Research Article - Biomedical Research (2017) Volume 28, Issue 1
Investigation of the survival time and quantification of therapeutic benefits for HIV patients with highly active antiretroviral therapy
Riying Lv1,2, Guojian Li1*, Jizhou Wu3, Yujia Zhu2, Jianlong Li4, Xionglin Qin4, Shixiong Li2, Shaoping Fu2, Qiongfen Qin2 and Chaoyu Xu21Guangxi Medical University, China
2Department of Infectious Diseases, Eighth Affiliated Hospital of Guangxi Medical University, Guigang, PR China
3Department of Infectious Diseases, the First Affiliated Hospital of Guangxi Medical University, Nanning, PR China
4Guangxi Guigang City Centers for Disease Control, Guigang, PR China
- *Corresponding Author:
- Guojian Li
Department of Infectious Diseases
Guangxi Medical University, Nanning, PR China
Accepted date: June 2, 2016
Abstract
Introduction of highly active antiretroviral therapy (HAART) has been proved to be very effective against HIV infection and AIDS progression. It has made a great contribution to decrease both HIVinduced AIDS mortality and morbidity. In this study, we aim to investigate the efficiency of HAART as the treatment for newly diagnosed HIV patients as well as survival time of patients. 3100 cases of newly diagnosed HIV patients receiving HAART were selected in this study for a period of 12 months. The level of viral RNA, numbers of CD4+ T cell and death cases were recorded monthly. With the 12 months of HAART treatment, the net increased numbers of CD4+ T cell were of 4.3 × 103 cell/ml in average (P<0.01). Viral load dropped to an undetectable level (below to 1.70 log copies/mL) after 8 months treatment. In conclusion, HIV/AIDS patients received significantly benefits by administrating HAART.
Keywords
HIV, Survival time, Antiretroviral therapy, Retrospective analysis, CD4+ T cells.
Introduction
The acquired immunodeficiency syndrome (AIDS) is a human disease caused by the human immunodeficiency virus (HIV), a lentivirus which belongs to a subgroup of retrovirus [1]. As the prefix “Lente” of lentivirus means “slow” in Latin, HIV infection in human causes progressive failure of the immune system, even results in life-threatening infection by opportunistic infections or the occurrence of cancers [2]. Without any intervention, the average survival time of HIV infected individuals is ranging from 9 months up to 11 years, depending on the subtype of HIV [3]. Since the first official publication described this virus [4,5], tremendous efforts had been contributed to elucidate the viral pathogenesis, discover specific drugs for HIV or develop a prophylactic vaccine. However, till now, there is still no vaccine available and only failed clinical trials were reported. Currently, treatment of HIV still heavily relies on the combined administration of different drugs which is named as the highly active antiretroviral therapy (HAART).
Although HIV cannot be completely cured, the introduction of HAART has been proved to be very effective against HIV infection and AIDS progression [6]. It has made a great contribution to decrease both HIV-induced AIDS mortality and morbidity [6]. Moreover, the survival time and life quality of HIV infected patients had been improved significantly as well [6].
In this study, an investigation of survival time and quantification of therapeutic benefits for the newly diagnosed HIV patients who were enrolled in the HIV antiviral treatment centres of Guigang City for HAART were conducted. During a 12-month monitoring period, the total amount of the CD4+ T cells, the net increased number of CD4+ T cells and viral copies in peripheral blood were quantified and compared. The overall survival rate was recorded as well.
Materials and Methods
Ethics statement
The protocol used in this study had been reviewed and approved by Medical Ethics Committee of Guigang City, Guangxi Province. All participating patients were formally informed for the purpose of using their samples in this study and a letter of consent had been signed by all involved patients.
Patients, inclusion criteria and exclusion criteria
3100 newly diagnosed HIV patients (2169 males and 931 females) who were enrolled in the HIV antiviral treatment centres of Guigang city from April 2007 to April 2014 were included in this study. Patients receiving HAART prior to enrolling in this centre were excluded. All patients were positive for anti-HIV antibody test and the CD4+ T cell number were less than 350 cell/μL. The average age of all patients was 33.8 ± 4.6 years (ranging from 18 to 68 years).
