- Biomedical Research (2010) Volume 21, Issue 1
Lecturing to 200 students and its effects on saliva flow rate, immunoglobulin a, lysozyme and salivary markers of adrenal activation
Edith Filaire1*, Alain Massart1, Deborah Nourrit1, Luis Rama L2 and Anna Teixeira21Laboratoire AMAPP UFRSTAPS- 2 allee du CHATEAU. BP 6237 45062 ORLÉANS CEDEX, FRANCE
2Centro de estudos biocineticos. Faculdade de ciencas do desporto e educaçao fisica. COIMBRA, PORTUGAL
- *Corresponding Author:
- Edith Filaire
Laboratoire AMAPP UFRSTAPS-2 allée du château
BP 6237 45062 Orléans cedex, France
Phone: +00330 -238417178
e-mail: edith.filaire@univ-orleans.fr
Accepted date: August 28 2009
Abstract
The aim of this study was to examine the effects of delivering a lecture to 200 students on salivary Immunoglobulin-A (S-IgA), salivary lysozyme (s-lys) and the association of these responses with salivary markers of adrenal activation. From 26 female professors, a total of eight unstimulated saliva samples were collected on two different days, (on a working day with a lecture, and on a working day without a lecture - control). They also completed the trait version of the state-trait anxiety inventory (STAI) to assess the dispositional anxiety on the control day and the state section of the stai fifteen minutes before their lecture and at the same hour on the control day. The STAI-State was significantly higher than the one noted the control day. This STAI score was negatively correlated with s-lys reported just before the lecture. Lecturing resulted in a significant decrease in S-IgA and s-lys concentrations but did not affect the saliva flow rate. These changes appeared to be associated with an increase in the concentrations of the stress markers, alpha-amylase, CgA and cortisol. The mechanism that leads to modify the activity of salivary alpha-amylase, lysozyme and chromogranin-A due to stress is not entirely understood and further investigations are needed to elucidate these mechanisms.
Keywords
Alpha-Amylase, Cortisol, Chromogranin-A, Lysozyme, Stress, Professor
Introduction
A number of studies have investigated the effects of stress on mental health and cardiovascular diseases and the relationship between stress and various components of the immune system. These studies have shown that chronic stress is associated with the suppression of a variety of immune parameters [1]. Salivary IgA (S-IgA) is a convenient and commonly used indicator of immune status. This parameter reportedly indicates the functional status of the entire mucosal immune system [2] and is considered to be an important immunological barrier of mucosal surfaces, complexing with luminal antigens and thus impeding penetration [3]. Early studies explored the relationship between S- IgA and long-term psychological stress or in individuals particularly prone to stress. Such studies consistently revealed stress-related downregulation of S- IgA [4], suggesting that prolonged psychological stress can compromise certain aspects of immune function. Paradoxically, acute responses to a psychological challenge include a rise in S-IgA concentration. This mobilization of S-IgA has been reported in response to acute laboratory psychological stress tests including mental arithmetic tests [5]. Effects on S-IgA were also reported for academic stress, exposure to chronic environmental stress and stressful life [6,7,8,9,10]. However, few studies have examined the s-iga response to occupational stress [11].
Another protein important in mucosal defence is lysozyme. It is a low-molecular-weight cationic protein that is synthesized in and continuously released from monocytes or macrophages, and widely distributed in human tissues and secretions [12]. Salivary lysozyme is primarily synthesized by the salivary glands [13]. It is considered to belong to a primitive defence system, known as the innate immune system. The relation between stress and lysozyme is not clear at present, because no significant correlation between self perceived stress levels and salivary lysozyme in nurses was detected [14]. On the other hand, nurses, who reported a higher level of professional stress, showed significantly lower secretion rates of S-IgA and lysozyme compared to controls [13]. More recently, S-IgA and lysozyme were found to be inversely associated with self-perceived occupational stress among the workers studied [15]. Perera, Uddin, and Hayes [16] reported decreased salivary lysozyme after academic examinations in 39 students and suggested that this marker could be a promising marker of the effect of stress on non-specific immunity.
Activation of the hypothalamo-pituitary-adrenal (HPA) axis and of the sympathetic-adrenomedullary (SAM) system are major components of the stress. The activities of the hpa axis and sam system can be biochemically evaluated by measuring catecholaminrs and cortisol, respectively. It should be noted that cortisol has been shown to inhibit transepithelial transport of S-IgA [17]. Direct measurements of salivary adrenaline and noradrenaline seem not to reflect sam activity [18]. Salivary alphaamylase (sAA) concentrations have been suggested as an indirect marker for sam activity under a variety of stressful conditions [19,20,21]. Alpha-amylase is produced by the serous acinar cells of the parotid and submandibular glands. It is one of the principal salivary proteins appearing as a number of isoenzymes. Amylase accounts for 10- 20% of the total salivary gland-produced protein content and is mostly synthesized by the parotid gland.
