Research Article - International Journal of Pure and Applied Zoology (2018) Volume 6, Issue 3
LANDLOCKED FALL CHINOOK SALMON EGG SIZE IS POSITIVELY RELATED TO HATCHING TIME
- *Corresponding Author:
- Michael E. Barnes
South Dakota Department of Game, Fish and Parks, McNenny State Fish Hatchery, 19619 Trout Loop, Spearfish, South Dakota, 57783 USA
E-mail: e.tyokumbur@mail.ui.edu.ng
Received 22nd October 2018; Accepted 1st November 2018; Published 07th November 2018
Abstract
Within fish species, there can be considerable variation in the sizes of eggs produced by individual females. Specifically within the family Salmonidae, the relationship between egg size and hatching time is uncertain. This study examined eggs obtained during the spawning of landlocked fall Chinook salmon from Lake Oahe, South Dakota, USA. Diameters were obtained from the eggs of 24 randomly-selected females spawned in October, 2017, and the number of days to hatch was recorded after incubation at 11°C. Mean egg diameter was 7.22 mm and ranged from 6.36 to 8.36 mm. Over 90% of the eggs survived, with mean hatching occurring from 46 to 49 days of incubation. Significant correlations were observed between egg diameter and time-to-initial-hatch (r=0.581; p=0.003), as well as between egg diameter and mean hatching time (r=0.450; p=0.027). There was no correlation between egg diameter and survival to hatch (r=-0.363; p=0.081). These results are the first reported relationship between egg size and time-to-hatch in landlocked fall landlocked fall Chinook salmon.
Keywords
Chinook salmon; Oncorhynchus tshawytscha; Egg size; Hatch time; Lake Oahe
Introduction
Compared to other freshwater fishes, trout and salmon have relatively large eggs with long developmental periods (Stickney, 1994). While temperature is the primary determinant of when eggs hatch (Blaxter, 1969; Alderdice and Velsen, 1978; Réalis-Doyelle et al., 2016), other factors may also be involved. For example, low dissolved oxygen levels can influence hatching time (Blaxter, 1969) possibly by activating hatching enzymes (Czerkies et al., 2001). Egg size has also been implicated as having an effect on hatching times (Pauly and Pullin, 1988; Gilliooly et al., 2002). For example, Thorpe et al. (1984) noted that larger Atlantic salmon (Salmo salar) eggs took longer to hatch than smaller eggs. Self et al. (2018) also reported that smaller steelhead trout (Oncorhynchus mykiss) eggs hatched sooner than larger eggs. However, no relationship between egg size and hatching time was observed for coho salmon (O. kisutch) or chum salmon (O. keta) by Beacham et al. (1985) and Beacham and Murray (1985). The lack of effect of egg size on hatching time has also been reported for Chinook salmon (O. tshawytscha), Arctic charr (Salvelinus alpinus), and Atlantic salmon (Kinnison et al., 1998; Einum and Fleming, 2000; Leblanc et al., 2016).
Landlocked fall Chinook salmon (O. tshawytscha) from Lake Oahe, South Dakota, USA have relatively unique reproductive characteristics (Barnes et al., 2000; Young et al., 2016). Compared to Chinook salmon in their native range, Lake Oahe salmon eggs are typically smaller and experience poorer survival (Barnes et al., 2000). There is no information regarding egg size and hatching time in landlocked fall Chinook salmon. Thus, the objective of this study was to ascertain if hatching time is influenced by egg size in in landlocked Chinook Salmon from Lake Oahe, South Dakota.
Materials and Methods
Spawning: Landlocked fall Chinook salmon from Lake Oahe, South Dakota were spawned on October 16, 2017 at Whitlocks Spawning Station near Gettysburg, South Dakota, USA. Milt was collected from males, pooled in a container, and kept on ice until use. Eggs from 24 females were used in this study. Each individual female was pneumatically spawned using compressed oxygen at low pressure to express the eggs into a mesh net. Lake water was added to the spawn to activate the sperm for fertilization. After 2 min eggs were rinsed with lake water and allowed to water harden in lake water for at least 1 h prior to shipment. This process was repeated for each of the spawns used in this study (24 times).
