Research Article - Biomedical Research (2017) Volume 28, Issue 21
Lactobacillus plantarum YS2 reduces oxazolone-induced colitis in BALB/c mice
Yu Qian1,2,3,4, Ruokun Yi1,2,3,4, Peng Sun1,2,3,4, Guijie Li1,2,3,4* and Xin Zhao1,2,3,4*
1Chongqing Collaborative Innovation Center for Functional Food, Chongqing University of Education, Chongqing, PR China
2Chongqing Engineering Research Center of Functional Food, Chongqing University of Education, Chongqing, PR China
3Chongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing, PR China
4College of Biological and Chemical Engineering, Chongqing University of Education, Chongqing, PR China
- *Corresponding Authors:
- Xin Zhao
Chongqing Collaborative Innovation Center for Functional Food
Chongqing University of Education, PR China
Guijie Li
Chongqing Collaborative Innovation Center for Functional Food
Chongqing University of Education, PR China
Accepted date: September 20, 2017
Abstract
By the animal model, the in vitro experiment anti-colitis effect of Lactobacillus plantarum YS2 (LPYS2) was measured. The BALB/c mice were induced colitis by oxazolone, and the serum and colon tissue levels were determined using kits and RT-PCR assay. The results showed that the DAI score of LP-YS2 treated mice was lower than control group. And the colon length and ratio of colon weight/colon length in colitis mice were also could be raised by LP-YS2 treatment. The MPO, NO, MDA colon tissue levels in LP-YS2 treated mice were higher than mice in control group, but GSH level was lower than control group mice. The cytokine levels of IL-2 in LP-YS2 treated mice was lower than mice in control group, but IL-10 level was higher than mice in control group. The nNOS, eNOS, c-Kit, SCF mRNA expressions in colon tissue of LP-YS2 treated mice were stronger than those in mice of control group, and iNOS, IL-8, CXCR2 expressions were weaker than those in mice of control group. From these results, LP-YS2 could be used as a probiotics which has anti-colitis effects.
Keywords
Lactobacillus plantarum, Oxazolone, Colitis, Cytokine, Expression
Introduction
Ulcerative Colitis (UC) is one type of Inflammatory Bowel Disease (IBD). The clinical manifestations of UC patients include diarrhea, purulent stools, abdominal pain, tenesmus and systemic symptoms in different degree in persistent or recurrent episodes. The pathology is changed into diffuse tissue reactions, including ulceration, crypt abscess, small vessel inflammation, goblet cell decrease, and infiltration of various inflammatory cells and nonspecific manifestation [1]. Due to the wide range, repeated attacks, poor treatment, disease progression, cancer possibility and other characteristics, the UC lesions has been identified as a modern refractory disease by WHO. The treatment of UC includes medication, nutrition, psychotherapy and surgical treatment. Among them, the drug treatment is the main method for the treatment of ulcerative colitis [2]. However, drug treatment has some side effects, which produces some influence on the patients.
Study has pointed out that the intestinal microflora imbalance is one of the important causes of UC, and the probiotics in active inflammatory bowel disease can alleviate colitis to certain extend. Remission by induction and prevention of recurrence and complications are purposes of the treatment of inflammatory bowel disease [3]. The intestinal flora runs through this cascade process. The remission of inflammatory bowel disease is very short for many patients who undergo the surgical treatment after repeated attacks. The pouch it is incurring after ulcerative colitis surgical procedures is usually caused by the decrease of the number of normal bacteria flora, such as lactic acid bacteria and bifid bacteria, around the pouch [4].
As a hapten, the sulfamethoxazole can induce contact allergic reactions in different parts of mice. The ulcerative colitis is an inflammatory bowel disease mediated by Th2 cell while the mouse colitis model induced by sulfamethoxazole is a kind of Th2 colitis mediated by the IL-4 colitis [5]. Ketones can produce a series of effects on the colon. NK-T (Natural Killer- T) cell resistance-CD3/and -CD28 secrets a large number of Th2 cytokines after simulation, which results in ulcerative colitis. The UC model is similar to the pathogenesis of human colitis [6]. Therefore, it is often used to detect the physiological activity of functional foods.
