Research Article - Journal of Biochemistry and Biotechnology (2021) Volume 4, Issue 5
Isolation and identification of Staphylococcus aureus from dairy farms in Bishoftu Town, Ethiopia
Sara Amanuel Bude*, Abdi kidane Mengesha
Department of Medicine, Addis Ababa University, College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia
- Corresponding Author:
- Sara Amanuel Bude
Department of Medicine
Addis Ababa University
College of Veterinary Medicine and Agriculture
Bishoftu, Ethiopia
E-mail: sara.amanuel@aau.edu.et
Accepted date: August 02, 2021
Citation: Bude SA, Mengesha AK. Isolation and identification of Staphylococcus aureus from dairy farms in Bishoftu Town, Ethiopia. J Biochem Biotech. 2021;4(5):9-13
Abstract
Across-sectional study was conducted from February, 2020 to March, 2020 in selected dairy farms in Bishoftu Town to isolate and identify Staphylococcus aureus from milk samples, swab (teat swab, tank swab and bucket swab) samples. The samples were transported to microbiology laboratory and, isolation and identification of an organism was based on morphological, cultural and biochemical characteristics. Accordingly a total of 120 samples of them 58 milk sample, 58 polled teat swab sample, 2 polled tank swab sample and 2 polled bucket swab samples. Those milk samples and swab sample were collected and cultured parallel on both Blood agar and nutrient agar. Out of total samples 68/120 were well grown on both Medias and sub cultured on nutrient agar for farther identification using Primary and secondary biochemical tests. Accordingly, 68 milk samples were shown typical large, round, golden yellow colonies with hemolysis when grown on blood agar, round shaped grape like clusters are seen under microscope after stained with grams stain, and catalase positive Staphylococcus aureus with bubble formation is observed and typical yellow pigmentation on mannitol salt agar which is selective media for genus Staphylococcus. Finally, 68 (56.67%) Staphylococcus aureus were identified with coagulase test with the clumping result observed. Hence, implementing hygiene conditions, creation of awareness on control and prevention of subclinical mastitis in dairy farms and conducting drug sensitivity test for Staphylococcus aureus is recommended.
Keywords
Bishoftu, Bovine, Dairy farms, Identification, Isolation, Staphylococcus aureus.
Introduction
Ethiopia is believed to have the largest livestock population estimated 52.13 million in Africa that represents a major national resource and form an integral part of the agricultural production system. Among these the dairy cows representing around 7.2 million (CSA) [1]. The dairy product like milk provides an important dietary source for the majority of rural and considerable number of the urban and per-urban population. Though milk production often does not satisfy the countries requirement, due to a multitude of factors, out of which disease of the mammary glands known as mastitis is among the various factors contributing to reduced milk production. According to their ports of (FAO, 2003), the total annual national milk production in Ethiopia ranges from 7,979,000 to 1,197,500 metric tons raw milk equivalents. Out of the total national milk production, between 85 and 89 percent is contributed from cattle. However, this amount is by far below the national demand for milk and milk products in the country, given the considerable potential for dairy farms income and employment generation from high value dairy products. Improvement of the dairy sector in Ethiopia can contribute significantly to poverty alleviation and nutrition in the country [2]. Nevertheless, the quality and quantity of milk in the country deteriorates due to various causes. Mastitis is one of these which are known, to cause a great deal of loss or reduction of productivity, to influence the quality and quantity of milk yield and to cause culling of animals at an unacceptable age in dairy cattle (Bradley and Green) [3]. Clinical mastitis is readily superficial and visually detected. It occurs, when the inflammatory response is strong enough to cause visible changes in the milk (clots and flakes),the udder (swelling), or the cow (off feed or fever) (Korhonen and Kaartinen, Bradley) [4,5]. Most estimates have shown a 30% reduction in productivity per affected quarter and a 15% reduction in production per cow/lactation, making the disease one of the costliest and serious problems affecting the dairy industry worldwide (Wallenberg and Vanior) [6]. Mastitis is worth studying as it incurs financial losses attributed to reduce milk yield, discarded milk following antibiotic therapy, early culling of cows, veterinary costs, drug costs, increased labor, death in peracute septicemia, and replacement cost [7]. In Ethiopia, different studies show the prevalence of the cause of clinical mastitis in different parts of the country. For example, in Harrarghe Zone, the predominant isolated bacteria were coagulase negative Staphylococcus species (CNS) (34.2%) followed by Staphylococcus aureus (24.2%) (Zeryehun and Abera) [8]. In other studies, the predominance of coagulase negative Staphylococcus (43.47%) and S. aureus (36.95%) was also reported in a study conducted in other parts of the country [9,10]. In a study conducted in Bishoftu town, the major pathogens isolated were Staphylococcus aureus (44.95%), S.intermedius (22%); S. haicus (9.2%), and other Staphylococcus spp. (23.9%); Streptococcus spp. (28.1%), and E. coli 9.8% [11,12]. Besides clinical mastitis is a frequently occurring and economically important disease for the dairy industry in our country, Ethiopia. For the control and prevention of the disease, proper isolation and identification of the responsible bacterial agents are necessary regarding which little studies are still done in the current study site. Therefore, the objective of this paper was to isolate and identify Staphylococcus aureus from mastitic cows in Bishoftu town.