Egg Incubation: After water hardening, eggs were transported approximately 4 h to McNenny State Fish Hatchery, rural Spearfish, South Dakota. Upon arrival at the hatchery, eggs were disinfected with 100 mg/L buffered free iodine (Syndel, Ferndale, Washington, USA) for 10 min. Eggs were then inventoried using water displacement (Piper et al., 1982) and placed into vertical-flow incubation trays (MariSource, Fife, Washington, USA) with discrete compartments for the spawn from each female. Each spawn was maintained discretely during spawning, fertilization, transportation, and incubation. Well water (11°C; total hardness 360 mg/L CaCO3; alkalinity as CaCO3, 210 mg/L; pH 7.6; total dissolved solids 390 mg/L) at 12 L/min was used throughout incubation. Daily treatments of 1,667 mg/L formalin (37% formaldehyde, 6 to 14% methanol; Paracide F, Syndel, Ferndale, Washington) for 15 min (Piper et al., 1982) were administered with a Masterflex model 7524-00 microprocessor peristaltic pump (Cole-Parmer Instrument Company, Vernon Hills, Illinois, USA). Dead eggs were removed on incubation day 30 (eyed egg stage) and the remaining viable eyed eggs were returned to their respective incubation tray.
Egg Measurement: Ten eyed eggs from each of the individual female spawns were then randomly selected and placed within round, wire-mesh, labeled baskets (8.9 cm diameter, 4.5 cm deep), and randomly placed into one of two semi-square 190 L tanks. Each tank contained 12 baskets, with each basket containing eyed eggs from an individual female. The diameters of three eggs from each spawn were randomly selected and measured to the nearest 0.01 mm using a digital caliper (SPC O/P Digimatic Caliper; Mitutoyo America Corporation, Aurora, Illinois, USA). Tank flows were set to 15 L/min using the same water source as the incubation stack. The egg baskets were checked daily, and the number of eggs that had hatch or died was recorded. Percent (%) egg survival to hatch was calculated dividing the initial number of eggs (10) by the number of eggs that survived and multiplying this amount by 100.
Data Analysis: Regression and correlation analysis was used to examine possible relationships between the variables. All data analysis was done using SPSS (9.0) statistical analysis program (SPSS, Chicago, Illinois, USA), with significance predetermined at p<0.05. This experiment was carried out within the American Fisheries Society Guidelines for the Use of Fishes in Research and within the guidelines of the Aquatics Section Research Ethics Committee of the South Dakota Department of Game, Fish and Parks.
Results
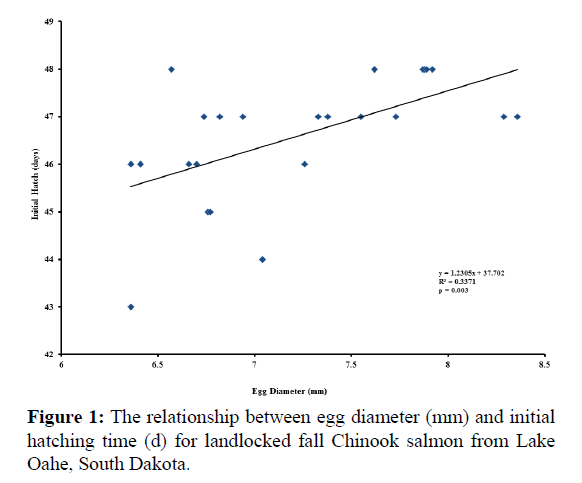
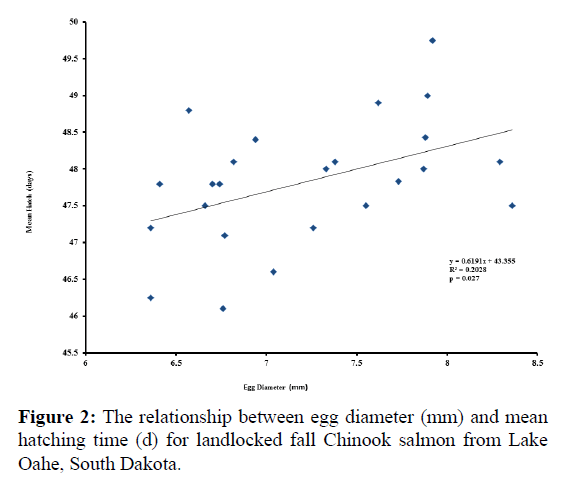
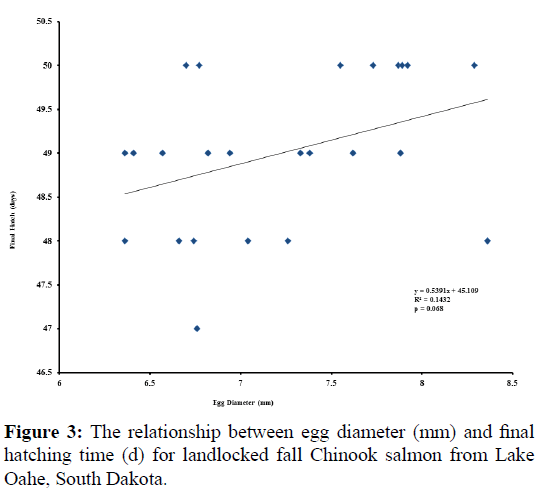
Mean egg diameter was 7.22 mm and ranged from 6.36 to 8.36 mm (Table 1). Over 90% of the eggs survived, with hatching occurring on average from 46 to 49 days of incubation.