China's Qinghai Tibet Plateau produces a special kind of naturally fermented food-Yak Yogurt due to unique geographical environment, fermented containers, fermented microorganisms and fermentation techniques [7]. Studies have shown that yak yogurt has the effects on antioxidation [8], lowering cholesterol [9] and improving immunity [10]. The lactic acid bacteria isolated from yak yogurt also have certain antioxidant effects [11] and intestinal biological activity [12]. The Lactobacillus plantarum is obtained by identification and separation and purification from Yushu Yak milk from Yushu Tibetan autonomous prefecture of Qinghai in this study, which is better than Lactobacillus bulgaricus in vitro anti-gastric acid and bile salt effects, and is named Lactobacillus plantarum YS-2 (LP-YS2). In this study, taking LP-YS2 as the research object, the preventive effect of LP-YS2 on oxazolone inducing the colitis was observed at the first time. Results of the study will accumulate certain theoretical basis for the further development of LP-YS2, conducive to the development and use of LP-YS2.
Materials and Methods
Microorganism strains
The Lactobacillus plantarum was isolated and identified from Yak yoghurt by our research team, and the lactic acid bacteria was named Lactobacillus plantarum YS2 (LP-YS2). This lactic acid bacteria was preserved in China Center for Type Culture Collection (CCTCC NO: M2016748, Wuhan, China), and Lactobacillus bulgaricus (LB, CCTCC AB 200048) was also purchased from CCTCC.
Animal experiment
The 50 BALB/c male mice were randomly divided into 5 groups: normal group, control group, LB group, LP-YS2-L group and LP-YS2-H group with 10 mice respectively. The normal group and the model group were fed normally without any other treatment; mice in group LB, group LP-YS2-L and group LP-YS2-H received intragastric administration of 2 ml of lactic acid bacteria fluid at 1 × 109, 1 × 108 and 1 × 109 CFU/ml daily for 26 d, respectively. On fifteenth day after the experiment, the abdominal area of all mice was shaved at 2 cm × 2 cm of area, and mice of normal group were coated with 0.2 ml of 99% ethanol. In the other group, the abdominal cavity was coated with a solution of 0.2 ml (3% mass ratio, and ethanol as solvent). On Nineteenth day after the start of the experiment, the mice go through the anesthesia (chloral hydrate 0.1 ml/10 g) after fasted for 24 h, and then the mice anus was inserted silicone tube blunt with depth of 3.5 cm. Mice of normal group were injected 50% ethanol solution of 0.15 ml, the mice in other groups were injected 1% oxazolone solution 0.15 ml (the mass ratio, 50% ethanol as solvent); the catheter pulled out after 20 s, and the mice were inverted for 30 s. On 26th d since the start of the experiment, all mice were killed by breaking the neck and then plasma and colon tissue of the mice was collected, and the weight and length of the colon were measured. In the meanwhile, Disease Activity Index (DAI) was determined using the formula: DAI=(score of weight loss+score of defecate character+score of hematochezia)/3 (Table 1) [5].
| Weight decrease (%) | Stool character | Hematochezia | Score |
|---|---|---|---|
| No decrease | Normal | Occult blood (-) | 0 |
| 1-5 | Semi sparse stool | Occult blood (-) | 1 |
| 6-10 | Semi sparse stool | Occult blood (+) | 2 |
| 11-15 | Sparse stool | Occult blood (+) | 3 |
| >15 | Sparse stool | Visible blood | 4 |
Table 1. DAI scoring standard.
MPO, NO, GSH, MDA and SOD colon tissues levels determination
The 2 ml normal saline was added to colon tissues (2 g), and the tissues were homogenated using high speed tissue homogenizer (T10, IKA, Staufen, Germany), and then the MPO, NO, GSH, MDA levels of colon tissues were measured by the experiment kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
IL-2 and IL-10 serum cytokines levels determination
The plasma was centrifuged at 3000 r/min for 10 min, and the serum was collected. The IL-2 and IL-10 cytokine levels in serum were measured by the experiment kits (Abcam, Cambridge, MA, USA).
