Research Article - Biomedical Research (2017) Volume 28, Issue 4
Investigating the anti-sarcoptic mange activity (in vivo) of propolis ointment in naturally infested rabbits
Dina Mahmoud Ahmed Metwally1,2*1Department of Zoology, Faculty of Science, King Saud University, Riyadh, KSA
2Department of Parasitology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- *Corresponding Author:
- Dina Mahmoud Ahmed Metwally
Department of Zoology
Faculty of Science
King Saud University
Kingdom of Saudi Arabia
Accepted on September 15, 2016
Abstract
Introduction: Sarcoptes scabiei (S. scabiei) var. cuniculi is the cause of sarcoptic mange in rabbits and results in considerable loss of weight, productivity, and wool quality.
Objective: The efficacy of 10% Propolis (bee glue) ointment on S. scabiei var. cuniculi was evaluated under in vivo conditions in pet rabbits with sarcoptic mange lesions compared to the currently used drug, ivermectin (400 μg/ kg, S/C).
Methods: In the in vivo condition, the efficacies of 10% Propolis ointment, 1% ivermectin (400 μg/kg, S/C), and Propolis ointment in combination with ivermectin were evaluated. Serum factors of the control and treated groups were tested to evaluate the toxic effects of Propolis on the liver.
Results: Topical application of 10% Propolis ointment showed complete recovery from clinical signs and complete absence of mite in microscopic examination from 10-15 days of treatment.
Conclusions: 10% Propolis ointment has antisarcoptic mange effects under in vivo conditions and may be used in clinical applications.
Keywords
Rabbits, Sarcoptes scabiei, Propolis ointment, In vivo.
Introduction
Mange is common in pet rabbits; it inhabits the epidermal layer of the skin and causes irritation, hypersensitivity reaction, inflammation, hyperkeratosis, seborrhoea, and alopecia as a result of the feeding behaviour of larvae and nymphs [1]. Mange lowers animal productivity and the quality of animal products and is often fatal if not treated [2]. S. scabiei var. cuniculi causes sarcoptic mange in rabbits. Infection with S. scabiei var. cuniculi induces relative protection against subsequent infection in rabbits [3]. Elimination of S. scabiei in rabbits is difficult compared to other domestic animals [4]. However treatment of sarcoptic mange with various acaricides like diazinon, deltamethrin, and ivermectin have achieved success [5], many of these chemical acaracides have side effects [6] such as resistance [7], toxicity [8], environmental contamination and persistence [8,9]. These limits have suggested a search for novel alternatives [10-13]. Propolis has lots of biological and pharmacological properties and its mechanisms of action have been widely investigated in vitro and in vivo in the last years. Propolis used as a popular remedies and is available in either pure or combined form with other natural products. Researchers have been focused on the study of its constituents and biological properties [14]. Propolis is used for upper respiratory tract infections, common cold and flu-like infections, and as dermatological preparations for wound healing [15]. Canine dermatophytosis resolved with topical propolis treatment [16]. Propolis is widely used in human and veterinary medicine as it has antimicrobial, antiviral, and antioxidant activities. The aim of the present study was to evaluate antisarcoptic mange activity of Propolis ointment under in vivo conditions.
Materials and Methods
Source of propolis
Propolis samples were obtained by contact with beekeepers in different zones of Riyadh, Saudi Arabia. Each sample was weighed, frozen at -20°C, ground with a mortar, and then stored at 4°C until use [17].
10% Propolis ointment preparation
Propolis extract was prepared as previously described [16] with modifications. Briefly, (5 g) was heated in a water bath with 50 g petroleum jelly (Vaseline) until melting and mixing. This mixture was used as the 10% propolis ointment for application twice/day to the skin lesion of each infested rabbit.
Experimental animals
Forty one-year-old and 2-2.4 kg weight naturally infested rabbits, of both sexes (eleven male and twenty nine female) were obtained from markets with crustaceous lesions on nose, lips, ears, legs and surround the eyes. Infestation was identified by taking skin scraping samples with a scalpel blade and they were microscopically examined for mobility and morphology of the mites [18]. Ten healthy rabbits free from any infestations were purchased and used as negative control. Mortality was checked daily. The animals were kept in wire-bottomed cages in a room under standard conditions of illumination with a 12 hour light-dark cycle and at a temperature of 25 ± 1°C for one week until the beginning of treatment. Animals were provided with tap water and a balanced diet ad libitum. The animals were divided into 5 groups, with 10 mice in each group. The five groups were group I (negative control), group II (positive control), group III (infected and treated with 10% Propolis ointment twice/day), group IV (1% ivermectin sterile solution (JaamectinTM, Jaapharm Canada Inc.) in a dose of (400 μg/kg, S/C), two injections with two weeks interval) [19], group V (infected and treated with both propolis ointment and ivermectin). Weekly, skin scrapings were collected from infected and recovered areas from each animal and were examined microscopically during the course of the experiment to detect sarcoptic mite.
