:570-9.
- Sharma A, Pandey S, Mohan K, et al. Cynoacrylate tissue adhesive augmented Tenoplasty: A new surgical treatment for bilateral severe chemical injuries. Cornea 1999;18:366-9.
- Anderson DF, Ellies P, Pires RT, et al. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567-75.
- Meller D, Maskin SL, Pires RT, et al. Amniotic membrane transplantation for symptomatic conjunctivochalasis refractory to medical treatments. Cornea. 2000;19:796-803.
- Soloman A, Tseng SC. Amniotic membrane transplantation for ocular surface diseases. Harefuah. 2000;139:134-40.
- Basti S, Rao SK. Current status of limbal conjunctival autograft. Curr Opin Ophthalmol. 2000;11:224-32.
- Gabler B, Lohmann CP. Hypopyon after repeated transplantation of humanamniotic membrane onto the corneal surface. Ophthalmology. 2000;107:1344-6.
- Adds PJ, Hunt SC, Dart JK. Amniotic membrane grafts, "fresh" or frozen? A clinical and in vitro comparison. Br J Ophthalmol. 2001;85:905-7.
- Erik Letko, Stephen U. Stechschulte, Kenneth R. Kenyon, et al. Amniotic membrane inlay and overlay grafting of corneal epithelium defects and stromal ulcers. Arch Ophthalmol. 2001;119:659-63.
- Ricardo JR, Cristovam PC, Filho PA et al.Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea. 2013;32:221-8
- Zhao Y, Ma L.Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34:592-600.
- Pedrotti E, Passilongo M, Fasolo A, et al. In Vivo Confocal Microscopy 1 Year after Autologous Cultured Limbal Stem Cell Grafts. Ophthalmology. 2015;122:1660-8.
- Sangwan VS, Matalia HP, Vemuganti GK et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54:29-34.
- Hernández-Bogantes E, Amescua G, Navas A, et al. Minor ipsilateral simple limbal epithelial transplantation (mini-SLET) for pterygium treatment. Br J Ophthalmol. 2015;0:1–3.
- Vazirani J, Lal I, Sangwan V. Customised simple limbal epithelial transplantation for recurrent limbal stem cell deficiency. BMJ Case Rep. 2015;14:2015.
- Bhalekar S, Basu S, Sangwan VS. Successful management of immunological rejection following allogeneic simple limbal epithelial transplantation (SLET)for bilateral ocular burns. BMJ Case Rep. 2013;14:2013.