Research Article - Biomedical Research (2017) Volume 28, Issue 5
L-asparaginase exerts anti-leukemia activity
Yuan Lu, Ling-zhen Wang*, Ren Zhong, Li-rong Sun and Xue-rong LiDepartment of Pediatric Hematology, the Affiliated Hospital of Qingdao University, Shandong, PR China
- *Corresponding Author:
- Ling-zhen Wang
Department of Hematology
The Affiliated Hospital of Qingdao University, PR China
Accepted date: February 03, 2017
Abstract
This study aimed to investigate the effects of L-Asparaginase (L-ASP) on leukemia cell proliferation, cell cycle distribution and geminin expression. Jurkat cells were treated with different concentration of LASP for 24 h, 48 h or 72 h. Cell proliferation was analysed by the CCK-8 assay, cell cycle distribution was examined by flow cytometry, and mRNA and protein expression levels of geminin were assessed by PCR and Western blot analysis. The results showed that L-ASP inhibited the proliferation of Jurkat cells in a dose- and time-dependent manner. In addition, L-ASP at 0.5-10 U/ml induced G1 phase arrest of Jurkat cells. Geminin mRNA and protein expression remarkably decreased in Jurkat cells treated with L-ASP at 1.0-10 U/ml. In conclusion, our study suggests that the anti-leukemia mechanism of LASP is related to the arrest of leukemia cells in G1 phase and the downregulation of geminin expression.
Keywords
L-ASP, Jurkat cells, Cell cycle, Geminin.
Introduction
L-Asparaginase (L-ASP) has been widely accepted as one of basic regimens used in the treatment of Acute Lymphoblastic Leukemia (ALL) [1,2]. The efficacy of L-ASP is generally thought to result from a rapid and complete depletion of asparagines in the plasma by hydrolysing this amino acid to aspartic acid. Leukemic cells have reduced expression of aspargine synthetase and thus need asparagines from the blood. However, asparagines deamination alone may be insufficient to induce cell death [1]. Additional mechanisms may account for anti-leukemia activity of L-ASP. For example, a recent study showed that L-ASP induced apoptosis and cytoprotective autophagy in leukemia cells [3].
In this study we mainly focused on the effects of L-ASP on leukemia cell proliferation and cell cycle progression. Our results suggest that the anti-leukemia mechanism of L-ASP is related to the arrest of leukemia cells in G1 phase and the downregulation of geminin expression.
Materials and Methods
Cells and reagents
The Jurkat cell line (Human T-lymphoblastic leukemia cell line) was kindly provided by the Cell Culture Laboratory of College of Animal Science of Zhejiang University. Jurkat cells were maintained at 37°C in a humidified 5% CO2 atmosphere in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 1X PenStrep. L-ASP was purchased from Qianhong Biopharma Co. Ltd (Changzhou, China).
CCK-8 assay
The cells in the logarithmic growth phase were plated in 96- well plates with 100 μl of cell suspension (at a concentration of 1 × 105/ml) in each well. The wells for test groups were added with 0.1, 0.5, 1.0, 5.0 and 10 U/ml L-ASP for treatment with 24 h, 48 h and 72 h, respectively. The wells for blank control group were added with the equal volume of medium. The CCK-8 kit (Sigma) was used to assess cell proliferation. The experiments were performed in triplicate.
Cell cycle analysis
The cells in test groups were treated with different concentration of L-ASP for 24 h, 48 h and 72 h. The G1 phase rate of the cell cycle was analysed by Flow Cytometry (FCM) based on the measurement of the DNA content of nuclei labelled with propidium iodide (Sigma). Samples were analysed using a FACSCanto II flow cytometer (Becton- Dickinson). The ratio of cells in G1 phase was expressed as mean ± SD. The experiments were performed in triplicate.
Western blot analysis
Total protein was extracted from cells and quantified using a protein assay kit. Aliquots of protein (50 μg) were separated by SDS-PAGE gel and transferred to nitrocellulose membranes. The membranes were blocked and then incubated overnight at 4˚C with geminin or GAPDH antibody (Santa Cruz Biotechnology) followed by incubation with horseradish perodixase conjugated anti-mouse secondary antibody (Santa Cruz Biotechnology). GAPDH was used as loading control. Immunoreactive bands were detected by ECL Western blotting detection system (Amersham Biosciences) and the density was analysed using imageJ software.
Statistical analysis
Data were expressed as mean ± standard deviation. The difference in means among multiple groups was compared using one-way ANOVA. The difference in means between two groups of multiple groups was compared using LSD-t test. A value of P<0.05 was considered statistically significant.