RT-PCR assay
The 2 ml normal saline was added to colon tissues (2 g), and the tissues were homogenated using high speed tissue homogenizer (T10), the tissue of mice was homogenized, and the total RNA of the colon tissue was extracted by RNAzol, and then the total RNA concentration was diluted to 1 μg/μL. The 2 μL diluted RNA extraction liquid is added oligodT18, RNase, dNTP, MLV enzymes with 1 μL and 10 μL of 5X buffer, the synthesized cDNA is realized at 37°C for 120 min, 99°C for 4 min, and 4°C for 120 min respectively. The mRNA expressions of nNOS, eNOS, iNOS, c-Kit, SCF, IL-8 and CXCR2 are amplified (Table 2) by reverse transcriptionpolymerase chain reaction method (one time at 94°C for 5 min, 30 cycles for 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s in order, and one time at 72°C for 5 min) while the GAPDH as reference is to simplify it. Finally, 1% ethidium bromide agar electrophoresis is adopted to check PCR amplification products and Image 1.44 software is used for semi-quantitative analysis [13].
| Gene Name | Sequence |
|---|---|
| nNOS | Forward: 5'-GAA TAC CAG CCT GAT CCA TGG AA-3' |
| Reverse: 5'-TCC TCC AGG AGG GTG TCC ACC GCA TG-3' | |
| eNOS | Forward: 5'-GGA GAG GCT GCA TGA CAT TG-3' |
| Reverse: 5'-GGT AGA GCC ATA GTG GAA TGA C-3' | |
| iNOS | Forward: 5'-AGA GAG ATC GGG TTC ACA-3' |
| Reverse: 5'-CAC AGA ACT GAG GGT ACA-3' | |
| c-Kit | Forward: 5′- AGA CCG AAC GCA ACT -3′ |
| Reverse: 5′- GGT GCC ATC CAC TTC A -3′ | |
| SCF | Forward: 5′- AAA CTG GTG GCG AAT -3′ |
| Reverse: 5′- CAC GGG TAG CAA GAA -3′ | |
| IL-8 | Forward: 5'-AGA AGC ATG GCC CAG AAA TCA-3' |
| Reverse: 5'-GGC CTT GTA GAC ACC TTG GT-3' | |
| CXCR2 | Forward: 5'-GAA CAA AGG CAA GGC TAA-3' |
| Reverse: 5'-AAC ATA ACA ACA TCT GGG CA-3' | |
| GAPDH | Forward: 5'-CGG AGT CAA CGG ATT TGG TC-3' |
| Reverse: 5′-AGC CTT CTC CAT GGT CGT GA-3′ |
Table 2. Sequences of reverse transcription-polymerase chain reaction primers were used in this study.
Statistical analysis
The experiment was carried out in three parallel experiments, and the results were averaged. The data were compared to significant differences using SAS9.1 statistical software (SAS Institute, Inc., Cary, NC, USA) and the one-way ANOVA method to analyse (P<0.05).
Results
DAI score, colon length and weight of mice
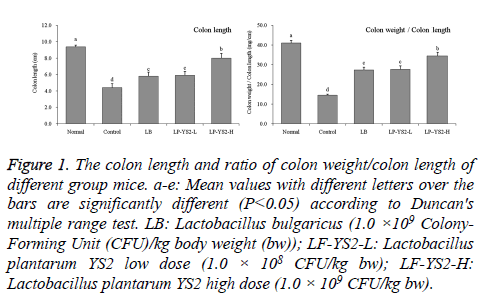
As shown in Table 3, at the 20th d, 22nd d and 25th d, the mice in control group had the highest DAI scores, the score of LPYS2- H treated mice was lower than that of LB and LP-YS2-L treated mice. The colon length of mice in normal, control, LB, LP-YS2-L and LP-YS2-H groups were 9.4 ± 0.2, 4.4 ± 0.5, 5.8 ± 0.5, 5.9 ± 0.5 and 8.0 ± 0.6 cm, respectively (Figure 1). The mice in normal mice had the highest ratio of colon weight/ colon length at 41.1 ± 1.2, but the ratio of mice in control group was lowest at 14.6 ± 0.5. LP-YS2-H (34.5 ± 1.9) treated mice showed than higher ratio than LB (27.4 ± 1.4) and LPYS2- L (27.7 ± 1.8) treated mice.