| Variable | Mean | SE | Minimum | Maximum |
|---|---|---|---|---|
| Egg diameter (mm) | 7.22 | 0.13 | 6.36 | 8.36 |
| Survival to hatch (%) | 91.3 | 2.4 | 60 | 100 |
| Initial hatch (day) | 46.6 | 0.3 | 43.0 | 48.0 |
| Mean hatch (day) | 47.8 | 0.2 | 46.1 | 49.8 |
| Final hatch (day) | 49.0 | 0.2 | 47.0 | 50.0 |
Table 1: Mean, SE, minimum, and maximum values for egg diameter, egg survival, and days to hatch for landlocked fall Chinook salmon from Lake Oahe, South Dakota.
Significant relationships were observed between egg diameter and initial hatch (p=0.003; Figure 1), as well as between egg diameter and mean hatch (p=0.027; Figure 2). The relationship between egg diameter and final hatch approached significance (p=0.068; Figure 3). There was no correlation between egg diameter and survival to hatch (r=-0.363, p=0.081).
Discussion
The positive relationship between egg size and hatching time observed in this study is supported by the results reported by Thorpe et al. (1984) and Self et al. (2018) in Atlantic salmon and steelhead trout respectively. However, Einum and Fleming (2000) reported no such relationship in a different population of Atlantic salmon. Kinnison et al. (1998) stated that egg size was not related to hatching time in a New Zealand population of Chinook salmon. Likewise, other studies have also found no relationship between egg size and hatching time in salmonids (Beacham and Murray, 1985; Beacham et al., 1985; Leblanc et al., 2016). What are some possible reasons for the discrepancies among the studies?
Egg size varies between populations of fish species, between fish within a population, and also within the spawn of individual females (Beacham and Murray, 1987; 1993; Einum, 2003; Leblanc, 2016; Self et al., 2018). This variation may possibly explain the different results among the studies examining egg size in relation to hatch timing. Indeed, in comparison to ocean-run Chinook salmon in their native range, the landlocked, completely freshwater Lake Oahe salmon population produces smaller females that exhibit dramatically different reproductive characteristics (Barnes et al., 2000; Young et al., 2016). The differing results may also be due to the different egg incubation temperatures used in each study (Réalis-Doyelle et al., 2016; Fuhrman et al., 2018). Lastly, different techniques have been used to determine egg size. For example, egg size was defined as egg mass by Beacham et al. (1985), Kinnison et al. (1998), and Self et al. (2018), whereas egg size was defined by egg diameter by Pauly and Pullin (1988) and this study.
In this study, the size of the landlocked fall Chinook salmon eggs was comparable with eggs from Chinook salmon in their native range Chinook salmon (Rounsefell, 1957; Beacham and Murray, 1988; Beacham and Murray, 1993). This is somewhat unusual. Lake Oahe salmon eggs are typically much smaller than those from their native range counterparts (Barnes et al., 2000; Young et al., 2016) likely because Lake Oahe Chinook salmon females are also smaller in weight (Barnes et al., 2000).
The water temperature used in this study was well within the optimal incubation temperature range of 5.5 to 16°C (Piper et al., 1982). In addition, the hatching time of 46 to 49 days (506 to 539 daily temperature units) is similar to the 510 to 529 daily temperature units needed for eggs from native range Chinook salmon incubated at 12.0°C (Beacham and Murray, 1988).
Conclusion
This is the first documentation of a positive relationship between egg size and hatching time in landlocked fall Chinook salmon from Lake Oahe, South Dakota, USA. This relationship is far from universal among salmonids, and may be unique to the Lake Oahe Chinook salmon population.
Acknowledgement
We thank Jill Voorhees for her assistance with this project.
References
- Aldedice, D.F. and Velsen, F.P.J., 1978. Relation between temperature and incubation time for eggs of Chinook salmon (Oncorhynchus tschawytscha). Can. J. Fish. Aquat. Sci., 35: 69-75.
- Barnes, M. E., Hanten R. P., Cordes R.J., Sayler W.A., Carreiro J., 2000. Reproductive performance of inland fall Chinook salmon. N. Am. J. Aquac., 62: 203- 211.