Biochemical analysis
To assess the toxic effects of propolis in the livers of the rabbits, serum samples were collected from the rabbits. Alanine Aminotransferase (AST), Aspartate Aminotransferase (AST), and Alkaline Phosphatase (ALP) were measured [19] using commercial kits (Roche) with a Reflotron® Plussy system machine (Roche, Mannheim, Germany) as well as serum cortisol levels [20] using commercial, coated tube radioimmunoassay kits (Pantex, Santa Monica, CA), and the results were statistically analysed for assessment before and after treatment.
Histopathological study
Tissue samples were fixed in 10% neutral formalin for 24 hours, and paraffin blocks were obtained and routinely processed for light microscopy. Slices of 4-5 μM thickness were obtained from the prepared blocks and stained with haematoxylin-eosin. The preparations were visualized using a Nikon microscope at a magnification of x400 [21].
Scanning electron microscope
Specimens of parasites were collected from skin lesions scraping in tubes, and fixed in (2.5%) buffered glutaraldehyde overnight at 4°C. The tubes were checked frequently, and then centrifuged for 5 min at 1500 rpm. The supernatant was removed, and the specimens were fixed in (1%) osmium tetroxide in the same buffer for one hour at 4°C. Dehydration was carried out in graded series of acetone from (30-100%). Specimens were dried at a critical point-drying apparatus, mounted on stubs and were coated with gold according to standard procedures [22]. The prepared specimens were examined by Zeiss DSM 940 electron microscope in the Electron Microscope Unite of King Saud University.
Statistical analysis
Data are presented as the means and standard errors of the mean or standard deviations using the Statistical Package for the Social Sciences (SPSS v.22, Chicago, IL, USA). The results are expressed as the means ± Standard Errors of the Mean (SEM). All statistical comparisons between the control and the treated groups were performed using One-Way Analysis of Variance (ANOVA) followed by Dunnett’s post-hoc test for multiple comparisons. Significance was assigned at the level of (P<0.05) [23]. Receiver Operating Characteristics (ROC) curve analysis was performed. Area Under the Curve (AUC), cut-off values, and degree of specificity and sensitivity were calculated.
Results
Macroscopic evaluation
The healing from clinical signs was observed after 7 days of treatment by 10% propolis ointment either alone (Figures 1a and 1b) or in combination with ivermectin (Figures 1c and 1d). Itching, scale formation, and anxiety were vanished. Moreover, smooth skin and new hair growth were observed after 15 days of treatment. Recovery was late till the 28th day of the experiment in ivermectin-treated group (Table 1). Untreated control rabbits exhibited signs of sarcoptic mange throughout the study (Figure 1e). Seven days post treatment; skin scrapings from group III and group V were contained dead mites. Fifteen days post treatment; the skin scrapings were negative for the presence of eggs, larvae and adult parasites. In group IV, dead mites were found in the microscopic examination until the end of the 28th day and eggs of sarcoptic mite were detected in scraping at the end of the experiment (Figure 1f).
Figure 1. Rabbits infested with S. scabiei var. cuniculi (a) sarcoptic mange in the skin of nose, ears, and around the eye before treatment. (b) Recovery from mange 10 days after treatment with propolis ointment, the skin had no scales and hair growth appeared. (c) Infested rabbit’s body surface with S. scabiei before treatment. (d) Recovery 10 days after treatment with propolis ointment in combination with ivermectin. (e) Control untreated rabbits: alopecia all over body surface. (f) Light microscopy of S. scabiei egg detected in scrabing from rabbits treated with ivermectin (x400).
| Treatment | Mean time (days) of face, nose, feet and body mange recovery |
|---|---|
| 10% Propolis ointment | 7-15 |
| Ivermectin (400 µg/kg, S/C) | 21-28 |
| 10% Propolis ointment+ivermectin (400 µg/kg, S/C) | 7-15 |
Table 1. Mean time on days for the treatment of infested rabbits with mites by 10% propolis ointment, ivermectin (400 μg/kg, S/C), and propolis ointment in combination with ivermectin.