Nakane, Asami , Yamada , Harada , Matsui , and Kanno [22] demonstrated that salivary chromogranin A (CgA) can be a quantitative index for monitoring the activity of the sympathetic nervous system too. CgA is a soluble protein that is stored and co-released by exocytosis along with catecholamines from the adrenal medulla and sympathetic nerve endings. Dimsdale, O’Connor, Ziegler and Mills [23] reported that the plasma CgA level correlates with the noradrenaline release rate. This result indicates that the plasma CgA level may be an index of the activity of the sympathetic/adrenomedullary system. Salivary CgA was shown to be produced by the human submandibular gland and secreted into saliva [24], and is considered to be a sensitive and reliable index for evaluating psychological stress. Yanaihara et al. [25] found that salivary CgA is a sensitive marker of the initial psychological phase of the stress response. Further, a rapid and sensitive elevation of salivary cga has been reported in response to psychosomatic stressors such as public speaking and driving a car [22,26].
All theses salivary biomarkers have made it possible to investigate the effect of stresses. Usually, academic stress research focuses on the effects of academic challenges (oral presentations, examinations) by students. There is an inconsistency in reports on the impact of teaching in academic staff. Moreover, studies related to teaching-related stress have mostly been conducted using subjective questionnaire or interview methods [27,28]. Therefore, the aim of the present study was to investigate the S-Iga and s-lys concentrations and to assess the responses of noninvasive salivary markers of adrenal activation in professors when they were lecturing to 200 students.
Materials and Methods
Participants
Twenty six female professors from two universities (age: 37.2 ± 3.0 years; height: 169.2 ± 3.5 cm; weight 59.2 ± 4.0 kg; year of experience: 14.6 ± 1.9 years) volunteered to participate in this study. All study participants were healthy and free of cardiovascular diseases as assessed by their medical history. The answers of the questionnaire administered prior to the experiment indicated that no subjects had a physical or mental illness, were pregnant or taking corticosteroids or oral contraceptives.
Participants received the saliva sampling materials along with both spoken and written instructions. Prior to data collection, according to the declaration of helsinki. The purpose of this study was explained thoroughly to each subject and informed consent was obtained from each individual.
Procedure
The study was carried out over two periods:
• On the day of the lecture (teaching day)
• On a working day without a lecture (control day).
As salivary cortisol, IgA, CgA or alpha-amylase activity showed a circadian rhythm 29, 30, 31, the experiment was performed between 10.00 a.m. and 12.30 p.m.
Hormonal assay
A written protocol on how to collect the saliva was given to the teachers. In addition, they were briefed on the collection method. Saliva samples were collected using the salivette system (Sarstedt Co, Nümbrecht, Germany) and cotton was placed under the tongue for 4 min in each subject. Samples were taken at 10.00 a.m. (S1), 12.00 a.m. (S2), and 12.30 p.m. (S3) and at 8.00 p.m. (S4) on the control day.
On the day of lecturing, saliva samples were also taken just before the lecture (10 a.m. S5), just after the lecture (12 a.m. S6), 30 min after the lecture (12.30 p.m. S7), and at 8 p.m (S8).
To avoid contamination of saliva with blood, participants were instructed not to brush their teeth before the morning saliva samples. Additionally, smoking, eating, and drinking beverages containing alcohol, caffeine, or fruit juices were not allowed for 60 min before sampling. The subjects were told not to undergo excessive physical activity for the 48h prior to the experiment and to refrain from any sporting activities at all 24h before the study. Besides these restrictions, participants were free to follow their normal daily routines on the sampling days.
Saliva samples were stored at -45°c until biochemical analysis. Tubes were centrifuged for 10 min at 3000 rpm to obtain clear saliva. Saliva volume was estimated by weighing to the nearest milligram and the saliva density was assumed to be 1.0 g.ml-1 [32]. Saliva flow rate (ml.min-1) was determined by dividing the volume of saliva by the collection time. The flow rate of saliva of valid samples should not be < 0.1 ml.min-1. Under basal conditions, the rate of saliva production is 0.5 ml.min-1 [33]. The concentration of S-IgA (mg.l-1) was determined by an enzyme-linked immunosorbant assay (elisa) method as previously described by Li and Glesson [34]. Salivary CgA, cortisol, and alpha-amylase were assayed using kits (YK070 Human CgA EIA kit; Yanaihara Institute, Shizuoka, Japan; cortisol EIA kit and alpha-amylase assay kit; Salimetrics inc., State College, PA, USA, respectively). Intraassay maximal coefficients of variation were 8.15% for CgA, 6.7% for alpha-amylase, and 3.65% for cortisol. Interassay maximal coefficients of variation were 12.42% for CgA, 5.8% for alpha-amylase, and 6.41% for cortisol. Alpha-amylase activity was expressed as international units/ml of saliva (u.ml-1). Chromogranin A and cortisol concentrations were expressed as nmol.l-1. The salivary lysozyme concentration was assayed using human EIA kit (biomedical technologies inc, stoughton, usa). Intraassay maximal coefficient of variation was 5.3%; interassay maximal coefficient of variation was 7%. Lysozyme activity was expressed as ng.ml-1.