Figure 1: The colon length and ratio of colon weight/colon length of different group mice. a-e: Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan's multiple range test. LB: Lactobacillus bulgaricus (1.0 ×109 Colony- Forming Unit (CFU)/kg body weight (bw)); LF-YS2-L: Lactobacillus plantarum YS2 low dose (1.0 × 108 CFU/kg bw); LF-YS2-H: Lactobacillus plantarum YS2 high dose (1.0 × 109 CFU/kg bw).
| Group | 20th d | 22th d | 25th d |
|---|---|---|---|
| Normal | 0.00 ± 0.00d | 0.00 ± 0.00d | 0.00 ± 0.00d |
| Control | 2.03 ± 0.31a | 2.52 ± 0.18a | 2.69 ± 0.23a |
| LB | 1.76 ± 0.21b | 1.85 ± 0.23b | 1.94 ± 0.19b |
| LP-YS2-L | 1.67 ± 0.24b | 1.83 ± 0.22b | 1.91 ± 0.20b |
| LP-YS2-H | 1.31 ± 0.20c | 1.42 ± 0.14c | 1.59 ± 0.17c |
Table 3. DAI score of different group mice.
Colon tissue levels of mice
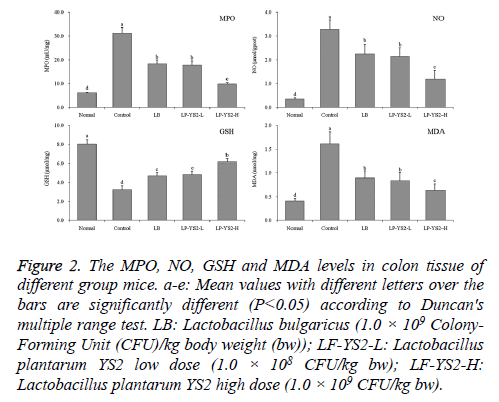
As shown in Figure 2, the mice in control group showed the highest MPO (31.25 ± 2.33 mU/mg), MDA (1.61 ± 0.25 nmol/mg) activities and NO (3.29 ± 0.39 μmol/gprot) levels, but lowest GSH (3.23 ± 0.41 μmol/mg) activity. The MPO, MDA activities and NO levels of LB, LP-YS2-L and LP-YS2- H treated mice were lower than mice in control group, but GSH activity was higher than mice in control group. Meanwhile, The MPO (9.82 ± 0.52 mU/mg), MDA (0.63 ± 0.14 nmol/mg) activities and NO (1.19 ± 0.36 μmol/gprot) level of LP-YS2-H were only higher than mice in normal group (6.20 ± 0.15 mU/mg, 0.35 ± 0.05 μmol/gprot, 0.41 ± 0.05 nmol/mg), but GSH (6.18 ± 0.32 μmol/mg) activity was also only lower mice in normal group (8.02 ± 0.47 μmol/mg).
Figure 2: The MPO, NO, GSH and MDA levels in colon tissue of different group mice. a-e: Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan's multiple range test. LB: Lactobacillus bulgaricus (1.0 × 109 Colony- Forming Unit (CFU)/kg body weight (bw)); LF-YS2-L: Lactobacillus plantarum YS2 low dose (1.0 × 108 CFU/kg bw); LF-YS2-H: Lactobacillus plantarum YS2 high dose (1.0 × 109 CFU/kg bw).
