Review Article - Journal of Public Health and Nutrition (2019) Volume 2, Issue 3
Lasting effect of nutritional and exercise interventions on body weight: A comprehensive literature review.
Marti T1, Foth D2, Weidlinger S1, Stute V3, Stute P1*1Department of Obstetrics and Gynecology, Inselspital Bern, Switzerland
2MVZ PAN Institut für endokrinologie und reproduktionsmedizin GmbH, Cologne, Germany
3Quantitative Risikomodellierung, Uhlandstrasse 11, Frankfurt am Main, Germany
- Corresponding Author:
- Dr. Petra Stute, MD
Section of Gynecologic Endocrinology and Reproductive Medicine
Department of Obstetrics and Gynecology
Inselspital Bern Friedbuehlstrasse 19, 3010 Bern Switzerland
Tel: (0)31-632-1303
E-mail: stutepe@web.de
Accepted date: October 29, 2019
Abstract
Objectives: Obesity is a serious public health concern. The purpose of this comprehensive review is to report on the effectiveness of nutritional and exercise interventions alone or in combination on body weight loss and maintenance in adults.
Methodology: A literature search was performed in 2018. 20 studies on nutritional and exercise interventions were identified and included into this review.
Results: Overall, 18 of 20 studies reported a total weight loss ranging between -0.6% to -9.0%. Diet in combination with exercise yielded the best results, exercise alone was the least effective. High-protein and/or low glycaemic index diets as well as the Mediterranean diet had a highly positive effect on body weight. However, the majority of studies reported body weight regain during follow-up.
Conclusion: Caloric restriction, especially in combination with physical activity, is the most effective way to achieve considerable weight loss in a short amount of time. However, after initial weight loss maintaining body weight in the long-term is a major challenge. Education and cognitive behavioral therapy contribute to long-term success of weight loss programs. Integrating subjective theories for being overweight/obese may improve the outcome.
Keywords
Obesity, Nutritional interventions, Exercise interventions, Long-term weight loss, Weight maintenance.
Introduction
Obesity has become a major public health concern. In 2016, worldwide more than 1.9 billion adults were overweight (body mass index (BMI) ≥ 25) of whom over 650 million were obese (BMI ≥ 30) corresponding to a prevalence of overweight in 39% and obesity in 13% adults, respectively. Obesity does not only affect adults but children as well. In 2016, 41 million children under the age of five years, and 340 million children and adolescents between the age of five and 19 years were overweight or obese, respectively. Overall, the prevalence of obesity increased threefold from 1975 to 2016 [1]. Obesity is associated with an increased risk to develop chronic non-communicable diseases (NCD) such as diabetes mellitus, cardiovascular disease, dementia and cancer. Furthermore, mortality has been shown to be increased in people with a BMI above a certain threshold [2]. As NCD risks increase with increasing BMI [1] weight reduction seems to be the appropriate way to approach these health risks. For most obese people maintaining weight loss is more difficult than initially losing weight. Unfortunately, both, persisting obesogenic environments and physiological adaptations such as reduced metabolic rate and increased appetite can lead to an at least partly regain of the lost body weight. However, it is uncommon that weight regain exceeds starting weight [3].
Thus, it is essential to maintain weight loss over time in order to achieve a long-term decrease in complications of obesity. The objective of this comprehensive literature review was to compare the effectiveness of different lifestyle interventions such as dietary and/or exercise interventions on initial and longterm weight loss.
Materials and Methods
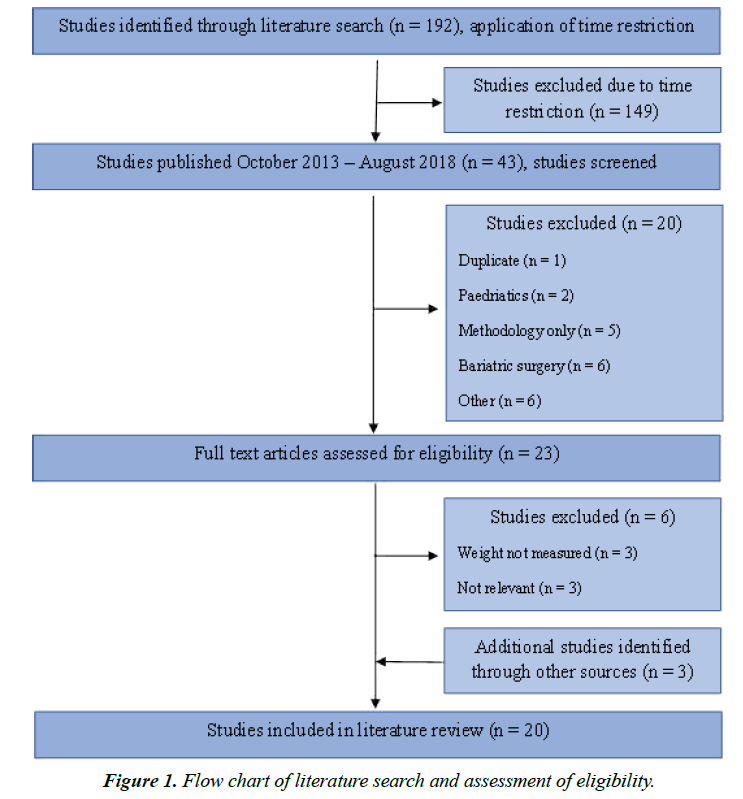
In August 2018, a literature search was performed using the data base Ovid Medline. The literature search was performed using multiple combinations of Mesh-terms and keywords related to the investigated topic. Included terms were “diet”, “nutrition”, “intervention”, “exercise”, “physical intervention”, “weight loss”, “weight maintenance”, “obesity”, “lasting”, “long-term”, “adult”, “follow-up” (Supplementary file 1). The search yielded 192 relevant articles. Only articles from October 2013 to August 2018 were included, since articles published from January 1990 to October 2013 were already covered by one of the included systematic reviews [4]. After application of the above-named time restriction, the final list comprised 43 relevant articles. After August 2018, three additional articles have been identified and included into this review.