Psychometric assessment
The state-trait anxiety inventory (STAI) was used to assess personal anxiety [35]. The test consisted of two separate, self-reported scales for measuring the distinct conceepts of state and trait anxiety. The STAI is one of the most commonly used scales to measure anxiety, and the trait anxiety reflects a predisposition to anxiety as determined by the personality pattern [36]. We used the Yversion of STAI translation in French [37]. After informed consent was obtained, each subject completed the trait section of the stai on the control day, and the trait anxiety score was calculated. Fifteen minutes before their lecture, the teachers completed the state section of the STAI, and the state section score was calculated. Each scale consists of 20 items. The teachers also completed the state section the control day at the same hour that the teaching day (9.45 a.m.).
Statistical analysis
Data analysis was performed using spss 16.0. Data are presented as means ± standard deviation (SD). A two-way repeated measures anova was used to compare the control and lecture data at 4 times [2 (day) x 4 (hour of sample)].
We used post-hoc bonferroni test. To correct for sphericity, greenhouse-geisser procedure was applied. Pairedsample tests were used to compare the data of the state section of the stai. Pearson product-moment correlations were used to test the relationships between anxiety components and biological parameters, and the relationships between biological parameters. An alpha-level of 5% was used in all the analyses.
Results
Psychological parameters
The STAI-trait was 38.5 ± 0.4. The STAI -state evaluated on the day of lecturing was 41.26 ± 1.6. This STAI-state was significantly higher than the one noted the control day (33.1 ± 1.8) (p < .01).
Salivary parameters
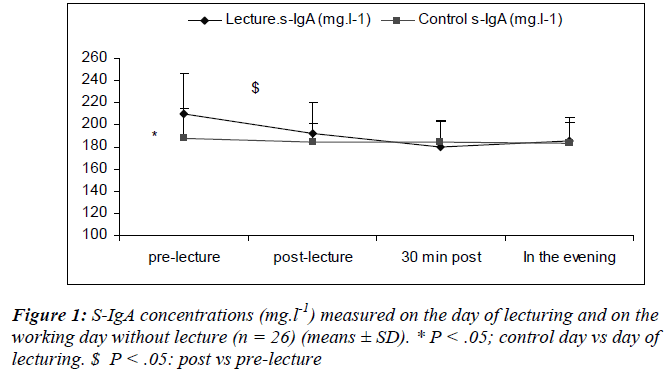
Salivary flow rates did not change significantly over time, neither on the day of lecturing nor during the control day and there was no differences between the two days (0.53 ± 1.7 and 0.54 ± 0.5 ml.min-1, respectively).
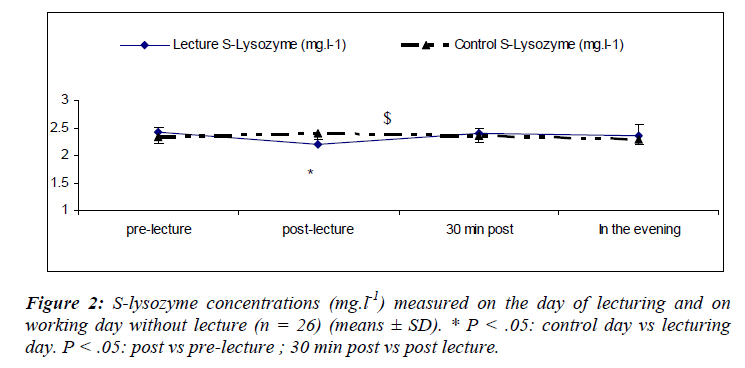
Salivary lysozyme
There was a main effect of sampling time [(F(3,75) = 7.49; P = 0.01)] where s-lys decreased after the lecture (S6) returning to within pre-lecture levels by 30 minutes (Figure 2).
We also noted a significant interaction effect for day and sampling [(F(3, 75) = 5.6; P = 0.002)] with lower s-lys concentrations at S2 during the lecturing as compared to the day of lecturing.