Cytokine levels in serum of mice
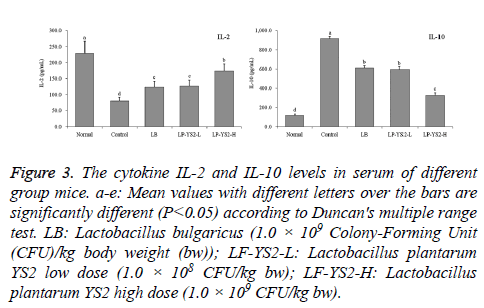
As shown in Figure 3, the IL-2 cytokine level of mice in normal mice (228.37 ± 38.46 pg/ml) was highest, but IL-10 level was lowest (118.78 ± 14.62 pg/ml). LP-YS2-H treated mice had the higher IL-2 level (174.36 ± 21.33 pg/ml) and lower IL-10 level (325.39 ± 27.66 pg/ml) than mice in LB (123.45 ± 18.71 and 611.68 ± 22.03 pg/ml), LP-YS2-L (126.78 ± 19.36 and 597.87 ± 29.63 pg/ml) and control groups (79.63 ± 11.85 and 917.68 ± 25.67 pg/ml).
Figure 3: The cytokine IL-2 and IL-10 levels in serum of different group mice. a-e: Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan's multiple range test. LB: Lactobacillus bulgaricus (1.0 × 109 Colony-Forming Unit (CFU)/kg body weight (bw)); LF-YS2-L: Lactobacillus plantarum YS2 low dose (1.0 × 108 CFU/kg bw); LF-YS2-H: Lactobacillus plantarum YS2 high dose (1.0 × 109 CFU/kg bw).
Colon tissue expression of colon tissue in mice
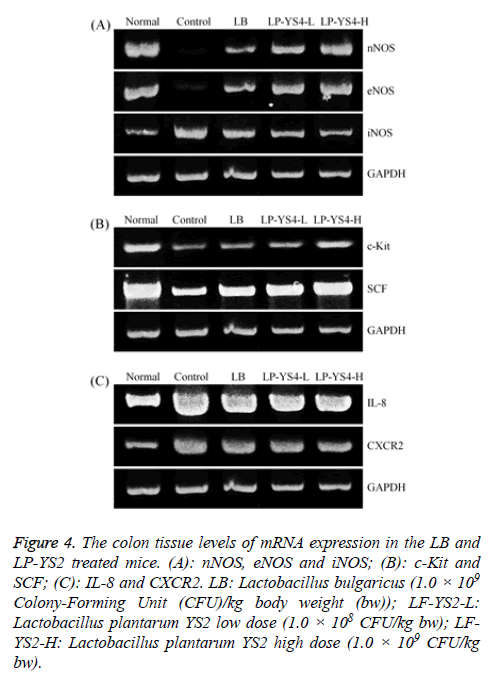
As shown in Figure 4A, the nNOS and eNOS expressions in colon tissue of mice in normal group were strongest, but iNOS expression was weakest. And the nNOS and eNOS expressions in LP-YS2-H treated mice were only weaker than those of mice in normal group, but stronger than those of mice in LB, LP-YS2-L groups; meanwhile, iNOS expression was only stronger than that of mice in normal group, but also weaker than those of mice in LB, LP-YS2-L groups.
Figure 4: The colon tissue levels of mRNA expression in the LB and LP-YS2 treated mice. (A): nNOS, eNOS and iNOS; (B): c-Kit and SCF; (C): IL-8 and CXCR2. LB: Lactobacillus bulgaricus (1.0 × 109 Colony-Forming Unit (CFU)/kg body weight (bw)); LF-YS2-L: Lactobacillus plantarum YS2 low dose (1.0 × 108 CFU/kg bw); LFYS2- H: Lactobacillus plantarum YS2 high dose (1.0 × 109 CFU/kg bw).
Colon tissue expression of c-Kit and SCF in mice
As shown in Figure 4B, the mice in control group had the weakest c-Kit and SCF expressions in colon tissue, LP-YS2-H treated mice showed the higher c-Kit and SCF expressions than those of mice in LB, LP-YS2-L and control groups.