Results
Of 46 studies, 20 were included into the comprehensive literature review (Table 1) [4-23]. The other studies were excluded as they, for example: involved participants under the age of 18 years, did not publish the participants’ body weight after the intervention period, or included surgical or pharmaceutical interventions, respectively (Figure 1).
| Author (year of publication) | Study design | Sample size and cohort characteristics | Study duration and follow-up | Description of intervention | Endpoints | Results | Comment |
|---|---|---|---|---|---|---|---|
| Astrup et al. (2015) | RCT | n=932; enrolment of families with at least one overweight or obese parent and at least one child aged 5-18 years, participants aged 18-65 year; n=773 for 6-month intervention; (n=256 in subgroup with dietetic instructions) | Initial 8-week low-calorie diet (LCD), then 6-month dietary intervention; then 6-month dietary instructions with follow-up for one subgroup | 8-week 800 kcal per day LCD period; If minimum weight loss of 8% randomisation to one of five diets, six counselling sessions during 6-month intervention (subgroup got dietetic instructions for 6 more months), maintenance of weight loss advised, further weight loss also allowed; NP/LGI: normal-protein, low glycaemic index; NP/HGI: normal-protein, high glycaemic index (GI); HP/LGI: high-protein, low glycaemic index; HP/HGI: high-protein, high glycaemic index; Control diet (medium protein content, no specific instructions on GI) | I. Changes in body weight II. Changes in dropout rate, body fat, sagittal diameter, CRP, LDL, HDL, triglycerides, blood pressure | Mean weight loss during 8-week LCD: 11 kg; Weight regain was 0.93 kg less in HP groups than in NP groups and 0.95 kg less in LGI groups than in HGI groups; Combination HP/LGI exerted additive effect that completely prevented weight regain; Subgroup with 6 further months of dietetic instructions:Â Weight regain was 2.0-2.8 kg less in HP groups than in NP groups (depending on analysis) but no consistent effect of GI on weight regain was found; Entire 14-month intervention: HP groups lost 7.3 kg in total compared to 4.5 kg in NP groups | Weight regain not given for every group but generalized, exact weight only displayed in graphic |
| Brown et al. (2015) | Pilot study | n=18; participants with serious mental illness and BMI > 25, mean age 47.3 (range 23-64) | 8 weeks; follow-up at 6 months | NEW-R (nutrition and exercise for wellness and recovery) with weekly 2 h sessions for 8 weeks: 1 h topics related to nutrition and physical activity, 20 min moderate intensity workout, 40 min healthy meal; participants provided with material to promote behavioural change e.g. recipes, elastic exercise bands | I. Changes in weight, changes in level of knowledge about nutrition and exercise | Mean weight at baseline: 229.2 pounds; Mean weight at immediate postintervention: 226.2 pounds (SD=52.01); mean weight loss: 3 pounds (p=0.12, t=1.66); Mean weight at 6-month follow-up: 219.2 pounds (SD=45.7); mean weight loss: 10 pounds (p=0.03, t=2.39) | Lack of control group, small sample size, lack of representative sample |
| Chmelo et al. (2016) | Pilot study | n=24; participants aged 65-79 years (mean age 70), sedentary (no training in last 6 months), BMI 27-35, non-smoker, weight stable and without severe illnesses | 5 months; follow-up 18 months after completion of intervention | Resistance training with (RT-CR) or without (RT) caloric restriction, 3 days per week for 5 months RT: 3 sets of 10 repetitions for each of 8 exercises on resistance machine at 70% 1RM (= maximal weight a person could lift with correct form in a single repetition) CR: reduction of daily caloric intake (subtracting 600 kcal from estimated daily energy needed for weight maintenance), meal replacements (2 per day), nutrition education (weekly), dietary behaviour modification advice | I. Changes in body weight, body and thigh composition | RT: mean weight at baseline 88.4 kg (SD=15), mean weight at 5 months 88.6 kg (SD=15), mean weight at 23 months 88.5 kg (SD=15) RT-CR: mean weight at baseline 83.9 kg (SD=12), mean weight at 5 months 76.8 kg (SD=9.7), mean weight at 23 months 81.6 kg (SD=10) | Parent study: Nicklas et al. (2015), RT vs. RT-CR; Chmelo et al. (2016): follow-up with 24 participants of parent study |
| De Vos et al. (2016) | RCT | n=407; female participants aged 50-60 years (mean age 55.7), BMI = 27 (mean BMI 32.4), free of knee osteoarthritis; n=247 at follow-up | 2.5 years (follow-up every 6 months); follow-up after 6.6 years | Meeting with dietician once every two weeks for the first month then maximal 4 hr per year (evaluation of current nutritional and physical activity habits, individual goal-setting, tailor-made advice was given for a low-fat or a low-calorie diet or both, as well as for physical activity), physical activity classes (20 group classes, 1 h per week, low-intensive sport activities) Control group: no intervention, free to undertake health-promoting activities at own initiative | I. Long-term weight change II. Changes in physical activity, nutritional habits, fat percentage and quality of life |
Control group: mean weight at baseline 89.2 kg (SD=13.6), change in weight compared to baseline +0.9 kg (6 months), +0.7 kg (12 months), +1.6 kg (18 months), +1.7 kg (24 months), +0.0 kg (30 months), +0.1 kg (80 months); Intervention group: mean weight at baseline 88.2 kg (SD=12.9), change in weight compared to baseline -0.8 kg (6 months), -0.6 kg (12 months), +0.8 kg (18 months), +0.9 kg (24 months), -0.5 kg (30 months), -0.5 kg (80 months); After 6 months, weight loss was significantly higher in intervention group, but over time, this difference decreased and became nonsignificant after 24 months | Parent study: De Vos et al. (2014); weight differences between groups given in numbers, exact weight only displayed in graphic; weight change not given for all different interventions individually |