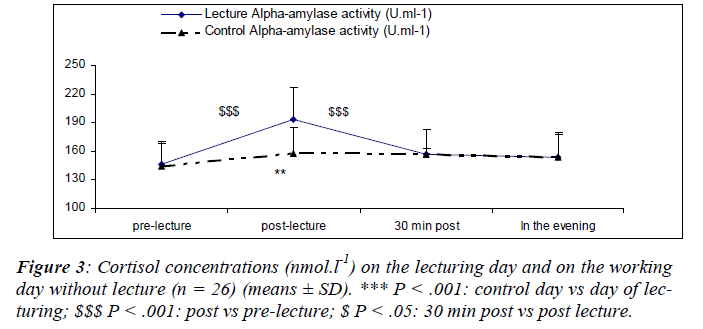
Stress markers
The mean cortisol concentrations during the resting day were in the standards of those found in the literature and presents a pronounced diurnal rhythm in accordance with the results of Fiet, Guechot, Gourmel, Villette and Cathelineau [30] (Figure 3).
A significant main effect for day [(F(1,25) = 40.4; P < 0.01)] was noted. A significant main effect for sampling time [(F(1,75) = 22.6; P = 0.03)] was also noted where cortisol concentrations increased after the lecture (S6). Cortisol concentrations noted 30 minutes after the lecture (S7) were higher than those noted just before the lecture. There was a significant interaction effect for day and sampling [(F(2,75) = 32.1; P = 0.03)]. Cortisol concentrations measured on the day of lecturing were significantly higher than the control values except in the evening.
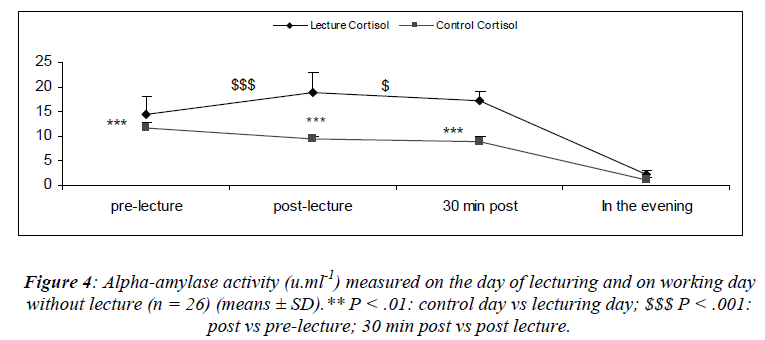
Stastically analysis revealed a significant main effect for day [(F(1,22) = 35.4; P = 0.01) and a main effect for sampling time [(F(3,66) = 15.23; P < 0.001)] where alphaamylase activity increased after the lecture (S6) returning to within pre-lecture levels by 30 minutes (Figure 4). We also noted a significant interaction effect for day and sampling [(F(3, 66) = 21.7; P = 0.02)] with lower alphaamylase activity at S2 during the control day as compared to the day of lecturing.
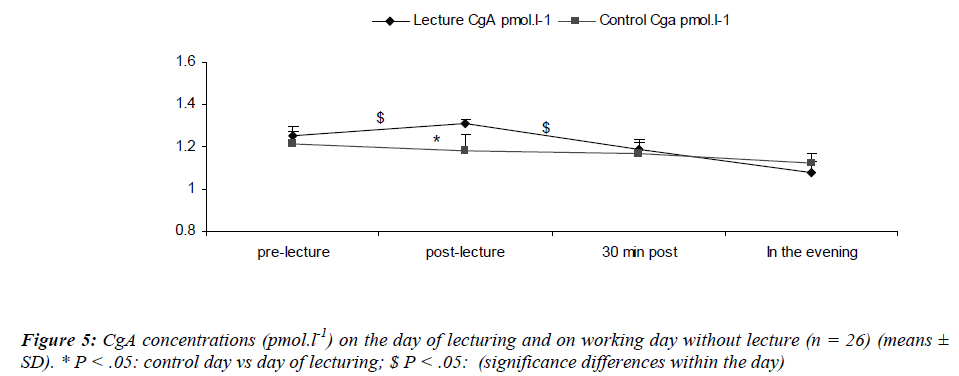
CgA concentrations during the resting day showed a diurnal rhythm in accordance with the results of Den, Toda, Nagasawa, Kitamura, Morimoto [29] (Figure 5). Stastical analysis revealed a significant main effect for sampling time [(F(3,75) = 4.7; P = 0.04)] where CgA increased after the lecture (S6) returning back within the pre-lecture levels by 30 minutes. We also noted a significant main effect for day [(F(1,25) = 5.87; P = 0.02)]. There was a significant interaction effect for day and sampling time [(F(2,75) = 9.9; P = 0.01)] with lower S-IgA concentrations at S2 during the control day as compared to the day of lecturing.
Correlation between hormonal parameters and the staiscores
On the day of lecturing, s-lys concentrations noted at S5 were negatively correlated with the STAI-state score (r = - 0.42; P < 0.05). We also reported at S5 a postive correlation between alpha-amylase activity and the STAI -State score (r = 0.81; P < 0.01).
No correlations were found between cortisol and the other biological parameters.