Materials and Methods
Study area
The study was conducted in dairy farms found in Bishoftu Town East Shoa zone of Oromia region., The area is located at 45 km along South East of Addis Ababa which is located at 9°N latitude and 40°E longitudes at an altitude of 1850 m above sea level in central high lands of Ethiopia. It has an annual rainfall of 866 mm of which 84% is in the long rainy season namely June to September. The dairy production system in the area is both intensive and extensive type. (NMSA, CSA, BTAO) [13,14]. The study animals were cross breeds cattle from dairy farms in Bishoftu Town.
Study design
A cross-sectional study design was conducted from February 2020 to May, 2020 in purposively selected dairy farms found in Bishoftu town, using microbiological procedure to isolate and identify Staphylococcus aureus from selected dairy farms.
Sampling method and transportation
A total of 120 samples were collected from dairy cattle farms of EMDDI (66) and from CVMAF (54). The sample size was fixed based on the representative samples taken from selected dairy cattle farms. Probability sampling (simple random) was used to select the population to be sampled. Samples were taken from two intensive dairy farms (EMDDI and CVMAF). Accordingly, 58 udder milk, 58 polled teat swab, 2 polled tank swab and 2 polled bucket swab were collected from dairy farms visits in and around Bishoftu.
Strict aseptic procedure was followed when collecting milk samples in order to prevent contamination with microorganisms present on the skin udder and teats. Teat ends were cleaned and disinfected with ethanol (70%) before sampling. Strict foremilks (first stream) were discharged to reduce the number of contamination of teat canal. Sterile test tubes with tight fitting cups were used. The test tube was labeled with permanent marker before sampling. To reduce contamination of teat ends during sample collection, the near teats were sampled first and then followed by the far ones [15].
Milk samples were collected from each of sub clinically mastitic non-blind quarters of the selected cows for bacterial isolation. About 10 ml of milk was aseptically collected from each quarter using sterile test tube and transported inside ice containing ice box and transported to laboratory. In the laboratory, sample was cultured immediately or stored at +4°C. And polled teat, polled bucket, and polled tank swabs from selected representative dairy cattle farms were collected using sterile cotton swabs from dairy cattle farms. Each sterile cotton swab was dipped into sterile distilled water prior to collection. Polled teat swab taken from a tips and from the body of the teat to make a representative sample. This was undertaken by rotating sterile applicator swab on those regions. Swabs from materials from dairy cattle farms (bucket and tank) were collected using sterile applicator swab through rotating on the body (inside) of the materials. After swab was taken, subsequently, it is put into a single screw capped test tube containing 10 milliliter of buffered peptone water containing sterile test tubes (ISO-17604 (2003)). Then, samples for culture were placed in racks for easy handling and held inside ice containing ice box properly packed and kept cold. Finally, it was transported to Addis Ababa University College of Veterinary Medicine Microbiology laboratory, Bishoftu.
Sample size determination
Purposive sampling technique was applied on all available dairy cows in the study area. A total of 58 dairy cows from intensive dairy farms in Bishoftu were selected conveniently based on the availability of dairy cows.