| Dombrowski et al.(2014) | Systematic review and meta-analysis (n=45 trials, all RCT or cluster RCT) | n=7788; participants were adults, had or had had BMI = 30, lost = 5% of body weight (within 24 months before weight loss maintenance treatment) (n=2949 after exclusion of pharmacological interventions) | Initial weight loss phase (n=42) ranged from 2-12 months (mean 4); follow-up ranged from 3-36 months (mean 12) from randomisation to weight loss maintenance intervention | Any behavioural/lifestyle, food replacement and/or supplement, alternative or pharmacological interventions, singly or in combination; Diet interventions: caloric restriction recommended most commonly; Exercise interventions: general increase in physical activity recommended, some specific recommendations including walking or resistance trainings | I. Weight at 12 months from randomisation to the weight loss maintenance intervention | Initial weight loss phase: mean weight loss-10.8 kg (-4.03 kg to -21.3 kg); Average difference in weight change between intervention and control group: 12 months (15 studies, 2949 participants, p=0.14): -1.56 kg (95% CI -2.27 to -0.86); 18 months (7 studies, I2=15%): -1.96 kg (95% CI -2.73 to -1.20) 24 months (2 studies, I2=0%): -1.48 kg (95% CI -2.27 to -0.69) 30 months (2 studies, I2=0%): -0.85 kg (95% CI -1.81 to 0.11) | Included due to separate arm for non-pharmacological interventions;Â weight differences between intervention and control given, but not weight regain after initial weight loss phase and total weight loss |
| Donini et al. (2014) | Prospective cohort study | n=464; participants aged 18-65 years, BMI>30 kg/m2, no eating disorder, no severe illnesses, not already engaged in regular physical activity; n=161 at follow-up | 4-year follow-up | Patients assigned to SNT or NPPRP according to their preference and availability; SNT (standard nutrition treatment): individualized diet, advice to increase physical activity; NPPRP (nutritional and psycho-physical reconditioning program): in addition to SNT 4 h sessions 2 days per week with physical reconditioning (ergometer, treadmill and resistance training), group cognitive-behavioural psychotherapy and educational activities | I. Changes in body weight, eating behaviour, physical activity performance, occurrence of obesity-related complications II. Risk of eating disorder, reasons for abandoning prescribed therapeutic plan, deviation from prescribed diet |
Weight loss was greater in the NPPRP group than in the SNT group (-8.08 ± 10 kg versus -3.02 ± 6.2 kg; p<0.05). BMI decreased equally more in the NPPRP group than in the SNT group (-3.02 ± 3.34 kg/m2 versus -0.88 ± 1.91 kg/m2; p<0.05) | Lack of randomisation |
| Duggan et al. (2017) | RCT | n=439; female participants, aged 50-75 years, postmenopausal, BMI = 25, sedentary, not taking hormonal therapy; n=156 at follow-up | 1 year; follow-up 18 months after completion of RCT | Participants randomly assigned to: Reduced-calorie dietary modification intervention (n=118), Moderate-to-vigorous intensity aerobic exercise intervention (n=117), Combined diet and exercise intervention (n=117), Control (no intervention; n=87) | I. Changes in biomarkers associated with angiogenesis (VEGF, PAI-1, PEDF) II. Changes in weight and BMI |
Diet and exercise: reduction in BMI -7.92% at 30 months vs. -13.7% at 12 months; Diet: reduction in BMI -6.64% at 30 months vs. -12.0% at 12 months; Exercise: reduction in BMI -2.11% at 30 months vs. -1.93% at 12 months; Control: reduction in BMI -2.88% at 30 months vs. +0.62% at 12 months | |
| Finocchiaro et al. (2016) | Cohort study | n=100; participants were female and in breast cancer follow-up, age = 70 years (mean 55.5), no involvement of vital organ, no metastases, no severe illnesses | 1 month; follow-up at 2 and 6 months | 4 meetings once a week of 2 hr including lectures, training sections and workshops lasting overall 1 month (content: importance of healthy lifestyle, recommendation of Mediterranean diet and brisk physical activity for at least 3 h per week, changes in food strategies) | I. Changes in anthropometrics data (weight, BMI, waist circumference) II. Changes in physical activity, adherence to Mediterranean diet |
Weight (p<0.001): at baseline 73.0 kg (SD=15.0), at 2 months 70.8 kg (SD=13.8; weight loss -2.2 kg (SD=3.2)), at 6 months 69.7 (SD=13.3; weight loss vs. baseline -3.4 kg (SD=3.8)) Weight loss>5% in 16% at 2 months and in 43% at 6 months BMI (p<0.001): at baseline 28.7 (SD=5.9), at 2 months 27.9 (SD=5.5), at 6 months 26.7 (SD=5.4) |
|
| Gilis-Januszewska et al. (2017) | Cohort study | n=262; participants aged >25 years, high diabetes risk (FINDRISC>14), no known diabetes, no chronic diseases; n = 105 completed all follow-ups | 4-month intervention; 6-month maintenance phase; follow-up at 1 and 3 years | 1 month with 11 sessions about diet and physical activity changes (goals: loss of initial weight, reduced intake of total and saturated fats, increased consumption of fruit, vegetables and fibre and increased physical activity), 3 months of physical activity sessions 2 per week, lastly 6 months maintenance phase with 6 motivational telephone sessions and 2 motivational letters | I. Changes in risk factors for type 2 diabetes (weight, BMI, waist circumference, systolic and diastolic blood pressure, fasting glucose, 2-h OGGT glucose, total cholesterol, HDL, serum triglycerides, FINDRISC) | Weight (p=0.048): at baseline 82.85 kg (SD=15.20), change from baseline to 1 year -2.27 kg (SD=5.25), change from baseline to 3 years -1.14 kg (SD=5.83) BMI (p=0.001): at baseline 31.10 kg/m2 (SD=4.93), change from baseline to 1 year -0.84 kg/m2 (SD=1.91), change from baseline to 3 years -0.35 kg/m2 (SD=2.18) | |
| Hinderliter et al. (2014) | RCT | n=144; participants aged >35 years (mean 52), BMI 25-39.9 (mean 33.1), sedentary, BP of 130-160/80-99 mmHg; n=140 at 16-week follow-up, n=124 at 1-year follow-up | 4 months; follow-up at 1 year (8 months after completion of intervention) | Participants randomised to diet (DASH: dietary approaches to stop hypertension; weekly sessions to learn how to buy and prepare the appropriate foods ? high fibre, whole grains, low fat dairy ? and how to stay motivated to follow the diet), diet plus behavioural weight management intervention (in addition weekly cognitive behavioural weight loss intervention, 3 times per week exercise sessions) or usual care (maintain usual dietary and exercise habits) | I. Persistence of changes in dietary habits, exercise behaviours, body weight and blood pressure 1 year after study enrolment | Weight at 16 weeks: diet -0.3 kg, diet plus behavioural weight management intervention -8.7 kg, usual care +0.9 kg; Weight at 1 year (compared to baseline): diet -1.5 kg, diet plus behavioural weight management intervention -6.3 kg, usual care -1.6 kg | |
| Lindstrom et al. (2013) | RCT | n=522; participants aged 40-64 years (mean:55), overweight (mean BMI 31.2), impaired glucose tolerance; n=366 at follow-up | Mean duration of active intervention 4 years (range 1-6 years); participants followed-up until diabetes diagnosis or maximal until 2009 (mean follow-up 9 months after intervention) | Participants randomised to intervention or control group; Control: received standard general lifestyle information; Intervention: counselling lessons with dietician (7 through the first year, then every 3 months, goal: low fat, high fibre), voluntary free-of-charge supervised exercise sessions (goal: at least 4 h physical activity per week) | I. Diagnosis of diabetes (based on OGTT) II. Changes in body weight, glycaemia, physical activity and diet |