To minimize the affection of general information to the final result, we conducted a systematic analysis for the gender, age and therapeutic protocol used to patients associated with their treatment outcomes. However, there was no significant difference observed.
Therapeutic protocol and drug dosage
The criteria for initiating HAART treatment was subject to The national HIV/AIDS diagnostic criteria and principles and The national free AIDS antiretroviral drugs program handbook of People’s Republic of China. For the triple-drug therapy based HAART, five different combinations of drugs were provided. They were: 1) AZT+3TC+NVP (applied in 969 cases), 2) D4T +3TC+NVP (applied in 1097 cases), 3) AZT+3TC+EFV (applied in 196 cases), 4) D4T+3TC+EFV (applied in 130 cases) and 5) DDI+D4T+NVP (applied in 710 cases).
For the dosage of these drugs, AZT was administered orally at 300 mg at an interval of 12 hours. A single dose of 200 mg single dose of NVP was administered daily for the first 14 days for patients. If no apparent side effect was observed, the dose of NVP would be increased to 200 mg with twice per day. For D4T dosage, 40 mg per dose with twice per day would apply to patients with weight more than 60 kg. Otherwise, the single dose of D4T will be reduced to 30 mg and with same administration frequency for those patients less than 60 kg. Moreover, the 3TC and EFV were administered at a single dose of 300 mg and 600 mg per day, respectively.
Plasma HIV RNA quantification
Plasma HIV RNA level was examined monthly via HIV-1 RNA Quantitative bDNA (v3.0) kit (Quest Diagnosis, Madison, NJ, USA ) in a Quantiplex HIV-1 RNA 3.0 bDNA system ( Bayer, Leverkusen, Germany ) according the manufacture’s instruction. Two independent plasma HIV RNA tests were conducted for each patient as the basal control before the initiation of HAART.
Flow cytometry assay for CD4+ T cells
The blood samples with EDTA from each patient were examined for CD4+ T cells via Flow Cytometry (FCM). Briefly, the lymphocyte separation medium (Lonza, Basel, Swiss) was used for separation of the PBMC from blood sample. Then, the PBMC was stained by anti-CD3-FITC (BD Bioscience, San Jose, CA, USA), anti-CD4-PE (BD Bioscience) and anti-CD8-PerP (BD Bioscience) antibodies as previously described [7,8]. FCM analysis for the T cell population of each sample was conducted in a FACS Calibur machine (BD Bioscience). The isotype control for each sample was also included as well.
Following-up and death rate surveillance
For all 3100 cases participating in this study, plasma HIV RNA level and CD4+ T cells number was examined monthly or bimonthly, respectively. The death of patient was set-up as the endpoint of following-up. The death cases were recorded bimonthly as well and used for calculating the survival rate.
Statistical analysis
Data are presented as means ± standard deviation (SD). The data of the CD4+ T cell number, viral copies and the survival rate were analysed by SPSS software (version 13.0) for the regression analysis. Comparisons between different groups were subjected to the Student’s t test. A two tailed P-value of less than 0.05 was considered significant.
Results
Toxicity and complications
The toxicity of triple drug therapy was reported from patients. The toxicity mainly included bloating, stomach ache, nausea, vomiting, diarrhoea, peripheral neuropathy (defined as limb pain and numbness), rush, central nervous system damage (head ache, insomnia and altered mental status), fever and hair loss.