- Beacham, T.D., Withler, F.C., Morley R.B., 1985. Effect of egg size on incubation time and alevin and fry size in chum salmon (Oncorhynchus keta) and coho salmon (Oncorhynchus kisutch). Can. J. Zool., 63: 847- 850.
- Beacham, T.D., and Murray, C.B., 1985. Effect of female size, egg size, and water temperature on developmental biology of chum salmon (Oncorhynchus keta) from the Nitinat River, British Colombia. Can. J. Fish. Aquat. Sci., 42: 1755-1765.
- Beacham, T.D. and Murray, C.B., 1987. Adaptive variation in body size, age, morphology, egg size, and developmental biology of chum salmon (Oncorhynchus keta) in British Columbia. Can. J. Fish. Aquat. Sci., 44: 244-261.
- Beacham, T.D., and Murray, C.B., 1988. Variation in developmental biology of sockeye salmon (Oncorhynchus nerka) and Chinook salmon (O. tshawytscha) in British Columbia. Can. J. Zool., 67: 2081-2089.
- Beacham, T.D., and Murray, C.B., 1993. Fecundity and egg size variation in North American Pacific salmon (Oncorhynchus). J. Fish Biol., 42: 485-508.
- Blaxter, J.H.S., 1969. Development: Eggs and Larvae. (Ed. W.S. Hoar and D.J. Randall). Fish physiology, vol. 3, Academic Press, New York. 177-252.
- Czerkies, P., Kordalski, K., Brzuzan, P., Luczynski, M., 2001. Critical dissolved oxygen concentrations causing precocious hatching in Coregoninae embyos. Aquaculture., 196: 151-158.
- Einum, S., and Fleming, I.A., 2000. Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature., 405: 565-567.
- Einum, S., 2003. Atlantic salmon growth in strongly food-limited environments: effects of egg size and paternal phenotype?. Environ. Biol. Fishes., 67: 263-268.
- Fuhrman, A.E., Larsen, D.A., Steel, E.A.. Young, G., Beckman, B.R., 2018. Chinook salmon emergence phenotypes: Describing the relationships between temperature, emergence timing, and condition factor in a reaction norm framework. Ecol. Freshwater Fish., 27: 350-362.
- Gilliooly, J.F., Charnov, E.L., West, G.B., Savage, V.M., Brown, J.H., 2002. Effects of size and temperature on developmental time. Nature., 417: 70-73.
- Kinnison, M.T., Unwin, M.J., Hershberger, W.K., Quinn, T.P., 1998. Egg size, fecundity, and development rate of two introduced New Zealand Chinook salmon (Oncorhynchus tshawytscha) populations. Can. J. Fish.Aquat. Sci., 55:1946‑953.
- Leblanc, C.A.L., Kristjánsson, B.K., Skúlason, S., 2016. The importance of egg size and egg energy density for early size patterns and performance of Arctic charr Salvelinus alpinus. Aquacult. Res., 47: 1100-1111.
- Pauly, D., and Pullin R.S.V., 1988. Hatching time in spherical, pelagic, marine fish eggs in response to temperature and egg size. Environ. Biol. Fishes., 22: 261- 271.
- Piper, R.G., McElwain, I.B., Orme, L.E., McCraren, J.P., Fowler, L.G., Leonard, J.R., 1982. Fish Hatchery Management. U.S. Fish and Wildlife Service, Washington, D.C.
- Réalis-Doyelle, E., Pasquet, A., De Charleroy, D., Fontaine, P., Teletchea, F., 2016. Strong effects of temperature on the early life stages of a cold stenothermal fish species, brown trout (Salmo trutta L.). PLoS One., 11: E0155487.
- Rounsefell, G.A., 1957. Fecundity of North American Salmonidae. Fish. Bull., 57: 451-468.
- Self, K.E., Schreck, C.B., Cogliati, K.M., Billman, E.J., Noakes, D.L.G., 2018. Egg size and growth in steelhead Oncorhynchus mykiss. J. Fish Biol., 93: 465-468.
- Stickney, R.R. 1994. Principles of Aquaculture. John Wiley & Sons, New York, USA.
- Thorpe, J.E., Miles, M.S., Keay, D.S., 1984. Developmental rate, fecundity and egg size in Atlantic salmon, Salmo salar L. Aquaculture., 43: 289- 305.
- Young, K.L., Barnes, M.E., Kientz, J.L., 2016. Reproductive characteristics of landlocked fall Chinook salmon from Lake Oahe, South Dakota. Prairie Nat., 48: 79-86.