Mean change of body weight in control group (compared to baseline):Â Â Â Â Â Â -1.1% (yr. 1), Â -0.9% (yr. 2), Â -0.6% (yr. 3), Â -0.5% (yr. 4), -0.4% (yr. 5), 0.0% (yr. 6), +0.2% (yr. 7), +0.1% (yr. 8), +0.6% (yr. 9), +0.7% (yr. 10) Mean change of body weight in intervention group (compared to baseline): Â Â Â Â Â Â -4.9% (yr. 1), -4.1% (yr. 2), -3.5% (yr. 3), -3.0% (yr. 4), -2.6% (yr. 5), -1.7% (yr. 6), -1.6% (yr. 7), -1.5% (yr. 8), -1.1% (yr. 9), -0.9% (yr. 10) | Weight only displayed in graphic |
| McRobbie et al. (2016) | RCT | n=330; participants were adults (= 18 years old, mean 46.1), had BMI 30-45 or BMI 28-30 plus comorbidities, mean weight 96.4 kg, not BMI>45, not lost>5% of their body weight in previous 6 months, not pregnant, not taking psychiatric medication; n=291 at follow-up | 8 weeks; follow-up at 2, 6 and 12 months (from baseline) | Participants randomised to intervention or control group (ratio 2:1); Control group (practice nurse intervention): best-practice intervention in primary care, incorporating national guidelines, 4 sessions over 8 weeks, 6- and 12-month follow-up sessions; Intervention group (Weight Action Programme WAP): standard cognitive-behavioural interventions, dietary advice and self-monitoring, close monitoring of exercise levels and caloric intake, non-judgemental support network 8 weekly group sessions followed by 10 monthly maintenance sessions | I. Weight change at 12 months II. Changes in BMI, waist circumference, blood pressure, proportion of participants losing at least 5%/10% of body weight, many others |
Weight control group: 98.3 kg (SD=16.6) at baseline, -1.0 kg (SD=1.6) at 1 month, -2.2 kg (SD=2.6) at 2 months, -2.1 kg (SD=4.3) at 6 months, -2.3 kg (SD=6.6) at 12 months; Weight intervention group: 95.5 kg (SD=15.8) at baseline, -1.0 kg (SD=1.7) at 1 month, -3.2 kg (SD=2.7) at 2 months, -5.0 kg (SD=5.4) at 6 months, -4.2 kg (SD=7.3) at 12 months; Treatment effect: -0.1 kg at 1 month (p=0.81), -1.0 kg at 2 months (p=0.009), -2.5 kg at 6 months (p<0.001), -1.9 kg at 12 months (p=0.04) | Use of Orlistat: 75 participants (23%) used Orlistat as part of their weight loss attempt, participants in WAP were more likely to use Orlistat than participants in the nurse arm (31% vs. 6%) |
| Mensinger et al. (2016) | RCT | n=80; participants were female, aged 30-45 years, BMI 30-45, physically inactive, pre-menopausal, on birth control if heterosexual, exclusion criteria include bariatric surgery and medication known to effect weight; n=72 at 6-month follow-up; n=40 at 24-month follow-up | 6 months; follow-up 6 and 24 months after randomisation | Participants randomised to weight-neutral or weight-loss approach, both groups had weekly 90-minutes sessions for 6 months; Weight-neutral (HUGS Programme for Better Health): emphasized principles of eating for well-being and pleasure (signs of hunger/satiety), size acceptance, importance of physical activity for personal fulfilment, social support network was created; Weight-loss (LEARN Program for Weight Management): weight loss as goal, focus on changing diet (caloric restriction) and lifestyle, social support network was created | I. Changes in blood pressure, lipid panels, blood glucose, BMI, weight, waist and hip circumference, distress, self-esteem, quality of life, dietary risk, fruit and vegetable intake, intuitive eating and physical activity | Weight-neutral program: 102.1 kg (SE=2.1) at baseline, 101.6 kg (SE=2.1) at 6 months (-0.56 kg from baseline), 101.3 kg (SE=2.2) at 24 months (-0.83 kg from baseline); Weight-loss program: 105.3 kg (SE=2.1) at baseline, 100.7 kg (SE=2.1) at 6 months (-4.6 kg from baseline), 101.6 kg (SE=2.2) at 24 months (-3.7 kg from baseline) | |
| Ortner et al. (2016) | RCT | n=124; participants 18-69 years old, BMI = 30, exclusion criteria include medication affecting weight control, newly diagnosed diabetes, CVD or hypertension; n=84 at 12-month follow-up | 12 months; follow-up 1 and 12 months after randomisation | Participants randomised to Mediterranean diet (MD) plus physical activity or standard hypolipemic diet (SHD) plus physical activity, both groups had intensive 5-day weight reduction program (education about nutrition and eating behaviour, physical activity - mainly walking - and behaviour modification), followed by 5 sessions (at week 1 and month 1, 3, 6 and 12); MD: energy intake restricted to 1573 kcal/day, 35% of calories from fat, rich in vegetables, fruits, whole grains and low in red meat, dietary supplements (extra virgin olive oil (2 tablespoons/day), nuts (1 handful/day), fish (3-4 portions/week) SHD: energy intake limited to 1287 kcal/day, rich in whole grains, fruits and vegetables and restriction of additional fats, sweets and high-fat snacks and low in red meat | I. Serum antioxidant capacity II. Changes in body weight and uric acid |
Change in weight; MD: 112.72 kg (SD=19.47) at baseline, 106.02 kg (SD=17.96) at month 1, 103.79 kg (SD=17.81) at month 12, from baseline to month 12 -8.93 kg; SHD: 111.51 kg (SD=21.30) at baseline, 106.61 kg (SD=20.11) at month 1, 106.19 kg (SD=21.93) at month 12, from baseline to month 12 -5.32 kg | |
| Pan et al. (2013) | Systematic review (n = 3 trials, all cohort studies) | n = 124988 (n = 50013 NHS, n = 52987 NHS II, n = 21988 HPFS); participants 18-65 years old, without obesity and chronic disease (diabetes, cancer, cardiovascular, renal, pulmonary or liver disease) at baseline | Duration not given; follow-up every 4 years | Assessment of usual intake of beverages and foods over the previous year at baseline (questionnaires administered at every follow-up), assessment of other lifestyle factors (including alcohol, smoking, physical activity, dietary factors), assessment of body weight every 2 years | I. Long-term changes of body weight | Relationship between beverage intake and weight change within each 4-year period (pooled results and multivariate-adjusted); Plain water: -0.13 kg (-0.17 to -0.08, p < 0.001); Coffee: -0.14 kg (-0.19 to -0.09, p < 0.001); Sugar-sweetened beverage: +0.36 kg (+0.24 to +0.48, p < 0.001); Fruit juice: +0.22 kg (+0.15 to +0.28, p < 0.001); Whole-fat milk: +0.02 kg (-0.06 to +0.10, p = 0.37); Low-fat milk: +0.02 kg (-0.04 to 0.09, p < 0.001); Tea: -0.03 kg (-0.05 to -0.01, p = 0.34); Diet beverage: -0.10 kg (-0.14 to -0.06, p = 0.03) | Only looked at beverages, results pooled and adjusted |