Results
L-ASP inhibited Jurkat cell proliferation
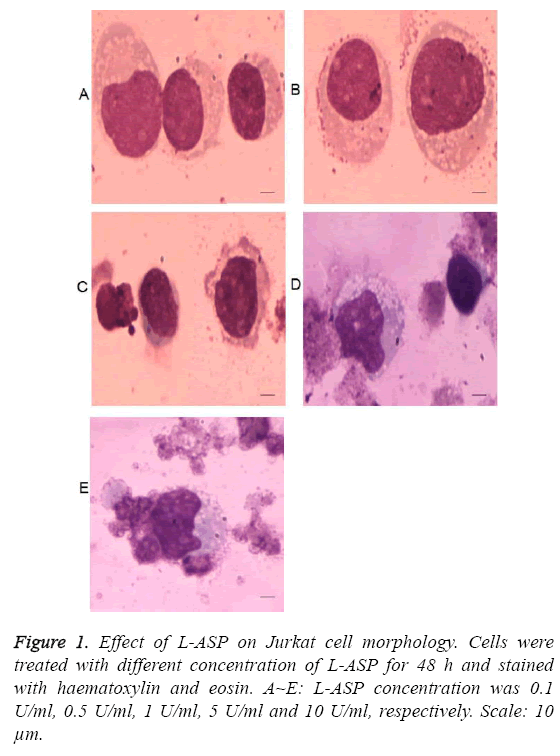
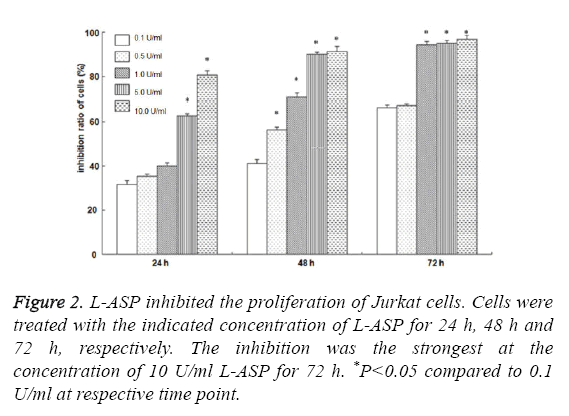
As shown in Figure 1, the morphology of Jurkat cells began to change after treatment with 0.5 U/ml L-ASP. The cytotoxicity effect of 10 U/ml L-ASP on Jurkat cells was most obvious, showing nuclear membrane shrinkage and deeply stained nuclei. The inhibition of cell proliferation was higher with increased exposure time and increased concentration (P<0.01, Figure 2). These data indicate that L-ASP inhibited Jurkat cell proliferation in a dose and time dependent manner.
L-ASP arrested Jurkat cells in G1 phase
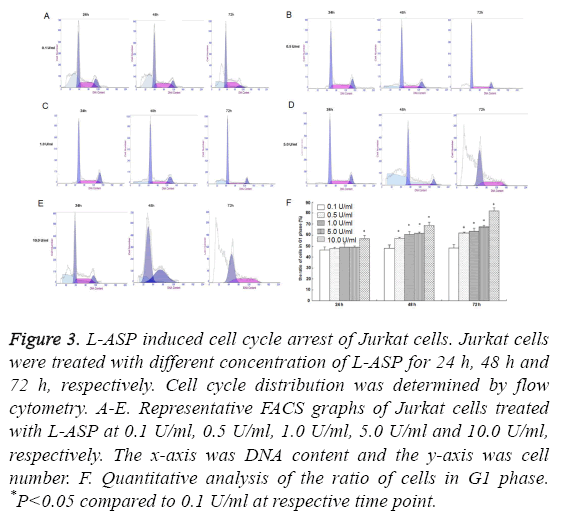
We found no difference in cell cycles of Jurkat cells treated with 0.1 U/ml L-ASP at different time points. 48 h after exposure to L-ASP at the concentration of 0.5 U/ml, the proportion of Jurkat cells in G1 phase increased markedly, and reached the peak in cells treated with 10 U/ml L-ASP for 72 h, showing a dose and time dependent manner (Figure 3).
Figure 3: L-ASP induced cell cycle arrest of Jurkat cells. Jurkat cells were treated with different concentration of L-ASP for 24 h, 48 h and 72 h, respectively. Cell cycle distribution was determined by flow cytometry. A-E. Representative FACS graphs of Jurkat cells treated with L-ASP at 0.1 U/ml, 0.5 U/ml, 1.0 U/ml, 5.0 U/ml and 10.0 U/ml, respectively. The x-axis was DNA content and the y-axis was cell number. F. Quantitative analysis of the ratio of cells in G1 phase. *P<0.05 compared to 0.1 U/ml at respective time point.