Colon tissue expression of IL-8 and CXCR2 in mice
As shown in Figure 4C, IL-8 and CXCR2 expressions in colon tissue of mice in normal mice were weakest, these expressions of mice in LP-YS2-H treated mice were only stronger than those of mice in normal group, and these expressions of mice in LP-YS2-H treated mice were weaker than those of mice in LB, LP-YS2-L and control groups.
Discussion
UC would lead to the loss of the body weight, diarrhea, bleeding and other symptoms, so weight, stool property, and hemafecia are taken as the standard DAI score that is used as a measure of the standard degree of colitis [14]. The DAI index showed that LP-YS2 could reduce the symptoms of LP-YS2 induced colitis, and the effect was better with the increase of concentration. The ratio of colon length to colon weight/colon length was also the criterion for the extent of colitis. The colon length of colitis mice was shorter than that of normal mice, and the colon weight/colon length ratio was lower [15].
The pathological mechanism of ulcerative colitis is still unclear, most of the research thinks that that many biochemical mediators, including cytokines, growth factors, NO and adhesion molecules play an important role in abnormal immune response on mediating. NO in physiological doses play an important protective role in the digestive system, but excessive production of NO or increased sensitivity to gastrointestinal smooth muscle can lead to ulcerative colitis [16]. MPO is an enzyme in neutrophils, determines the degree of neutrophil infiltration by determining the activity of MPO that is a reliable indicator of neutrophil infiltration in the organization. The study indicates that the MPO activity for colitis mice was significantly higher than mice in normal group [17]. At the same time, the experimental colitis study showed increased lipid peroxidation, decreased free radical scavenging ability, decreased GSH activity, and increased MDA as the end product of lipid peroxidation for colitis model mice [18].
The local immune tolerance of intestinal mucosa is broken, and the disorder of immune response is caused by the bacterial antigen in the gut cavity, which leads to the dysregulation of immune effector T cells as one of the important pathogenesis of UC [19]. The immune effector T cells are mainly CD4+ Th cells. After activation, they can differentiate into different subsets, including Th1, Th2, Th17 and Treg. These 4 groups of cells play different roles in the development, persistence and remission of UC inflammation by secreting a series of cytokines (such as IL-2, IL-4, IL-17, and IL-10) [20]. Under UC symptoms, levels of IL-2 decreased and IL-10 increased substantially [21].
There are two main types of NOS in vivo, cNOS (including nNOS and eNOS) and inducible (iNOS). The main mucosal layer of UC is iNOS. cNOS is the dependent enzyme for calcium ion and calmodulin in neuron of airframe part. Vascular endothelial cells have stable activity, and release persistently small amounts of NO as neurotransmitters, which play a role in local blood flow regulation with a protective effect on intestinal mucosa [22]. iNOS is an inducible enzyme that is not expressed in resting state and is enhanced when cells are stimulated by inflammation; At the same time, the expression levels of nNOS and eNOS increased under UC state [23].
ICC colitis appeared excessive autophagy, which lead into the programmed death, decreased organelles expressing in pathological microscopic structure. The reduction of the expression of c-Kit presents in molecular biology in case of a large number of reduced or absent autophagy [24]. SCF is a multifunctional growth factor, and activates the tyrosine kinase combined with c-Kit that leads to a series of phosphorylation process, and play an important role in the regulation of proliferation, differentiation and migration process in a variety of Kit positive cells (including ICC) [25]. The proliferation and differentiation of ICC are affected when mutations in the loci encoding SCF are mutated and the SCF/Kit signaling pathway is impaired [26].