Discussion
While sufficient reports are available on chronic stress in lecturers, yet very little work related to acute stress, specifically on lecturers has so far been conducted. This is the first study examining the evolution of salivary IgA, cortisol, lysozyme, CgA concentrations and alphaamylase activity during the day of lecturing in relation with anxiety. The findings of this study demonstrate specific psychological and physiological responses to lecturing. Regarding psychological responses, the pattern of findings suggests that lecturing to 200 students is emotionally challenging. In fact, the state of anxiety was statistically higher (+17%) during lecturing compared to the control situation. Regarding physiological processes, two different components within the response pattern could be identified. First, lecturing to 200 students resulted in a significant decrease in S-IgA and s-lys concentrations but did not affect the saliva flow rate. These changes appeared to be associated with increases in the secretion of the stress markers alpha-amylase, cortisol and CgA. Secondly, there were anticipatory responses which could be identified by comparing the concentrations of the two different situations (lecturing day versus control day). Lecturing in front of 200 students did not affect the saliva flow rate and these values are in agreement with those of Guyton [33]. It has been shown that psychological factors, such as anxiety, perceived stress, and emotional state are associated with low saliva flow [38,39]. However, any evidence of the association between psychological factors and the flow of saliva is somewhat contradictory. While some studies seem to support that academic examination stress is associated with a reduction in unstimulated saliva flow rate [40], others reported that stress is associated with an increase in saliva flow [41]. In our study, it is not surprising that saliva flow rate does not show detectable changes when salivettes are used, as also reported by Guinard, Zoumas-Morse and Walchak [42], who assumed that the use of salivettes leads to a rather uniform stimulation of salivary flow due to tactile stimulation by the presence of the cotton roll in the oral cavity.
STAI was used to assess the personal anxiety and the state score of anxiety just before the lecture. Our results indicated that this state score was raised more than one observed on the control day (41.26 ± 1.6 vs 33.1 ± 1.8), suggesting that the lecturers perceived a higher level of anxiety before the lecture than the day of work without lecture.
Acute and chronic stresses have been reported to increase the activity of HPA axis with subsequent rise in cortisol concentrations [43]. In our study, adrenocortical responses to the lecturing situation were found to increase for the sample. Cortisol concentrations remained elevated 30 minutes after the lecture, showing that the return of cortisol to baseline, as reported by Webb, Weldy, Fabianke- Kadue, Orndorff, Kamimori, and Acevedo [44] , can take some times after the stressor. Moreover, salivary cortisol concentrations were noted to be higher prior to and after the lecture than those noted in the control day at the same time. These results are in line with previous studies, which reported increased cortisol concentrations during anticipation of stressful experiences such as academic examinations, dental procedures and public speaking [45]. Giving a lecture to 200 students influences the secretions of proteins in mucosal immunity. In fact, the present study revealed that lecturing decreased significantly the concentrations of salivary IgA and lysozyme. S-lys is part of the innate immune system and is a cationic protein with wide antimicrobial activities [46]. Perera et al.[16] have indicated s-lys as a promising marker of the effects of stress in non-specific immunity, demonstrating negative relations between perceived stress and s-lys. In our study, the salivary lys concentrations noted after the lecture indicated a negative correlation with the STAI state scores (r = - 0.42; P< 0.05). Until now the mechanisms responsible for regulating the concentrations of slysozyme have not been elucidated. A few authors have attributed the reduction in s-lys concentrations in response to psychological stress to an increased secretion of glucocorticoids [16,47]. In the present study, no correlations were observed between cortisol and lys concentrations. Thus, we can put forward the hypothesis that the decreases are unlikely to be attributable to glucocorticoids. Further investigations are needed to understand the relation between anxiety and lysozyme and the mechanisms responsible for regulating s-lys.
Secretory IgA in saliva, the main immunological defence of mucosal surfaces, had repeatedly been shown to be sensitive to psychological variables [48]. Changes in saliva IgA concentration in response to psychological stress also appear equivocal, with some workers reporting decreases, some reporting increases and others no effect [10,41,49]. Our results show that the lecture activates the hypothalamic-pituitary-adrenocortical and sympathoadrenomedullary systems. It has been shown that increased secretion of cortisol and alpha-amylase inhibit both cell-mediated and humoral immunity. IgA and lysozyme are the main factors in humoral and mucosal immunity. Thus, it is biologically plausible that lecturing to the students may cause a decrease in the production of secretory IgA and lysozyme.
In the present study, we evaluated alpha-amylase and CgA to reflect changes in sympathetic activity [20,50]. Moreover, salivary CgA is considered to be a sensitive psychological stress markers in the adults [51] .We noted in our study a significant increases in alpha-amylase activity and CgA concentrations in response to the lecture (Figures 4 and 5). It was reported that the concentrations of cga were elevated during mental arithmetic tasks, examination or oral presentations [10,22]. These studies revealed that, in short-term stressful situations, such as an oral presentation, salivary CgA concentrations increase and peak immediately before, and decrease immediately after the event. Even if there are some studies that shown the interest of cga as psychological marker of stress, no studies have dealt with the association of IgA, cortisol, and CgA with stress before and after giving a lecture to 200 students.