L-ASP downregulated geminin expression
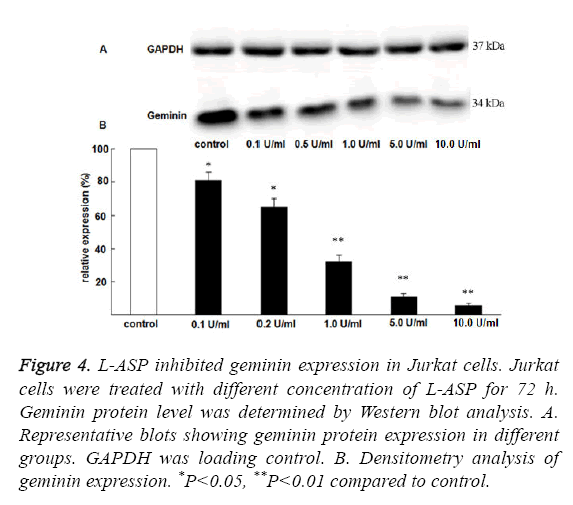
To understand the mechanism how L-ASP arrested Jurkat Cells in G1 phase, we examined the expression of geminin, a wellknown cell cycle regulatory protein. Geminin expression was significantly downregulated by L-ASP in a dose dependent manner (Figure 4).
Figure 4: L-ASP inhibited geminin expression in Jurkat cells. Jurkat cells were treated with different concentration of L-ASP for 72 h. Geminin protein level was determined by Western blot analysis. A. Representative blots showing geminin protein expression in different groups. GAPDH was loading control. B. Densitometry analysis of geminin expression. *P<0.05, **P<0.01 compared to control.
Discussion
ALL is a disease more frequent in children and greatly threatens the health of children [4,5]. The incidence of ALL in China has been on the rise recently [6,7]. L-ASP is widely used in the treatment of multiple malignant blood system diseases, especially in ALL in children. In this study we aimed to understand the mechanism by which L-ASP exerts inhibition on leukemia cell proliferation.
CCK-8 assay is a very good method to determine cell proliferation and toxicity [8,9]. Using CCK-8 assay we found that L-ASP substantially inhibited Jurkat cell proliferation in a dose and time dependent manner. Our results are consistent with previous report that L-ASP inhibited the proliferation of eight haematological malignant tumor cell lines [10].
The progression of cell cycle is closely associated with cell differentiation, growth, apoptosis and carcinogenesis. There are two cell cycle checkpoints during cell cycle progression, including checkpoint in G1/S phase and checkpoint in G2/M phase [11,12]. The distribution in cell cycle determined by FCM showed that the proportion of cells in S phase decreased after treatment with L-ASP, demonstrating that DNA synthesis of leukemia cells was inhibited. The arrest of cells in G1 phase resulted in prolonged cell cycle and decreased cell proliferation. Similar results showed that L-ASP induced cell cycle arrest of tumor cells [13,14]. Furthermore, human leukemia cell lines such as Molt-4 and HL-60 had higher proportion of cells in G1 phase after treatment with L-ASP [15]. Taken together, these data suggest that L-ASP regulates cell cycle progression of human leukemia cells to inhibit their proliferation.
Geminin acts as initiating signal for the transition from G1 phase to S phase, and plays important role in cell growth and proliferation [16]. Abundant studies showed that geminin was overexpressed in multiple types of tumor cells [17,18]. The length of transition from G0 phase to G1 phase was decreased in cells overexpressing geminin [19]. In this study, we found that the expression of geminin protein was substantially decreased in Jurkar cells after treatment with L-ASP, suggesting that the arrest of cell cycle in G1 phase by L-ASP is probably mediated by the downregulation of geminin expression.
Interestingly, a recent study reported that L-ASP induced the apoptosis of glioblastoma cells by the dissipation of mitochondrial membrane potential and the activation of effector caspases [20]. Another study showed that L-ASP induced the apoptosis of human leukemia MOLT-4 cells via the activation of intrinsic apoptotic pathway [21]. Further studies are needed to investigate the mechanism by which L-ASP induces the apoptosis of leukemia cells in vivo.
In summary, this study investigated the effect of L-ASP on leukemia cell proliferation and explored the underlying mechanism. Our data revealed a potential new mechanism that L-ASP inhibits leukemia cell proliferation by downregulating geminin expression and inducing G1 cell cycle arrest. These findings suggest that L-ASP could be utilized as novel drugs for the treatment of leukemia.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Horvat TZ, Pecoraro JJ, Daley RJ, Buie LW, King AC, Rampal RK, Tallman MS, Park JH, Douer D. The use of Erwinia asparaginase for adult patients with acute lymphoblastic leukemia after pegaspargase intolerance. Leuk Res 2016; 50: 17-20.
- Egler RA, Ahuja SP, Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J Pharmacol Pharmacother 2016; 7: 62-71.