The sample size was calculated according the formula given (Thrusfield) using 74.7% previous prevalence of clinical and subclinical mastitis (overall) in the same area [16,17].
5% absolute precision and 95% confidence level.
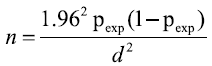
Where n=required sample size, Pexp=expected prevalence d=desired absolute precision and 1.96 is multiplier of 95% CI.
Study methodology
Sample type: A total of 120 samples containing four sample types were collected from selected dairy cattle farms in Bishoftu town and its surroundings. These sample types include udder milk, teat swab (polled), bucket swab from bucket (polled), and tank swab from total collected milk (polled) from selected dairy farms was taken.
Milk sample and swab sample collection: Milk sample was collected both from clinical and subclinical mastitis separately. The udder of the animal was thoroughly cleaned with water (Chauhan and Agarwal) [18]. The teat orifice was also cleaned using cotton soaked in 70% ethyl alcohol [19]. After discarding a few streams of milk, by holding the sterile collection bottle nearly horizontal, 5 ml milk was collected according to the procedures recommended by National Mastitis Council (NMC) [20]. The milk samples were polled together from all the 4 quarters in case all are involved. Then the samples were labeled based on temporary ID given to a cow. And polled teat, polled bucket, and polled tank swabs from selected representative dairy cattle farms were collected using sterile cotton swabs from dairy cattle farms. Each sterile cotton swab was dipped into sterile distilled water prior to collection. Polled teat swab taken from a tips and from the body of the teat to make a representative sample. This was undertaken by rotating sterile applicator swab on those regions. Swabs from materials from dairy cattle farms (bucket and tank) were collected using sterile applicator swab through rotating on the body (inside) of the materials. After swab was taken, subsequently, it is put into a single screw capped test tube containing 10 milliliter of Buffered Peptone Water (BPW) containing sterile test tubes. Then, samples for culture were placed in racks for easy handling and held inside ice containing ice box properly packed and kept cold. And both milk and swab sample were transported to Addis Ababa University College of Veterinary Medicine Microbiology laboratory, Bishoftu.
Bacteriological identification and examination: Both milk sample and swab samples were bacteriologically examined according to the procedures employed [19,20]. A loopful of pooled milk sample and swab sample (pooled teat swab, pooled bucket and tank swabs ) were collected from each infected quarter then inoculated on Blood Agar base (BA) (Oxoid, UK) enriched with 7% defibrinated sheep blood. The milk sample and swab samples was inoculated on an enriched Tryptone Soya Broth (TSB) (Oxoid, Hampshire, England) then incubated aerobically at 37°C for 24 hours to amplify the bacterial growth. Then inoculated on blood agar plate and incubated aerobically at 37°C for 24-48 hours. The plates were examined for growth, morphology and haemolytic characteristics on blood agar. Presumptive colonies were selected and subcultured on Nutrient Agar (NA) (Oxoid, Hampshire, England) and incubated aerobically at 37°C for 24-48 h to get a pure culture. Final identification of Staphylococcus species was done based on Gram reaction (Gram-positive), Cellular Morphology (coccus) and arrangements of the bacteria, catalase test, growth characteristics on Mannitol Salt Agar, (MSA) tube coagulase tests (by using rabbit plasma). Colonies that were identified as Staphylococcus by Gram-staining reaction and catalase test were streaked on Mannitol Salt Agar (MSA) plates and incubated at 37°C and examined after 24-48 hours for growth and change in the color of the medium. The presence of growth and change of pH in the media (red to yellow color) were regarded as confirmative identification of staphylococci. Phenol red PH indicator detected the acidic metabolic product of mannitol. Fermentation of mannitol by S. aureus causes yellow discoloration of the medium, colonies that develop weak or delayed yellow color after 24 hours of incubation.
To identify the pathogenic Staphylococcus from non-pathogenic coagulase test was used. The tube coagulase test was performed in sterile tubes by adding 0.5 ml of selected isolates of Staphylococcus grown on Tryptone Soya Broth (TSB) at 37°C for 24 hours to 0.5 ml of fresh rabbit plasma. After mixing by gentle rotation, the tubes were incubated at 37°C along with a negative control tube containing a mixture of 0.5 ml of sterile TSB and 0.5 ml of rabbit plasma. Clotting was evaluated at 30 minutes intervals for the first 4 hours of the test and then after 24 hours incubation. The reaction was considered positive if any degree of clotting from a loose clot to a solid clot that is immovable when the tube is inverted (tilted) was visible within the tube and no degree of clotting would be taken as negative [19].