IL-8 is a member of the CXC subfamily with inflammatory activity and chemotaxis, and a variety of inflammatory cells and epithelial cells secrete IL-8 [27]. Both CXCR1 and CXCR2 are IL-8 receptors, and IL-8 and CXCR2 can participate in the development of inflammatory bowel disease through interaction. For patients with UC, the peripheral blood IL-8 is increased significantly. Combined with high expression of CXCR2 in the intestinal tissues, the expression of CXCR2 in intestinal tissues could be up-regulated after the combination of IL-8 and CXCR2 [28]. The expression of CXCR2 in intestinal tissues of patients with IBD is significantly upregulated, and the up regulation of CXCR may be mainly involved in the pathogenesis of UC, and the down regulation of CXCR expression may provide a new target for the treatment of UC [29].
Conclusion
In this study, the anti-colitis effects of Lactobacillus plantarum YS2 were determined using the BALB/c mice after inducing colitis by oxazolone. LP-YS2 could decrease the DAI score, and also could increase the colon length after the mice induced colitis. Meanwhile, LP-YS2 raises the ratio of colon weight/ colon length in colitis induced mice. The MPO, NO, MDA levels in colon tissues of LP-YS2 treated colitis mice were lower than untreated colitis mice (control group), but GSH level was higher than untreated colitis mice. The IL-2 cytokine serum level of colitis mice was lower than LP-YS2 treated colitis mice and IL-2 level of colitis mice was higher than LPYS2 treated colitis mice. By RT-PCR assay, LP-YS2 could raise nNOS, eNOS, c-kit, SCF mRNA expressions and reduce iNOS, il-8 and CXCR2 expressions compared to the mice in control group. Based on these results, LP-YS2 had a good anticolitis effects, it could be used us a probiotics for colon health.
Acknowledgements
The present research was supported by Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0820), the Program for Innovation Team Building at Institutions of Higher Education in Chongqing (CXTDX201601040) and Research Project of Chongqing University of Education (KY2015TBZC), China.
Conflict of Interest
There is no conflict of interest.
References
- Shivakumar BM, Chakrabarty S, Rotti H, Seenappa V, Rao L, Geetha V, Tantry BV, Kini H, Dharamsi R, Pai CG, Satyamoorthy K. Comparative analysis of copy number variations in ulcerative colitis associated and sporadic colorectal neoplasia. BMC Cancer 2016; 16: 271.
- Jedel S, Hoffman A, Merriman P, Swanson B, Voigt R, Rajan KB, Shaikh M, Li H, Keshavarzian A. A randomized controlled trial of mindfulness-based stress reduction to prevent flare-up in patients with inactive ulcerative colitis. Digestion 2014; 89: 142-155.
- Martín R, Chain F, Miquel S, Motta JP, Vergnolle N, Sokol H, Langella P. Using murine colitis models to analyse probiotics-host interactions. FEMS Microbiol Rev 2017; 41: 49-70.
- Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. AM Fam Physician 2017; 96: 170-178.
- Xie L, Xing ZH, Jiang RX, Li JB, Zhou W. Experimental study on oxazolone-induced ulcerative BALB/C mice. Prog Mod Biomed 2009; 9: 1057-1059.
- Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 2002; 17: 629-638.
- Chen X, Zhao X, Wang H, Yang Z, Li J, Suo H. Prevent effects of Lactobacillus fermentum HY01 on dextran sulfate sodium-induced colitis in mice. Nutrients 2017; 9: 545.
- Qian Y, Zhao X, Li YC, Zhou YL, Du MY, Kan JQ. Study of Lactobacillus from natural fermented yak yogurt in Tibetan plateau on antioxidant capacity. Sci Technol Food Ind 2014; 35: 119-122.
- Wu CS, Shu C, Li J, Qian Y, Suo HY. The research progress and prospect of Yak yogurt lactic acid bacteria. Food Ind 2012; 2012: 135-139.
- Wu CS, Li J, Qian Y, Suo HY. Research progress of Yak milk and fermented Yak milk and their nutritional value. J Dairy Sci Technol 2012; 35: 43-46.
- Zhou XF, Yi RK, Zhao X. Skin anti-aging effect of mice of Lactobacillus fermentum Zhao. J Chongqing Univ Edu 2016; 29: 160-167.