Histopathological studies
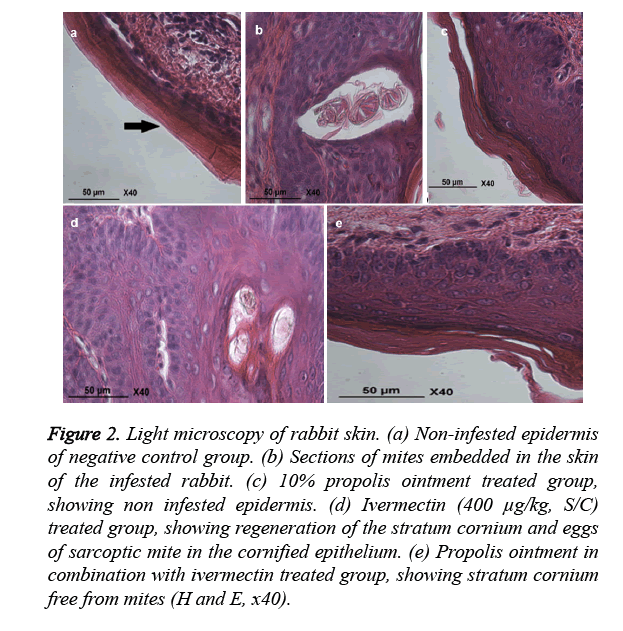
The histopathological studies on healthy control group skin revealed non infested epidermis (Figure 2a). The positive control group skin revealed heavy infiltration of eosinophil’s, lymphocytes, and mast cells in the epidermis and dermis of skin. There was hyper keratinization and sloughing of the epidermal layer. Section of embedded mites in the damaged tissue was also seen (Figure 2b). The skin of propolis group revealed complete healthy skin formation (Figure 2c), ivermectin group skin revealed egg in cornified epithelium (Figure 2d), and rabbit’s skin in 10% propolis ointment with ivermectin (400 μg/kg, S/C) treated group showed stratum corneum free from mites (Figure 2e).
Figure 2. Light microscopy of rabbit skin. (a) Non-infested epidermis of negative control group. (b) Sections of mites embedded in the skin of the infested rabbit. (c) 10% propolis ointment treated group, showing non infested epidermis. (d) Ivermectin (400 μg/kg, S/C) treated group, showing regeneration of the stratum cornium and eggs of sarcoptic mite in the cornified epithelium. (e) Propolis ointment in combination with ivermectin treated group, showing stratum cornium free from mites (H and E, x40).
Scanning electron microscopy (SEM)
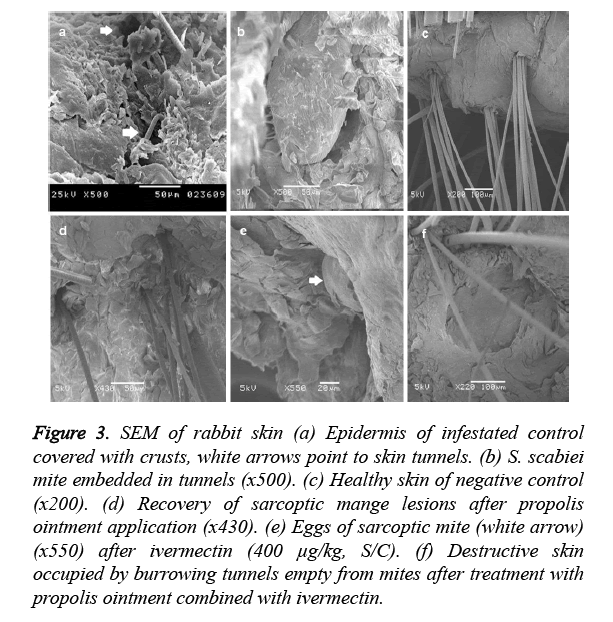
The skin of group I showed numerous cracks of mite penetration (white arrow) (Figure 3a) in the epidermis and embedded mites in the keratinized layer (Figure 3b). Group II showed completely formed stratum cornium (Figure 3c).
Figure 3. SEM of rabbit skin (a) Epidermis of infestated control covered with crusts, white arrows point to skin tunnels. (b) S. scabiei mite embedded in tunnels (x500). (c) Healthy skin of negative control (x200). (d) Recovery of sarcoptic mange lesions after propolis ointment application (x430). (e) Eggs of sarcoptic mite (white arrow) (x550) after ivermectin (400 μg/kg, S/C). (f) Destructive skin occupied by burrowing tunnels empty from mites after treatment with propolis ointment combined with ivermectin.