Studies also reported results according to the effect of the sam system activities on salivary constituents such as alpha- amylase. Early studies also show that salivary alphaamylase is responsive to various types of situations including socially and cognitively oriented laboratory tasks and physical exercise [21,52,53]. However, the data are controversial. In fact, Borgeat, Chagon and Legault [54] noted no effect of stress on salivary alpha-amylase activity in a laboratory situation, using mental arithmetics as a stressor. Bosch, Brand, Ligtenberg, Bermond and Hoogstraten Amerongen [41] reported higher levels of alphaamylase 30 min before an examination compared with baseline, which has been explained by an anticipation of the stressor beforehand. Van Stegeren, Wolf and Kindt [55] also reported increased alpha-amylase activity during anticipation of stressful experiences. These discrepancies could be explained by the different experimental designs used [21]. In our study as in the one of Nater et al. [56], participants were monitored in real life during the day whereas in other studies as in the one of Van Stegeren et al. [55], subjects were tested in a laboratory in the afternoon. Moreover, in this last study, the participants came to the laboratory for a psychological experiment, and knew that they were going to be emotionally challenged. These two differences might contribute to the discrepancy of our data and their findings.
The few studies that focused on a joined response between cortisol and saa levels in a stress paradigm did not find any significant correlation. In fact, Chatterton et al. [52] did not find a correlation between these variables after physiological stress induction (exercise and exposure to heat and cold). Nater, Rohleder, Schlotz, Ehlert and Kirschbaum [21] also reported no relationship between cortisol and saa using a psychological employed in a controlled laboratory environment. In line with these studies, we did not find correlations between alphaamylase activity and cortisol concentrations.
The lack of correlations between these two parameters suggests that alpha-amylase reflects the reaction of a different system than the HPA axis. However, it is clear that the hpa axis and the sympathetic nervous system (SNS) work in coordination to generate the physiologic changes associated with the stress response [57]. Nevertheless, the mechanisms that lead to increased activity of salivary alpha- amylase due to the stress are not entirely understood [53] and further research is needed to elucidate these mechanisms.
In conclusion, this study found specific emotional responses and physiological response patterns of different physiological systems to deliver lecture as compared to a control condition (working day without lecture). This study was restricted to lecturers working in the university since 15 years. Therefore, results may not generalize to other populations. Data were obtained over a single working day, and patterns may vary with more sustained measurement. However, it appears that the biomarkers evaluated in this study might be useful as putative markers of stress. Lecturing to 200 students results in temporary decreases in S-IgA and s-lys without affecting the saliva flow rate. These results seem to be associated with increases in alpha-amylase activity, cortisol and CgA concentrations. the mechanism that leads to modify activity of salivary alpha-amylase, chromogranin A, and lysozyme due to stress are not entirely understood and further research is needed to elucidate these mechanisms.
References
- Evans P, Clow A, Hucklebridge FH. Stress and im- mune system. Psychologist 1997; 10: 303-307.
- Mestecky J. Saliva as a manifestation of the common mucosal immune system. Ann N Y Acad Sci 1993; 694: 184-194.
- Gleeson M, Pyne DB. Exercise effects on mucosal im- munity. Immunol Cell Biol 2000; 78: 536-544.
- Evans P, Hucklebridge FH, Clow A, Doyle A. Secre- tory Immunoglobulin A as a convenient biomarker in health survey work. In: Health psychology and quality of life research. Rodrigues M, ed. Spain: Health Psy-chology Dept, University Of Alicante. 1995; 541-549.
- Willemsen G, Ring C, Mckeever S, Carroll D. (2000) Secretory Immunoglobulin A and cardiovascular activ- ity during mental arithmetic: effects of task difficulty and task order. Biol Psychol 2000; 52: 127-141.
- Goyette SR, Deluca A. Semester-long student-directed research project involving enzyme immunoassay: ap-propriate for immunology, endocrinology, or neurosci-ence courses. Life Sci Educ 2007; 6: 332-342.
- Ng V, Koh D, Mok By, Chia S, Lim L. Stressful life events of dental students and salivary Immunoglobulin A. J Dental Educat 2003; 67: 1091-1094.
- Ohira H. Social support and salivary secretory Immu- noglobulin A response in women to stress of making a public speech. Percept Mot Skills 2004: 98; 1241-1250.
- Otsuki T, Sakaguchi H, Hatayama T, Takata, A, Hyodoh F, Tsujita S, Ueki A Morimoto K. Secretory IgA in saliva and academic stress. Int J Immunopathol Pharmacol 2004; 17: 45- 48.