The complications and numbers of corresponding cases during the therapy were listed as follows: skin damage (835 cases), trush (646 cases), oral hairy leukoplakia (344 cases), persistent diarrhoea for more than one month (349 cases), recurrence fever for more than one month (827 cases), recurrence bacterial infection except Pneumonia (55 cases), nontuberculous mycobacterial infection (4 cases), oesophageal candidiasis (50 cases), extra pulmonary cryptococcosis (18 cases), Y. pneumocystis pneumonia (72 cases), disseminated fungal disease (6 cases), Cytomegalovirus infection (3 cases), extra pulmonary tuberculosis (33 cases), recurrence bacterial pneumonia (69 cases), chronical HSV infection (21 cases), shingles (211 cases), toxoplasmosis (6 cases), other opportunistic infections and tumours (66 cases).
The changes of the total CD4+ cells in HIV infected patients receiving HAART
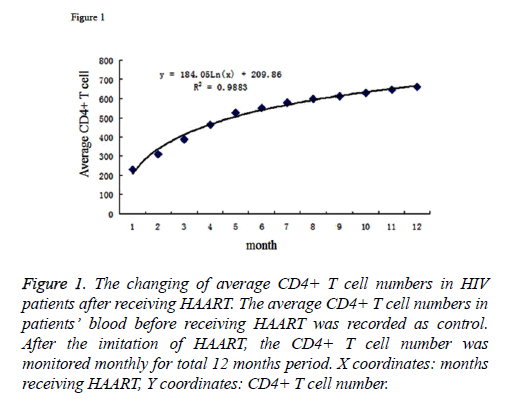
The average number of CD4+ T cells in patients’ blood before HAART was 2.31 × 105 cell/ml. After receiving the therapy, the numbers of CD4+ T cells were monitored in every month. The cell numbers were stably increasing; and at time point of the 12th month, it reached to an average number of 6.61 × 105 cell/ml, which showed a statistically significant increase compared to non-treatment. By inputting the average CD4+ T cell number as Y coordinate and months after therapy as the X coordinate to generate the graph, we performed the regression analysis for the X and Y coordinates. The results were listed as Figure 1. The logarithmic curve function could be described as: y=184.94ex+2.6.72, R2=0.9864. Based on this logarithmic curve, we got the relation between the net increased T cell number and the time after receiving therapy. The result was described as Figure 2.
Figure 1: The changing of average CD4+ T cell numbers in HIV patients after receiving HAART. The average CD4+ T cell numbers in patients’ blood before receiving HAART was recorded as control. After the imitation of HAART, the CD4+ T cell number was monitored monthly for total 12 months period. X coordinates: months receiving HAART, Y coordinates: CD4+ T cell number.
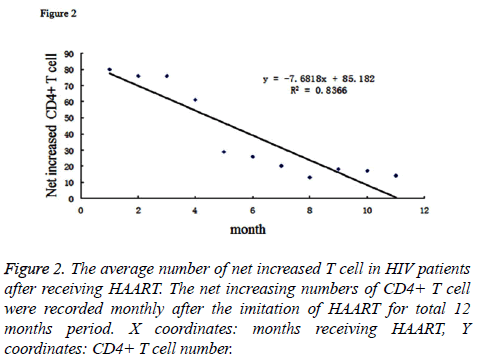
By analysing the Figure 2, it could demonstrate that the average number of net increased T cells became decrease as the time receiving HAART increased. If we performed the regression analysis for the net increased T cell (Y coordinates) and the time receiving HAART (X coordinates), their relation could be described as the linear function: y=-7.6818x+85.182, R2=0.8366. Taken together, our data indicated that the HAART was very effective in the early stage of treatment which could be reflected by net increased number of CD4+ T cell in patients’ blood, however, as the time elapsed, the efficiency reduced. Reasons why caused the decreased efficiency of HAART could be due to generation of drug-resistant mutation of virus, or poor compliance of the patients for taking drugs.