Ethical approval consideration: The study was carried out in compliance with relevant guidelines and regulations stated by the Ethical Clearance Committee of the college of veterinary medicine and agriculture, Addis Ababa University. The purpose of the study was explained to all dairy farm owners/managers who participated in the study and verbal consent was obtained from the dairy farm owners before collection of milk and swab samples.
Data analysis
Data was entered to MS Excel spreadsheet and checked for accuracy. After validation it was transferred and processed using computer software SPPS version 20 for analysis. Pearson’s chi square test was used when appropriate to analyze the proportion of categorical data. 95% confidence were computed and the 95% confidence level was used and result were considered significant of p<0.05.
Results
Out of 120 total samples collected from different sample sources and processed for S.aureus of this, the isolation rate. S.aureus was 68 (56.67%) (Tables 1 and 2).
| Variables | N | NP | Prevalence (%) | X2 | P-values | |
|---|---|---|---|---|---|---|
| Age | Adult | 93 | 55 | 59.14% | 1.0295 | 0.31 |
| Young | 27 | 13 | 48.15% | |||
| Body | Good | 92 | 52 | 56.52% | 0.0034 | 0.954 |
| Condition | poor | 28 | 16 | 57.14% | ||
| Lactating | Early (1-3) | 28 | 15 | 53.57% | 0.1425 | 0.706 |
| Period | Late (3-5) | 92 | 53 | 57.61% | ||
| Sample | Milk | 58 | 32 | 55.17% | 3.163 | 0.367 |
| Types | Sample | |||||
| Teat swabs | 58 | 32 | 55.17% | |||
| Tank swabs | 2 | 2 | 2.94 | |||
| Bucket swabs | 2 | 2 | 2.94 | |||
| Farm | EMDDI | |||||
| Name | CVMAF | 54 | ||||
Note: N: Number of animal examined; NP: Number of positive samples; X2: Chi square.
Table 1. Proportions of S.aureus in different age, body condition, lactating period and sample types.
| Result | Frequency | Percentage |
|---|---|---|
| Positive | 68 | 56.67% |
| Negative | 52 | 43.33 |
| Total | 120 | 100 |
Table 2. The percentage result of S.aureus.
Discussion
Staphylococcus aureus that causes mastitis is a complex disease commonly caused by bacteria and the difference in results could be due to variations in herd size, management practices, proportion of exotic gene inheritance, agro-climates and other risk factors might have contributions to the observed differences in proportion of mastitis among the findings of other scholars [21-24].
Staphylococcus aureus is mostly resistant to different food processing method which cannot be easily eliminated from foods by heat treatment (in pasteurized foods) or by competition with other flora (in fermented foods) unlike other Staphylococcus species [25]. Hence, the surveillance of food for microbial contamination is vital for the protection of public health and consumer interests [26,27]. Safe food production also has important economic role in increasing competition in global market (Hamid and Owni) [28].
The bacteria can gain access to raw milk and milk products either by direct excretion from udders having clinical and subclinical staphylococcal mastitis or by contamination from food handlers [26]. The present study was undertaken to investigate Staphylococcus aureus from intensively selected dairy farms of Bishoftu town. A total of 120 sample (milk samples, teat swabs, tank swabs and bucket swabs sample) 68 (56.67%) were Staphylococcus aureus positive. The present report is lower than findings of different scholars from different areas of Ethiopia [29-31]. This variation will be due to difference in herd size, management practices, and genetic difference and environmental factors [32].
From bacteriological positive samples 83.6% (106/120) were shown typical yellow pigmentation on mannitol salt agar which is selective media for genus Staphylococcus [19]. The present isolates of Staphylococcus species was higher than the previous report of which was 31.19 and 36.9% respectively [31-33]. From further laboratory confirmation 68(56.67%) isolates of Staphylococcus aureus were identified. The predominant or high proportion of Staphylococcus aureus in the present study was supported by the finding of Hundera et al. [29-34]. High prevalence of Staphylococcus aureus is due to its contagious nature and has been adapted to survive in the udder and establish chronic and subclinical infections [35]. From there it is shed into the milk, which serves as a source of infection for healthy cows during the milking process [36,37].