| Poulsen et al. (2015) | RCT | n = 147; participants aged 18-65 years (mean age 43), waist circumference = 80 cm for women and = 94 cm for men, mean BMI 29.1; n = 110 at follow-up (n = 132 for intention to treat analysis) | 26 weeks; 12-month follow-up | Participants randomised to New Nordic Diet (NND) or Average Danish Diet (ADD) in 3:2 ratio, after completion of 26-week intervention participants from ADD were introduced to NND as well; 9 meetings with dietician during intervention (healthy eating, compliance with diet), 2 meetings with dietician during follow-up (assessment of dietary compliance, advice on healthy eating); During intervention maintenance of physical activity habits, during follow-up encouraged to increase physical activity level; NND: more calories from plant foods and fewer from meat, more foods from the sea and lakes and more foods from the wild countryside (participants supplied with recipes, menu plans and a few central NND ingredients) | I. Changes in body weight II. Changes in waist and hip circumference, sagittal diameter, blood pressure, compliance and satisfaction with the diets |
Weight at baseline: NND 91.8 kg (SE = 16.3), ADD 90.9 kg (SE = 19.3); Change in weight during intervention: NND -6.2 kg (SE = 0.5), ADD -3.0 kg (SE = 0.6), adjusted difference -3.3 kg (-5.0 to -1.6, p<0.001); Change in weight during follow-up: NND +4.6 kg (SE = 0.5), ADD +1.1 kg (SE = 0.7), adjusted difference +1.8 kg (+0.1 to +3.4, p<0.041) Intention to treat analysis: NND regained 3.2 kg (SE 1.3, p = 0.011), ADD regained 1.1 kg (SE 1.2), NND total weight change -1.7 kg (SE 0.6, p = 0.0063), ADD total weight change -1.8 kg (SE 0.6, p = 0.0076), no difference between groups (p = 0.88) | |
| Stumm et al. (2016) | RCT | n=60; participants were aged 30-60 years, diagnosed with metabolic syndrome, without severe illnesses | 12 months with follow up | Active Body Control (ABC): telemonitoring of daily physical activity and calorie intake with individual feedback by experienced coaches; Diet intervention: low-calorie (reduction by 500 kcal), preferring carbohydrates with low glycaemic indexes; Exercise intervention: daily moderate but regular physical activity; Counselling weekly during first year (parent study) and monthly (= ABC continued) or no more (= ABC discontinued) during second year | I. Changes in body weight II. Weight loss maintenance, prevention of weight regain through continuation of monitoring and coaching | Weight change ABC continued: -11.4% (-13.7 to -9.3, p<0.0001) over 12 months, -8.5% (-11.0 to -6.1, p<0.0001, +2.8% during ABC continuation) over 24 months; Weight change ABC discontinued: -13.4% (-15.8 to -11.1, p<0.0001) over 12 months, -9.0% (-11.6 to -6.4, p<0.0001, +4.4% during ABC discontinuation) over 24 months | Parent study: Luley et al. (2014), 12-month intervention with ABC programme; Stumm et al. (2016): 12-month intervention with or without continuation of ABC programme |
| Tapsell et al. (2017) | RCT | n = 377; participants were aged 25-54 years, BMI 25-40 (n = 298 completed intervention, n = 178 completed follow-up) | 3 months; follow-up at 12 months after randomisation | Participants randomised to usual care (C), intervention (I) and intervention plus food supplement (IW), counsel sessions monthly for the first 3 months, then every 3 months; C: general advice; I: interdisciplinary advice (dietitian => changes in food choices and increase in physical activity; exercise physiologist => exercise guidance following exercise assessment; psychologist => development of workbook for participants and training of health coaches; health coaches => scripted 15-min calls at quarterly intervals); IW: interdisciplinary advice plus 30 g walnuts per day | I. Changes in body weight II. Changes in blood pressure, fasting blood glucose, HbA1C, and lipids, and changes in diet, exercise and psychological parameters |
Weight change C: 91.8 kg (SD = 14.7) at baseline, 90.0 kg (SD = 14.1) at 3 months, 87.8 kg (SD = 14.9) at 12 months, total weight loss -4.4%; Weight change I: 91.9 kg (SD = 15.2) at baseline, 90.3 kg (SD = 15.3) at 3 months, 86.5 kg (SD = 17.8) at 12 months, total weight loss -5.9%; Weight change IW: 91.4 kg (SD = 15.6) at baseline, 88.3 kg (SD = 14.7) at 3 months, 87.9 kg (SD = 14.2) at 12 months, total weight loss -3.8% | |
| Washburn et al. (2014) | Systematic Review (n = 20 trials, all RCT) | n = 2635; participants aged 18-65 years, overweight or obese (BMI = 25) | Active weight loss period of > 6 months (n = 6) or active weight loss with a follow-up period of any duration (n = 14) | Comparison of diet (D), aerobic exercise (AE) and resistance exercise (RE), singly or in combination | I. Changes in body weight II. Changes in body composition (fat mass, fat-free mass, waist circumference) and chronic disease risk factors (including total, HDL and LDL cholesterol, triglycerides, insulin, glucose, HbA1C and blood pressure) | 40% of trials reported significantly greater long-term weight loss with diet compared with aerobic exercise (results for differences in weight regain were inconclusive); Diet&aerobic exercise resulted in significantly greater weight loss than diet alone in 50% of trials; Weight regain was similar in diet and diet&aerobic exercise groups (55% of loss); Fat-free mass tended to be preserved when interventions included exercise | |
| Wing et al. (2016) | RCT | n = 599; participants aged 18-35 years, BMI 21-30 | 3-year follow-up | Participants randomised to control (C), self-regulation plus small changes (SRs) or self-regulation plus large changes (SRl), all received monthly feedback on weight, quarterly newsletters and personalized feedback reports; Self-regulation: frequent self-weighing to cue behaviour changes, 10 meetings over 4 months then 2 4-week online refresher per year; Small changes: reduce intake and increase activity (both by 100 kcal/day); Large changes: focused on losing 2.3-4.5 kg initially (500-1000 kcal deficit during initial 8 weeks) to buffer against expected weight gain, increase physical activity C: only 1 initial meeting | I. Changes in body weight from baseline over follow-up II. Changes in body weight from baseline to 2 years, proportion gaining at least 0.45 kg from baseline, proportion developing obesity | Change in weight from baseline to 2 years: +0.54 kg in C group, -0.77 kg in SRs group, -1.50 kg in SRl group; Change in weight over mean of 3-year follow-up: +0.26 kg (SE = 0.22) in C group, -0.56 kg (SE = 0.22) in SRs group, -2.37 kg in SRl group; Participants developing obestiy at least once during follow-up: 16.9% in C group, 7.9% in SRs group, 8.6% in SRl group | |
| Note: AE: Aerobic exercise; BMI: Body mass index [kg/m²]; CR: Caloric restriction; HGI: High glycaemic index; HP: High protein; LCD: Low-calorie diet; LGI: Low glycaemic index; NP: Normal protein; RCT: Randomised controlled trial; RT: Resistance training | |||||||
Table 1. Overview of studies included into the review.