The changing of viral RNA copies in plasma and its relation with CD4+ T cell numbers
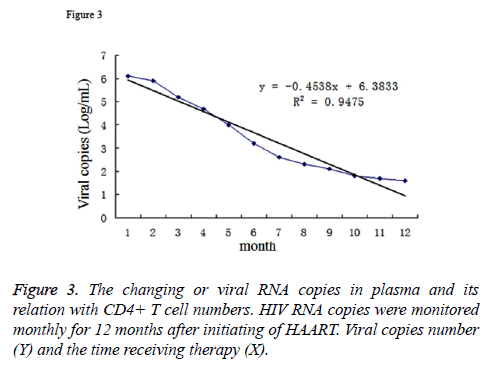
In the patients receiving HAART therapy, we also monitored HIV RNA copy numbers in their blood. Since the initiation of the therapy, the RNA level decreased from 6.1 log copies/mL to about 1.7 log copies/mL. The relevance between the viral copies (Y) and the time receiving therapy (X) can be described as the function: y=-0.4538x+6.3833, R2=0.9475 (Figure 3). Furthmore, we analysed the correlation of viral RNA copies and the CD4+ T cell numbers by SPSS. The result demonstrated that R2=-0.867, which demonstrated the CD4+ T cell number was negatively correlated with the viral copy numbers.
The overall survival rate of patients receiving HAART
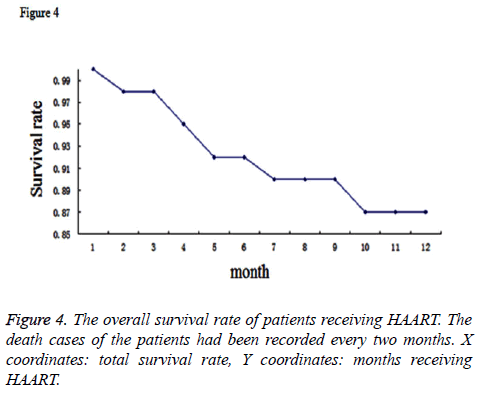
During the whole therapy period, death cases of the patients had been documented as well. There were 190 death cases reported totally. Moreover, 76 cases ceased the treatment and other 527 cases were lost to follow-up for more than 6 months. These above cases were not included in the prediction model. To make a prediction model for the time of therapy and the surviving rate, we conducted the COX analysis for the surviving data of the patients and generated a survival curve by Kaplan Meier method (Figure 4). This curve demonstrated that the total survival rate in two years for HIV patients receiving HAART could reach to 87%.
Discussion
Since the first official announcement of HIV [4,5], tremendous efforts had been made to develop a HIV specific drug for therapeutic purpose. The zidovudine (AZT), the first nucleoside reverse transcriptase inhibitor (NRTI) approved in United States in 1987, was once demonstrated promise results for the healing of HIV [9]. Unfortunately, people further found that HIV has the potential to develop drug-resistance mutation during the long term treatment of AZT due to the low fidelity of reverse transcriptase [10]. Since drugs targeting different step of HIV replication were unavailable before 1997, solely or combined administration of NRTI lead to the development of multi-drug resisted mutations [11]. Therefore, HAART had been developed by combining drugs targeting different step of HIV replication, such as HIV entry inhibitor, NRIT, nonenucleoside reverse transcriptase inhibitor NNRIT, integrase inhibitor as well as protease inhibitor [12-16].
It had been demonstrated that HAART could delay the progression of AIDS effectively and improved the life quality of the patients. Application of HAART could minimize the viral load in HIV patients’ blood or even to an undetectable level. The reduced viral load could help the patients maintaining or improving their immune function via increase the CD4+ T cells, which is necessary to prevent the opportunistic infections and extend the surviving time of patients. In our study, we found that the total CD4+ T cell number increased in every patient receiving the HAART. The absolute increasing cell number level was much higher in the first 4 to 5 months but reduced to a lower level soon in the following months. In the contrast, the viral load reduced stably as a linear trend.
The CD4+ T cell is the major target of HIV. However, it is still unclear that HIV only infects a very small proportion of CD4 T cells (estimated at 1 in 100 to 1 in 1,000) could finally overwhelm the renewal capacity of CD4+ T cell and result in the destruction of the whole immune system [2]. In our study, although patients receiving HAART indeed showed a reduced plasma viral load and improved CD4+ T cell numbers, there was still death cases reported. Our data suggested that the death were mainly occurred during the first 6 months after therapy. Since then, the death rate started to decline, which is consistence with previous reports. Moreover, it was reported that the overall survival rate was benefited from the earlier initiation of HAART [17]. These death cases observed in here may be due to a later diagnosis and initiation of HAART.