Conclusion
The overall high prevalence of mastitis in the present study signifies the effect of mastitis in Bishoftu dairy farms. Staphylococcus aureus was mastitis inducing pathogens detected from clinical mastitis in this study. Primary and secondary bacterial identification tests were employed for isolation and identification that confirms high isolation rate showed despite little attention and invisible loss from clinical mastitis.
Based on the above conclusions the following points are recommended:
• Improving hygienic measure, appropriate major of management based on their production system in the dairy farms.
• Training should be given to dairy farm owners and dairy farm worker on the cause of mastitis with appropriate interventions.
• Staphylococcus aureus mastitis prevention and control strategy should be initiated and promoted.
• Awareness creation to the farmer about the usage of anti- microbial agents and its withdrawal period.
• Further research should be focused on prevalence and molecular characterization of S.aureus bacterial isolate for serotyping and their population structure, genetics.
Competing Interests
The authors declare that they have no competing interests.
Author’s Contributions
Sara Amanuel Bude involved in the design of the study, bacterial isolation, data organization and analysis, and manuscript drafting and revision; Abdi Kidane Mengesha collected sample, conducted bacterial isolation, identification and drafted the manuscript.
Acknowledgement
The authors acknowledge Addis Ababa University and the owners of dairy farms in andaround Bishoftu town.
References
- Central Statistical Agency (CSA) : “Report on livestock and livestock characteristics,” The Federal Democratic Republic of Ethiopia, Statistical Bulletin, Central Statistical Agency, Addis Ababa, Ethiopia, 2015;570.
- Ahmed MA, Ehui S, Assefa Y. Dairy development in Ethiopia. Intl Food Policy Res Inst. 2004.
- Bradley AJ, Green MJ. Aetiology of clinical mastitis in six Somerset dairy herds. Vet Rec. 2001;148(22):683-6.
- Korhonen HJ, Kaartinen L. Changes in the composition of milk induced by mastitis. The Bovine Udder and Mastitis. 1995.
- Bradley AJ. Bovine mastitis: An evolving disease. Vet J. 2002;164(2):116-28.
- Wallenberg GJ, Vanderpoel HM, Vanior JT. Viral infection and bovine mastitis. J Vet Micro. 2002;88(1):27-45.
- Quinn PJ. Clinical veterinary microbiology. 1994.
- Zeryehun T, Abera G. Prevalence and bacterial isolates of mastitis in dairy farms in selected districts of Eastern Harrarghe zone, Eastern Ethiopia. J Vet Med. 2017;2017(1):1-7.
- Sori T, Hussien J, Bitew M. Prevalence and susceptibility assay of Staphylococcus aureus isolated from bovine mastitis in dairy farms of Jimma town, South West Ethiopia. J Anim Vet Adv. 2011;10(6):745-749.
- Dabash H, Petros A, Fekadu A. Prevalence and identification of bacterial pathogens causing bovine mastitis from crossbred of dairy cows in North Showa Zone of Ethiopia. Glob Vet. 2014;13(2):189-195.
- Kubota M, Hayashi T, Iwasaki K, et al. Rapid and effective method for separation of Staphylococcus aureus from somatic cells in mastitis milk. J Dairy Sci. 2007;90(9):4100-4107.
- Birhanu M, Leta S, Mamo G, et al. Prevalence of bovine subclinical mastitis and isolation of its major causes in Bishoftu Town, Ethiopia. BMC Res Notes. 2017;10(1):1-6.
- National Meteorological Services Agency (NMSA): National Meteorological Services Agency of Adama Station, unpublished data. 2010.
- Bude SA, Mengesha AK. Isolation and Identification of Staphylococcus Aureus from Dairy Farms in Bishoftu Town, Ethiopia.
- Quinn PJ, Carter ME, Markey BK, Carter GR. Clinical veterinary microbiology. Mosby International Limited. 1999;96.
- Thrusfield M. Veterinary epidemiology. (3rd edn), Blackwell Science, United Kingdom. 2005:158.