- Takatsuji K., Sugimoto Y, Ishizaki S, Ozaki Y, Ma- tsuyama E, Yamaguchi Y. The effects of examination stress on salivary cortisol, Immunoglobulin A, and chromogranin A in nursing students. Biomed Res 2008; 29: 221-224.
- Zeier H, Brauchli P, Joller-Jemelka H. Effects of work demand on immunoglobulin-a and cortisol in air traffic controllers. Biol Psychol 1996; 42: 413-423.
- Moutsopoulos HM, Karsh J, Wolf Ro, Tarpley TM, Tylenda A, Papadopoulos NM. Lysozyme determina- tion in parotid saliva from patients with sjogren’s syn- drome. Am J Med 1980; 69: 39-42.
- Yang Y, Koh, D, Ng V, Lee Cy, Chan G, Dong F, Goh SH, Anantharaman V, Chia SE. Self perceived work re- lated stress and the relation with salivary IgA and ly- sozyme among emergency department nurses. J Occup Environ Med 2002; 59: 836-841.
- Ng V, Koh D, Chan G, Ong HY, Chia SE, Ong CN. Are salivary Immunoglobulin A and lysozyme bio-markers of stress among nurses? J Occup Envir Med 1999; 41: 920-927.
- Yu SF, Jiang KY, Zhou WH, Wang S. Relationship between occupational stress and salivary S-IgA and ly-sozyme in assembly line workers. Chin Med J 2008; 121: 1741-3.
- Perera S, Uddin M, Hayes JA. Salivary lysozyme: a noninvasive marker for the study of the effects of stress on natural immunity. Int J Behav Med 1999; 4: 170- 178.
- Sabbadini E, Berczi I. The submandibular gland: a key organ in the neuro-immuno-regulatory network? Neuroimmunomodulation 1995; 2: 184–202.
- Schwab Ko, Heubel G, Bartels H. Free epinephrine, norepinephrine and dopamine in saliva and plasma of healthy adults. Eur J Clin Chem Clin Bioch 1992; 30: 541-544.
- Bosch JA, De Geus EJ, Veerman EC, Hoogstaten J, Nieuw Amerongen AV. Innate secretory immunity in response to laboratory stressors that evoke distinct pat- terns of cardiac autonomic activity. Psychosom Med 2003; 65: 245-258.
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis ED, Stroud LR. Salivary α- amylase in biobehavioral re- search. Ann N Y Acad Sci 2007; 1098: 122- 144.
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kir- schbaum C, Ehlert U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psy-chophysiol 2005; 55: 333-342.
- Nakane H, Asami O, Yamada Y, Harada T, Matsui N, Kanno T. Salivary chromogranin a as an index of psy- chosomatic stress response. Biomed Res 1998; 18: 401-406.
- Dimsdale JE, O’Connor DT, Ziegler M, Mills P. Chromogranin A correlates with norepinephrine release rate. Life Sci 1992; 51: 519-525.
- Saruta J, Tsukinoki K. Sasaguri K, Ishii H, Yasuda M, Osamura YR, Watanabe Y, Sato S. Expression and lo- calization of chromogranin a, purification and charac- terization from catecholamine storage vesicles of hu- man pheochromocytoma. Hypertension 2005; 6: 2-12.
- Yanaihura N, Nishikawa Y, Hoshino M. Evaluation of region-specific radioimmunoassays for rat and human Chromogranin a: measurement of immunoreactivity in plasma, urine and saliva. in: Kanno T, Nakazato Y, Kumakura K. (ed.) The adrenal chromaffin cell. Hok- kaido University Press, Sapporo, Japan, 1998; 305-313.
- Nakane H, Asami, O, Yamada Y, Ohira H. Effect of negative air ions on computer operation, anxiety and salivary chromogranin a-like immunoreactivity. Int J Psychophysiol 2002; 46: 85-9.
- Brouwers A, Tomic W. A longitudinal study of teacher burnout and perceived self-efficacy in classroom man- agement. Teaching Teacher Educ 2000; 16: 253-259.
- Grayson J, Alvarez H. School climate factors relating to teacher burnout: a mediator model. Teaching Teacher Educ 2008; 24: 1349-1363.
- Den R, Toda M, Nagasawa S, Kitamura K, Morimoto K. Circadian rhythm of human salivary chromogranin A. Biomed Res 2007; 28: 57-60.
- Fiet J, Passat P, Guechot J, Gourmel B, Villette JM, Cathelineau G. Interet du dosage du cortisol dans la salive. Nouv Pres Med 1981; 10: 2664.
- Rohleder A, Nater UM.Determinants of salivary alpha- amylase in humans and methodological considerations. Psychoneuroendocrinology 2009; 34: 469-85.