It had been confirmed that using HAART could restrain the replication of HIV to a low level for long term. Thus, it could restore the immune function and extent the patient’s life. However, application of HAART alone cannot clear the HIV completely due to existence of pro-virus in memory T cells or virus entering the central nerve system. Once the HAART stops, the virus starts to replicate again and the viral load in blood will recover to the pre-treatment level. However, the side effects and toxicity of long term application of HAART still need to be considered, as well as the causing of the drugresistant HIV mutant. In conclusion, our data demonstrated that HAART are highly effective in newly diagnosed HIV patients and the HIV/AIDS patients received significant benefits from application of HAART.
References
- Weiss RA. How does HIV cause AIDS? Sci J 1993; 260: 1273-1279.
- Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med 2009; 60: 471-484.
- UNAIDS World 2007 AIDS epidemic update. 2007.
- Gallo RC, Sarin PS, Gelmann EP, Robert-Guroff M, Richardson E, Kalyanaraman VS, Mann D, Sidhu GD, Stahl RE, Zolla-Pazner S, Leibowitch J, Popovic M. Isolation of human T-cell leukaemia virus in acquired immune deficiency syndrome (AIDS). Sci J 1983; 20: 865-867.
- Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Sci J 983; 20: 868-871.
- Michaels SH, Clark R, Kissinger P. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 339: 405-406.
- Wu W, Rochford R, Toomey L, Harrington W, Feuer G. Inhibition of HHV-8/KSHV infected primary effusion lymphomas in NOD/SCID mice by azidothymidine and interferon-alpha. Leuk Res 2005; 29: 545-555.
- Zhang YJ, Patel D, Nan Y, Fan S. Inhibition of primary effusion lymphoma engraftment in SCID mice by morpholino oligomers against early lytic genes of Kaposi's sarcoma-associated herpesvirus. Antivir Ther 2011; 16: 657-666.
- Wright K. AIDS therapy. First tentative signs of therapeutic promise. Nature 1986; 23: 283.
- Richman DD. Susceptibility to nucleoside analogues of zidovudine-resistant isolates of human immunodeficiency virus. Am J Med 1990; 88: 8-10.
- Schmit JC, Cogniaux J, Hermans P, Van Vaeck C, Sprecher S, Van Remoortel B, Witvrouw M, Balzarini J, Desmyter J, De Clercq E, Vandamme AM. Multiple drug resistance to nucleoside analogues and nonnucleoside reverse transcriptase inhibitors in an efficiently replicating human immunodeficiency virus type 1 patient strain. J Infect Dis 1996; 174: 962-968.
- Bai Y, Xue H, Wang K, Cai L, Qiu J, Bi S, Lai L, Cheng M, Liu S, Liu K. Covalent fusion inhibitors targeting HIV-1 gp41 deep pocket. Amino Acids 2013; 44: 701-713.
- Das K, Arnold E. HIV-1 reverse transcriptase and antiviral drug resistance. Part 1. Curr Opin Virol 2013; 3: 111-118.
- Das K, Arnold E. HIV-1 reverse transcriptase and antiviral drug resistance. Part 2. Curr Opin Virol 2013; 3: 119-128.
- Metifiot M, Marchand C, Pommier Y. HIV integrase inhibitors: 20-year landmark and challenges. Adv Pharmacol 2013; 67: 75-105.
- Wensing AM, van Maarseveen NM, Nijhuis M. Fifteen years of HIV Protease Inhibitors: raising the barrier to resistance. Antiviral Res 2010; 85: 59-74.
- Palella FJ Jr, Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, Holmberg SD and HIV Outpatient Study Investigators. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med 2003; 138: 620-626.