- Zeryehun T, Aya T, Bayecha R. Study on prevalence, bacterial pathogens and associated risk factors of bovine mastitis in small holder dairy farms in and around Addis Ababa, Ethiopia. J Anim Plant Sci. 2013;23(1):50-55.
- Chauhan RS. Textbook of veterinary clinical and laboratory diagnosis. Jaypee Bros. 1995.
- Quinn PJ, Carter ME, Markey B, et al. Clinical veterinary microbiology microbial disease, Black well sciences. Wolf Spain. 2002;2:261-267.
- NMC, Newsletter Udder. 1997.
- Nemeghaire S, Argudín MA, Haesebrouck F, et al. Epidemiology and molecular characterization of methicillin-resistant Staphylococcus aureus nasal carriage isolates from bovines. BMC Res Notes. 2014;10(1):1-9.
- Botrel MA, Haenni M, Morignat E, et al. Distribution and antimicrobial resistance of clinical and subclinical mastitis pathogens in dairy cows in Rhône-Alpes. Foodborne Pathog Dis. 2010;7(5):479-487.
- Wyder AB, Boss R, Naskova J, et al. Streptococcus spp. and related bacteria: their identification and their pathogenic potential for chronic mastitis–a molecular approach. Vet Sci Res. 2011;91(3):349-357.
- Birhanu M, Leta S, Mamo G, et al. Prevalence of bovine subclinical mastitis and isolation of its major causes in Bishoftu Town, Ethiopia. BMC Res Notes. 2017;10(1):1-6.
- Abunna F, Abriham T, Gizaw F, et al. Staphylococcus: isolation, identification and antimicrobial resistance in dairy cattle farms, municipal abattoir and personnel in and around Asella, Ethiopia. J Vet Sci Technol. 2016;7(6):223-229.
- Kalorey DR, Shanmugam Y, Kurkure NV, et al. PCR-based detection of genes encoding virulence determinants in Staphylococcus aureus from bovine subclinical mastitis cases. J Vet Sci. 2007;8(2):151-154.
- Bude SA, Mengesha AK. Isolation and identification of Staphylococcus aureus from Dairy Farms in Bishoftu Town, Ethiopia.
- Hamid, O, Owni, O. Microbiological properties and sensory characteristics of white cheese collected in Zalingei area. J. Anl and Vet Sci. 2007;2(1): 61-65.
- Hundera S, Ademe Z, Sintayehu A. Dairy cattle mastitis in and around Sebeta, Ethiopia. Intern. J Appl Vet. Med. 2005;3(4):1525-1530.
- Lakew M, Tolosa T, Tigre W. Prevalence and major bacterial causes of bovine mastitis in Asella, South Eastern Ethiopia. Trop Anim Health Prod TROP ANIM HEALTH PRO. 2009;41(7):1525-1530.
- Wubishet Z, Ararsa D, Alemayehu L. Bovine mastitis in selected districts of Borena zone, Southern Ethiopia. Bull Anim Health Prod Afr. 2013;61(2):173-179.
- Ayele Y, Gutema FD, Edao BM, et al. Assessment of Staphylococcus aureus along milk value chain and its public health importance in Sebeta, central Oromia, Ethiopia. BMC Microbiol. 2017;17(1):1-7.
- Tadesse A, Chanie M. Study on the occurrence of bovine mastitis in Addis Ababa dairy farms and associated risk factors. Adv Biol Res. 2012;6(4):151-158.
- Mekonnen H, Workineh S, Bayleyegn M, et al. Antimicrobial susceptibility profiles of mastitis isolates from cows in three major Ethiopian dairies. Rev Med Vet. 2005;156(7):391.
- Beyene T, Hayishe H, Gizaw F, et al. Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia. BMC Res Notes. 2017;10(1):1-9.
- International Organization for Standardization (ISO): Microbiology of Food and Animal Feeding Stuffs Carcass Sampling for Microbiological Analysis (ISO 17604), Geneva. 2003:1-17.
- National Mastitis Council (NMC): Microbiological Procedures for the Diagnosis of Bovine Udder Infection. National mastitis council (3rd edn), Arlington, Virginia, USA, 1990.