- Song P, Ye L, Fan J, Li Y, Zeng X, Wang Z, Wang S, Zhang G, Yang P, Cao Z, Ju D. Asparaginase induces apoptosis and cytoprotective autophagy in chronic myeloid leukemia cells. Oncotarget 2015; 6: 3861-3873.
- Moricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig WD. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008; 111: 4477-4489.
- Wiemels JL, Kang M, Chang JS, Zheng L, Kouyoumji C, Zhang L, Smith MT, Scelo G, Metayer C. Backtracking RAS mutations in high hyperdiploid childhood acute lymphoblastic leukemia. Blood Cells Mol Dis 2010; 45: 186-191.
- Taub JW, Konrad MA, Ge Y, Naber JM, Scott JS, Matherly LH, Ravindranath Y. High frequency of leukemic clones in new-born screening blood samples of children with B-precursor acute lymphoblastic leukemia. Blood 2002; 99: 2992-2996.
- Mayer SP, Giamelli J, Sandoval C, Roach AS, Fevzi Ozkaynak M, Tugal O, Rovera G, Jayabose S. Quantitation of leukemia clone-specific antigen gene rearrangements by a single-step PCR and fluorescence-based detection method. Leukemia 2005; 13: 1843-1852.
- Zhou ZX, Zhang BG, Zhang H, Huang XZ, Hu YL, Sun L, Wang XM, Zhang JW. Drug packaging and delivery using perfluorocarbon nanoparticles for targeted inhibition of vascular smooth muscle cells. Acta Pharmacol Sin 2009; 30: 1577-1584.
- Olsnes AM, Ryningen A, Ersvaer E, Bruserud O. In vitro induction of a dendritic cell phenotype in primary human Acute Myelogenous Leukemia (AML) blasts alters the chemoline release profile and increases the levels of T cell chemotactic CCL17 andCCL22. Interferon Cytokine Res 2008; 28: 297-310.
- Li BS, He YY, Luo CY, Jiang H, Shen SH, Jiang LM, Zhang B, Gu LJ. Relationship between asparagine synthetase expression level and cell sensitivity to L-asparaginase in human leukemia cell lines. J Exp Hematol 2010; 18: 559-563.
- Viallard JF, Lacombe F, Belloc F, Pellegrin JL, Reiffers J. Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology. Cancer Radiother 2001; 5: 109-129.
- Takeshi K, Masanobu K, Junko T, Yokoyama A, Suzuki S, Kato N, Onozawa M, Chiba K, Hashino S. Rapid degradation of Cdt1 upon UV-induced DNA damage is mediated by SC Complex. J Biol Chem 2004; 279: 27315-27319.
- Lorenzi PL, Llamas J, Gunsior M, Ozbun L, Reinhold WC. Asparagine synthetase is a predictive biomarker of L-asparaginase activity in ovarian cancer cell lines. Mol Cancer Ther 2008; 7: 3123-3128.
- Ueno T, Ohtawa K, Mitsui K, Kodera Y, Hiroto M. Cell cycle arrest and apoptosis of leukemia cells induced by L-asparaginase. Leukemia 1997; 11: 1858-1861.
- Krejci O, Starkova J, Otova B, Madzo J, Kalinova M, Hrusak O, Trka J. Upregulation of asparagine synthetase fails to avert cell cycle arrest induced by L-asparaginase in TEL/AMIL-1 positive leukaemic cells. Leukemia 2004; 18: 434-441.
- Wharton SB, Hibberd S, Eward KL, Crimmins D, Jellinek DA. DNA replication licensing and cell cycle kinetics of oligodendroglial tumours. Br J Cancer 2004; 91: 262-269.
- Caillat C, Perrakis A. Cdt1 and geminin in DNA replication initiation. Subcell Biochem 2012; 62: 71-87.
- Xouri G, Lygerou Z, Nishitani H, Pachnis V, Nurse P. Cdt1 and geminin are down-regulated upon cell cycle exit and are over-expressed in cancer-derived cell lines. Eur J Biochem 2004; 271: 3368-3378.
- Montanari M, Boninsegna A, Faraglia B, Coco C, Giordano A, Cittadini A, Sgambato A. Increased expression of Geminin stimulates the growth of mammary epithelial cells and is a frequent event in human tumors. J Cell Physiol 2005; 202: 215-222.
- Karpel-Massler G, Ramani D, Shu C, Halatsch ME, Westhoff MA, Bruce JN, Canoll P, Siegelin MD. Metabolic reprogramming of glioblastoma cells by L-asparaginase sensitizes for apoptosis in vitro and in vivo. Oncotarget 2016; 7: 33512-33528.
- Husain I, Sharma A, Kumar S, Malik F. Purification and characterization of glutaminase free asparaginase from Pseudomonas otitidis: Induce apoptosis in human leukemia MOLT-4 cells. Biochimie 2016; 121: 38-51.