- Cole AS, Eastoe JE. Biochemistry and oral biology. London: Wright, 1988; 476-477.
- Guyton AC. Secretory functions of the alimentary tract. in: Textbook of medical physiology. 8TH ed. Philadel- phia: WB Saunders, 1991; 771.
- Li TL, Gleeson M. The effect single and repeated bouts of prolonged cycling and circadian variation on saliva flow rate, Immunoglobulin A and alpha-amylase responses. J Sports Sci 2004; 22: 1015-1024.
- Spielberger CD. Manual for the State-Trait Anxiety Inventory: STAI. Palo Alto, Ca: Consulting Press, 1983.
- Gotlib IH, Cane DB. Self-report assessment of depres-sion and anxiety. in: Kendall PC, Watson D eds. Anxi- ety and depression; distinctive and overlapping features. Academic Press, San Diego, 1989; 131-170.
- Gauthier J, Bouchard S. Adaptation canadienne- française de la forme revisee du State-Trait Anxiety In- ventory de Spielberger. Can J Behav Sci 1993; 25: 559-578.
- Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dental Res 2000; 79: 1652-1658.
- Gemba H, Teranaka A, Takemura K. Influences of emotion upon parotid secretion in human. Neurosc Let- ters 1996; 211: 159-162.
- Queiroz CS, Hayacibara MF, Tabchoury CP, Marcon- des FK, Cury JA. Relationship between stressful situa- tions, salivary flow rate and oral volatile sulfur- containing compounds. Eur J Oral Sci 2002 ; 110: 337-340.
- Bosch JA, Brand HK, Ligtenberg TJM, Bermond B, Hoogstraten Amerongen AV. Psychological stress as a determinant of protein levels and salivary-induced ag-gregation of streptococcus gordonii in human whole sa-liva. Psychosom Med 1996; 58: 374-382.
- Guinard JX, Zoumas-Morse C, Walchak C. Relation between parotid saliva flow and composition and the perception of gustatory and trigeminal stimuli in foods. Physiol Behav 1997; 63: 109-118.
- Vedhara K, Cox NK, Wilcox GK, Perks P, Hunt M, Anderson S, Lightman SL, Shanks NM. Chronic stress in elderly care of dementia patients and antibody response to influenza vaccination. Lancet 1999; 353: 627-631.
- Webb HE, Weldy ML, Fabianke-Kadue EC, Orndorff GR, Kamimori GH, Acevedo E0. Psychological stress during exercise: cardiorespiratory and hormonal responses. Eur J Appl Physiol 2008; 104: 973-981.
- Vivian NG, Koh D, Mok BY, Chia SE, Lim LP. Sali- vary biomarkers associated with academic assessment stress among dental under graduates. J Dental Educ 2003; 67: 1091-1094.
- West N, Pyne DB, Renshaw G, Cripps AW. Antim- icrobial peptides and proteins, exercise and innate mu-cosal immunity. Fems Immunol. J Med Microbiol 2006; 48: 293-304.
- Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The rela- tionship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg 2005; 101: 1873-1876.
- Yang Y, Koh, D, Ng V, Lee CY, Chan G, Dong F, Goh SH, Anantharaman V, Chia SE. Self perceived work re- lated stress and the relation with salivary IgA and lysozyme among emergency department nurses. J Occup Environ Med 2002; 59: 836-841.
- Kiecolt-Glaser JK, Garner W, Speicher CE, Penn GM, Holliday J, Glaser R. Psychosocial modifiers of immu- nocompetence in medical students. Psychosom Med 1984; 46: 7-14.
- Taupenot L, Harper KL, O’Connor DT. The chromo- granin-secretogranin family. N Engl J Med 2003; 348: 1134-1149.
- Lee T, Shimizu T, Iljima M, Obinata K, Yamashiro Y, Nagasawa S. Evaluation of psychosomatic stress in children by measuring salivary chromogranin A. Acta Paediat 2006; 95: 935-939.
- Chatterton RT, Vogelsong KM, Lu YC, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol 1996; 16: 433-448.
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity-associations with adrenergic activity. Psychoneuroendocrinology 2006; 31: 49-58.
- Borgeat G, Chagon G. Legault Y. Comparison of the salivary changes associated with a relaxing and with a stressful procedure. Psychophysiology 1984; 21: 690-698.
- Van Stegeren AH, Wolf O, Kindt M. Salivary alpha- amylase and cortisol responses to different tasks: im- pact of sex. Int J Psychophysiology 2008; 69: 33-40.
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 2007; 32: 392-401.
- Ziegler DR, Cass WA A, Herman JP. Excitatory influ- ence of the locus coeruleus in Hypothalamic-Pituitary- Adrenocortical axis responses to stress. J Neuroendocrinol 1999; 11: 361-369.