Study characteristics
Of selected studies (Supplementary file 2), twelve were randomized controlled trials (RCT) [5-12,14-16,21], five were prospective cohort studies [17,18,20,22,23], and three were systematic reviews [4,13,19] including one meta-analysis [19]. Sample size ranged from 18 [17] to 124,988 subjects [13]. Four studies only considered female participants [5,11,21,22]. In general, age range of subjects was broad, e.g. all adults (above age 18) [10, 19], and 18 to 65 years [4,7,13,14,20], respectively, while one study focused on geriatric (age 65-79 years) [18] and another one on younger patients (age 18-35 years) [16]. All but one study (BMI range 21-30) [16] included overweight and obese subjects only. In and exclusion criteria also differed. For example, some studies excluded participants with chronic or severe diseases [6,13,18,20,22,23], BMI above 45 [10], eating13 disorder [20], or hormone therapy [21]. In contrast, others specifically included patients with serious mental illness [17], metabolic syndrome [6], high risk to develop diabetes mellitus [9,23], or physical inactivity [8,10,11,18,20,21]. All but one study [13] provided the duration of intervention ranging from one month [22] to six years [9], and of follow-up ranging from six months [17,22] to almost seven years [5]. Body weight change expressed as relative (%) or absolute change (kg, BMI) was an endpoint in all studies. Additional endpoints comprised changes in body composition [4,5,7,18], abdominal circumference [4,7,10,11,14,22,23], serum lipids [4,7,11,15,23], blood pressure [4,7,8,10,11,14,15,23], and physical activity and nutrition habits [5,8,9,11,15,20,22], respectively. All but two studies (with dietary interventions only [7,13]) examined the impact of dietary interventions combined with exercise on weight loss and weight maintenance, applying either caloric restriction [4- 6,10-12,16,18-22] or not [8,9,14,15,17,23]. Frequently, lifestyle interventions were complemented by, e.g. cognitive behavioral therapy [8,10,20], self-monitoring [6,10,16], and social support and/or motivation [7,10,11,15,23], respectively.
Impact of lifestyle interventions on body weight loss and maintenance
Overall, 18 in 20 studies reported a total body weight loss ranging from -0.6% to -9.0% (Table 2) [5,6]. Of those, 12 studies reported an initial body weight loss but weight regain during follow-up [6-11,14,16,18,19,21,23]. The remaining two studies were systematic reviews and rather compared the effectiveness of lifestyle interventions than individual body weight changes [4,13].
| Type of intervention | Description of intervention types | Studies | ||
|---|---|---|---|---|
| Diet only (2 studies) | Comparison of different diets | Astrup et al. (2015) | ||
| Comparison of different beverages | Pan et al. (2013) | |||
| Diet and exercise  (18 studies) | With caloric restriction (12 studies) |
Comparison of different diets | Low fat vs. low caloric vs. low fat plus low caloric diet | De Vos et al. (2016) |
| Low fat vs. Mediterranean diet | Ortner et al. (2016) | |||
| Effect of Mediterranean diet | Finocchiaro et al. (2016) | |||
| Comparison of different combinations of diet and exercise | Diet with/without exercise | Donini et al. (2014) | ||
| Exercise with/without diet | Chmelo et al. (2016) | |||
| Diet vs. exercise vs. diet plus exercise | Duggan et al. (2017) | |||
| Washburn et al. (2014) | ||||
| Weight-neutral/small changes vs. weight-loss/large changes | Mensinger et al. (2016) | |||
| Wing et al. (2016) | ||||
| McRobbie et al. (2016) | ||||
| Other | Low glycaemic index with/without active body control | Stumm et al. (2016) | ||
| Any intervention vs. control | Dombrowski et al. (2014) | |||
| Without caloric restriction (6 studies) | Comparison of different diets | Effect of “healthy” diet | Gilis-Januszewska et al. (2017) | |
| Lindstrom et al. (2013) | ||||
| Brown et al. (2015) | ||||
| Effect of New Nordic diet | Poulsen et al. (2015) | |||
| Other | “Healthy” diet with/without cognitive behavioural therapy | Hinderliter et al. (2014) | ||
| Diet with/without food supplement | Tapsell et al. (2017) | |||
| Total: 20 studies | ||||
Table 2. Overview of interventions.
Dietary interventions only: A systematic review on the impact of dietary interventions only found a decrease of body weight when choosing beverages such as water, coffee and tea but an increase with beverages such as whole-fat and low-fat milk, and even more with sugar-sweetened beverages and fruit juices [13]. In a more recent RCT [7], diets with either average protein content, high protein content, low glycaemic index (GI) or high GI were compared to each other. During the initial study phase when all subjects simply followed a low-caloric diet body weight decreased by about -11.9%. However, during the intervention and post-intervention phase body weight increased again in all groups ranging from -4.9% to -7.9%. Intergroup comparisons revealed a lower body weight regain in subjects consuming either a diet with high protein content or low GI. Body weight remained stable in participants consuming a diet with high protein content combined with low GI.