- Qian Y, Suo H, Du M, Zhao X, Li J, Li GJ, Song JL, Liu Z. Preventive effect of Lactobacillus fermentum Lee on activated carbon-induced constipation in mice. Exp Ther Med 2015; 9: 272-278.
- Zhao X, Wang Q, Li GJ, Chen F, Qian Y, Wang R. In vitro antioxidant, anti-mutagenic, anti-cancer and anti-angiogenic effects of Chinese Bowl tea. J Func Food 2014; 7: 590-598.
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002; 20: 495-549.
- Pandey A, Verma S, Kumar VL. Metformin maintains mucosal integrity in experimental model of colitis by inhibiting oxidative stress and pro-inflammatory signaling. Biomed Pharmacother 2017; 94: 1121-1128.
- Saijo H, Tatsumi N, Arihiro S, Kato T, Okabe M, Tajiri H, Hashimoto H. Microangiopathy triggers, and inducible nitric oxide synthase exacerbates dextran sulfate sodium-induced colitis. Lab Invest 2015; 95: 728-748.
- Kadri CJ, Pereira JA, Campos FG, Ortega MM, Bragion CB, Martinez CA. Anti-inflammatory effects of enemas containing an oily extract of curcumin in an experimental model of diversion colitis. Histol Histopathol 2017; 32: 161-169.
- Patel PP, Trivedi ND. Effect of karanjin on 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis in Balb/c mice. Indian J Pharmacol 2017; 49: 161-167.
- Sun X, Cai Y, Fleming C, Tong Z, Wang Z, Ding C, Qu M, Zhang HG, Suo J, Yan J. Innate γδdT17 cells play a protective role in DSS-induced colitis via recruitment of Gr-1+CD11b+ myeloid suppressor cells. Oncoimmunology 2017; 6: 1313369.
- Zou Y, Li WY, Wan Z, Zhao B, He ZW. Huangqin-tang ameliorates TNBS-induced colitis by regulating effector and regulatory CD4(+) T cells. Biomed Res Int 2015; 2015: 102021.
- Maakowska WD, Swora CE, Poniedziaaek B, Adamski Z, Dobrowolska A. Usefulness of selected laboratory markers in ulcerative colitis. Eur Cytokine Netw 2015; 26: 26-37.
- Dijkstra G, van Goor H, Jansen PL, Moshage H. Targeting nitric oxide in the gastrointestinal tract. Curr Opin Investig Drugs 2004; 5: 529-536.
- Vallance BA, Dijkstra G, Qiu B, van der Waaij LA, van Goor H, Jansen PL, Mashimo H, Collins SM. Relative contributions of NOS isoforms during experimental colitis: endothelial-derived NOS maintains mucosal integrity. Am J Physiol Gastrointest Liver Physiol 2004; 287: 865-874.
- Dai YC, Zheng L, Zhang YL, Chen X, Chen DL, Wang LJ, Tang ZP. Jianpi Qingchang decoction regulates intestinal motility of dextran sulfate sodium-induced colitis through reducing autophagy of interstitial cells of Cajal. World J Gastroenterol 2017; 23: 4724-4734.
- Ren H, Han J, Li Z, Xiong Z. Stem cell factor/Kit signal insufficiency contributes to hypoxia-induced intestinal motility dysfunctions in neonatal mice. Dig Dis Sci 2017; 62: 1193-1203.
- Feng J, Gao J, Zhou S, Liu Y, Zhong Y. Role of stem cell factor in the regulation of ICC proliferation and detrusor contraction in rats with an underactive bladder. Mol Med Rep 2017; 16: 1516-1522.
- Stojkovic B, McLoughlin RM, Meade KG. In vivo relevance of polymorphic Interleukin 8 promoter haplotype for the systemic immune response to LPS in Holstein-Friesian calves. Vet Immunol Immunopathol 2016; 182: 1-10.
- Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S, Ericksen R, Frucht H, Fox JG, Wang TC. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 2013; 144: 155-166.
- Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol 2008; 84: 1213-1221.