Dietary interventions with caloric restriction and/or exercise: Four studies compared various combinations of diet and exercise [4,18,20,21]. Diet in combination with exercise yielded the best results with body weight loss ranging from -2.7% [18] to -8.7% [20], followed by diet only with body weight loss ranging from -2.5% [20] to -6.6% [21]. Exercise alone was only partly effective with body weight changes ranging from +0.1% [18] to -2.1% [21]. During follow-up there was at least some body weight regain in most study arms ranging from +0.1% to +5.8% [18]. Three studies investigated the impact of defined diets in combination with exercise on body weight [5,12,22]. Both, the Mediterranean Diet and the Hypolipemic Diet (lowfat diet) induced a decrease in body weight. Herein, body weight loss ranged from -4.7% [22] to -7.9% [12] with Mediterranean Diet, and from -0.6% [5] to -4.8% [12] with Hypolipemic Diet, respectively. In those two studies, body weight decreased quite fast, while in the third one applying another low-fat diet [5] body weight loss only stabilized at -0.6% at intervention termination after 30 months but then remained stable until follow-up at 80 months. The magnitude of lifestyle changes needed to yield body weight loss was reported by three studies [10,11,16]. Small lifestyle changes included a healthy lifestyle and some exercise merely for enjoyment, whereas large lifestyle changes comprised caloric restriction and moderate-to-high physical activity. Large lifestyle changes induced a body weight loss ranging from -3.3% [16] to -4.4% [10], while small lifestyle changes yielded smaller results ranging from -0.8% [11, 16] to -2.3% [10].
The impact of active body control (ABC) was analyzed by another RCT [6] comparing a low-caloric, low GI diet with and without ABC. ABC comprised telemonitoring of daily physical activity and calorie intake as well as regular individual feedback by experienced coaches. During the first year, diets were complemented with ABC in both groups and total body weight loss was comparable (about -9%). During the second year ABC was only continued by one group. Not surprisingly, body weight regain was lower when ABC was continued (+2.8% vs. +4.4%). Finally, a systematic review and meta-analysis including 45 RCT investigating any lifestyle interventions, food supplements, alternative therapies or pharmacological interventions, alone or in combination, supported the above mentioned results [19]. Caloric restriction was the most commonly recommended dietary intervention and a general increase in physical activity was the most commonly recommended exercise intervention, followed by more specific recommendations such as walking or resistance training. In general, the mean difference in body weight change between intervention and control group at follow-up favored the intervention group ranging from -0.85 kg (at 30 months) to -1.96 kg (at 18 months), respectively.
Dietary interventions without caloric restriction and/or exercise: Dietary interventions without caloric restriction were quite heterogeneous. For example, total body weight loss was greater with the Average Danish Diet (-2.1%) compared to the New Nordic Diet (-1.7%) due to a greater weight regain with the New Nordic Diet (+5.1%) during follow-up [14]. Three studies investigated the effect of what was referred to as “healthy diet” (low-fat, high-fibre diet) combined with a general increase of exercise on body weight [9,17,23]. Total body weight was found to decrease by -0.9% [9] to -4.4% [17] with or without body weight regain during follow-up ranging from +4% [9] to -3.1% [17]. In another RCT, a low-fat, high-fibre dietary intervention was combined with physical activity and cognitive behavioral therapy and compared to diet alone [8]. Total body weight loss was higher in the combined intervention group (-6.7%) than in the diet only and control groups (-1.6% vs. -1.7%), respectively, with body weight regain also being higher in the combined intervention group (+2.6% vs. none). When walnuts (30 g/day) were added as a food supplement to diet and exercise, total body weight loss was lower with walnuts (-3.8%) than with diet and exercise alone (-5.9%). Body weight remained stable in all groups during follow-up [15].
Education and cognitive behavioral therapy: In order to support body weight loss and weight maintenance seven out of nine studies [8,10,12,15-17,20-22] that reported successful further weight loss after the initial weight loss applied educational lessons or consultations with a dietitian [8,10,12,15,17,20,22], partly combined with cognitive behavioral therapy [8,10,20], respectively. Total weight loss ranged from -1.6% [8] to -8.7% [20] with cognitive behavioral therapy having a particularly supporting impact; total weight loss was -6.7% [8], -4.4% [10] and -8.7% [20], respectively, after one year [8,10] and four years of follow-up [20]. Most other studies that included some kind of education [5,9,11,14,18,23] at least partly achieved weigh loss maintenance after a follow-up of up to ten years [9]. This suggests a positive impact of knowledge about nutrition, exercise and lifestyle patterns on long-term weight loss.
Discussion
The World Health Organization (WHO) recommends a diet with limited energy intake from total fats, limited salt and free sugars consumption and an increased intake of fruits, vegetables, whole grains and nuts. For physical activity the WHO recommends to engage in adequate levels of exercise throughout one’s life acknowledging that different types and amounts of physical activity are needed to achieve different outcomes like e.g. body weight control [24]. However, these recommendations are quite general. So, for the individual the question remains which lifestyle intervention is the most effective to accomplish body weight reduction and body weight maintenance.
Our comprehensive review found that: 1) Diet in combination with exercise yielded the best results, diet alone partly achieved good results, and exercise alone was the least effective; 2) Large lifestyle changes induced greater body weight loss while smaller lifestyle changes mainly maintained body weight; 3) Diets with highly positive effect on body weight comprised high-protein and/or low-GI diets as well as Mediterranean Diet; 4) Defined diets like Hypolipemic diet (low-fat), New Nordic Diet, Average Danish Diet, and “healthy diet” were effective for body weight control; 5) Water, unsweetened coffee and tea decreased body weight, whereas sugar-sweetened beverages, fruit juice and milk increased body weight, and 6) Walnuts as food supplement had no or very little effect on body weight.
This supports the findings of a previous systematic review concluding that dietary interventions in combination with exercise are the most effective way for long-term weight loss, followed by dietary interventions only, while exercise alone does not seem to be very effective [2]. In respect to diet type those with caloric restriction are most efficient to induce the greatest body weight loss in a short amount of time (-3.4% [10] to -13.7% [21]). However, in order to achieve long-term body weight loss and body weight maintenance, (considerable) caloric restriction may be not the best solution as most studies reported (significant) weight regain after initial weight loss (+0.9% [11] to +7% [7]). This is not surprising as with total body weight loss also lean body mass decreases which mainly define basal metabolic rate. As a consequence, basal metabolic rate decreases and appetite increases with the resulting hyperphagia leading to an increased lean and fat body mass (collateral fattening) [25]. Thus, long-term maintenance of body weight after initial weight loss is the biggest challenge. Six out of 20 of the selected studies applied several strategies to overcome this problem. Interventions during maintenance phase included education about nutrition [7,10,12,14], social support or motivation [10,23], and further lifestyle interventions (mostly nutritional and exercise interventions in combination) [19]. Total weight loss was >4% in half of those studies [7,10,12], even though there was some weight regain during maintenance phase ranging from +0.8% [10] to +7% [7]. This indicates that cognitive behavioural therapy might increase patients’ adherence to the weight loss program as well as support a sustained long-term change of behaviour patterns.
The strength of our review is to provide a comprehensive overview on different (combined) interventions to reduce and then maintain body weight. It also highlights that counselling patients about realistic results of weight loss programs is essential in order not to avoid feelings of failure. Obviously, this review also has some limitations. Study designs including sample sizes, cohorts’ characteristics, investigated interventions, intervention duration and follow-up were heterogeneous making it difficult to compare initial and long-term effectiveness of interventions. Accordingly, studies with a short follow-up period of e.g. six months achieved better results than studies with a long followup period of three years or even more as weight regain would not be registered in the former studies. Furthermore, in some studies results were either generalized [7,19], pooled [13] or only displayed in graphics and therefore not very exact [5,7,9]. For the individual, it is hard to predict which strategy for body weight maintenance would fit best. For example, illness perception in people with overweight or obesity has been shown to differ [26]. Accordingly, the predominant theories for being overweight/obese were health behaviour and psychosocial factors. Overweight/obese people with psychosocial theories on illness causes were more likely to have emotional or disinhibited eating patterns. Furthermore, cognitive control of eating patterns increased with age in both sexes. Thus, successful weight reduction programs should integrate subjective illness perceptions to improve therapeutic outcome, preferably right from the weight loss program start.
Conclusion
In conclusion, caloric restriction is the most effective way to achieve considerable weight loss in a short amount of time, especially in combination with physical activity. However, after initial weight loss maintaining body weight in the longterm is a major challenge. Adjunct education and cognitive behavioural therapy contribute to long-term success of weight loss programs. In addition, integrating subjective theories for being overweight/obese may improve the outcome.
Acknowledgements
The authors would like to thank the secretary J. Wilhelm for her administrative support.
Conflict of Interest
All authors declare that they have no conflicts of interest with the contents of this article.
Authors’ Contributions
Tiziana Marti: Literature search, manuscript writing; Dolores Foth: manuscript editing and advice; Susanna Weidlinger: manuscript editing and advice; Volker Stute: manuscript editing and advice; Petra Stute: project development, supervision, funding, manuscript editing and finalization.
References
- World Health Organization. Obesity and overweight. 2018.
- Segula D. Complications of obesity in adults: a short review of the literature. Malawi Med J. 2014;26:20-24.
- Lean M, Hankey C. Keeping it off: the challenge of weight-loss maintenance. Lancet Diabetes Endocrinol. 2018;6:681-683.
- Washburn RA, Szabo AN, Lambourne K, et al. Does the method of weight loss effect long-term changes in weight, body composition or chronic disease risk factors in overweight or obese adults? A systematic review. PLoS One. 2014;9:e109849.
- de Vos BC, Runhaar J, van Middelkoop M, et al. Long-term effects of a randomized, controlled, tailor-made weight-loss intervention in primary care on the health and lifestyle of overweight and obese women. Am J Clin Nutr. 2016;104:33-40.
- Stumm G, Blaik A, Kropf S, et al., Long-Term Follow-Up of the Telemonitoring Weight-Reduction Program "Active Body Control". J Diabetes Res. 2016.
- Astrup A, Raben A, Geiker N. The role of higher protein diets in weight control and obesity-related comorbidities. Int J Obes (Lond). 2015;39:721-726.
- Hinderliter AL, Sherwood A, Craighead LW, et al. The long-term effects of lifestyle change on blood pressure: One-year follow-up of the ENCORE study. Am J Hypertens. 2014;27:734-741.
- Lindström J, Peltonen M, Eriksson JG, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013;56:284-293.
- McRobbie H, Hajek P, Peerbux S, et al. Tackling obesity in areas of high social deprivation: clinical effectiveness and cost-effectiveness of a task-based weight management group programme - a randomised controlled trial and economic evaluation. Health Technol Assess. 2016;20:1-150.
- Mensinger JL, Calogero RM, Stranges S, et al. A weight-neutral versus weight-loss approach for health promotion in women with high BMI: A randomized-controlled trial. Appetite. 2016;105:364-374.
- Ortner Hadžiabdić M, Vitali Čepo D, Rahelić D, et al. The Effect of the Mediterranean Diet on Serum Total Antioxidant Capacity in Obese Patients: A Randomized Controlled Trial. J Am Coll Nutr. 2016;35:224-235.
- Pan A, Malik VS, Hao T, et al. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond). 2013;37:1378-1385.
- Poulsen SK, Crone C, Astrup A, et al. Long-term adherence to the New Nordic Diet and the effects on body weight, anthropometry and blood pressure: a 12-month follow-up study. Eur J Nutr. 2015;54:67-76.
- Tapsell LC, Lonergan M, Batterham MJ, et al. Effect of interdisciplinary care on weight loss: a randomised controlled trial. BMJ Open. 2017;7:e014533.
- Wing RR, Tate DF, Espeland MA, et al. Innovative Self-Regulation Strategies to Reduce Weight Gain in Young Adults: The Study of Novel Approaches to Weight Gain Prevention (SNAP) Randomized Clinical Trial. JAMA Intern Med. 2016;176:755-762.
- Brown C, Read H, Stanton M, et al. A pilot study of the Nutrition and Exercise for Wellness and Recovery (NEW-R): A weight loss program for individuals with serious mental illnesses. Psychiatr Rehabil J. 2015;38:371-373.
- Chmelo EA, Beavers DP, Lyles MF, et al. Legacy effects of short-term intentional weight loss on total body and thigh composition in overweight and obese older adults. Nutr Diabetes. 2016;6:e203.
- Dombrowski SU, Knittle K, Avenell A, et al. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646.
- Donini LM, Cuzzolaro M, Gnessi L, et al. Obesity treatment: results after 4 years of a Nutritional and Psycho-Physical Rehabilitation Program in an outpatient set