Group III showed healthy skin layers (Figure 3d). Group IV skin revealed the existence of S. scabiei eggs (Figure 3e). Group V showed destructed stratum cornium (Figure 3f).
Biochemical analysis
The levels of total protein of rabbits in ivermectin (400 μg/kg, S/C) treated group were significantly (P ≤ 0.05) increased compared to the corresponding values of healthy control. No significant differences were noted between the healthy control group and the treated groups in the levels of albumin and globulin. ALP, ALT, AST and cortisol were the most affected parameters. ALP was significantly decreased in the treated groups (P ≤ 0.05) compared to the healthy control group. AST and ALT levels were significantly increased in the positive control group (P ≤ 0.05) compared to those of the healthy control group. AST was significantly decreased (P ≤ 0.05) in ivermectin (400 μg/kg, S/C) treated group. ALT levels were significantly decreased (P ≤ 0.05) in the treated groups compared to those of the healthy control group. Cortisol levels were significantly increased (P ≤ 0.05) in the positive control and ivermectin treated groups compared to those of the healthy control group while it was significantly decreased (P ≤ 0.05) in 10% propolis ointment groups (groups III and V). The percentage change of the measured parameters in treated groups compared to the healthy control (Table 2). ROC analysis showed satisfactory values of area under the curve, sensitivity and specificity (Table 3). Result revealed that the application of propolis had no aggressive effects on liver factors.
| Parameters | Group | N | Mean ± S. D. | % Change compared to (-ve control) |
P-value |
|---|---|---|---|---|---|
| Total protein (g/dl) |
(-ve) Control | 10 | 5.40 ± 0.84 | 100.00 | |
| (+ve) Control | 10 | 5.80 ± 0.42 | 107.41 | 0.556 | |
| 10% Propolis ointment | 10 | 5.00 ± 0.94 | 92.59 | 0.556 | |
| ivermectin | 10 | 6.60 ± 0.52 | 122.22 | 0.002* | |
| Propolis+ivermectin | 10 | 4.80 ± 0.79 | 88.89 | 0.215 | |
| Albumin (g/dl) |
(-ve) Control | 10 | 3.80 ± 0.42 | 100.00 | |
| (+ve) Control | 10 | 3.40 ± 0.52 | 89.47 | 0.151 | |
| 10% Propolis ointment | 10 | 3.60 ± 0.52 | 94.74 | 0.705 | |
| ivermectin | 10 | 4.00 ± 0.00 | 105.26 | 0.705 | |
| Propolis+ivermectin | 10 | 3.40 ± 0.52 | 89.47 | 0.151 | |
| Globulin (g/dl) |
(-ve) Control | 10 | 1.60 ± 0.52 | 100.00 | |
| (+ve) Control | 10 | 2.40 ± 0.52 | 150.00 | 0.066 | |
| 10% Propolis ointment | 10 | 2.20 ± 0.79 | 137.50 | 0.227 | |
| ivermectin | 10 | 2.40 ± 0.52 | 150.00 | 0.066 | |
| Propolis+ivermectin | 10 | 2.00 ± 1.15 | 125.00 | 0.570 | |
| Alkaline phosphatase (IU/L) |
(-ve) Control | 10 | 114.20 ± 30.77 | 100.00 | |
| (+ve) Control | 10 | 29.60 ± 11.64 | 25.92 | 0.001* | |
| 10% Propolis ointment | 10 | 30.00 ± 5.96 | 26.27 | 0.001* | |
| ivermectin | 10 | 37.20 ± 13.80 | 32.57 | 0.001* | |
| Propolis+ivermectin | 10 | 62.60 ± 5.06 | 54.82 | 0.001* | |
| AST (IU/L) |
(-ve) Control | 10 | 48.60 ± 1.94 | 100.00 | |
| (+ve) Control | 10 | 63.06 ± 2.30 | 129.76 | 0.001* | |
| 10% Propolis ointment | 10 | 46.60 ± 3.80 | 95.88 | 0.479 | |
| ivermectin | 10 | 38.30 ± 4.51 | 78.81 | 0.001* | |
| Propolis+ivermectin | 10 | 45.80 ± 3.45 | 94.24 | 0.200 | |
| ALT (IU/L) |
(-ve) Control | 10 | 45.00 ± 2.05 | 100.00 | |
| (+ve) Control | 10 | 50.68 ± 2.77 | 112.62 | 0.001* | |
| 10% Propolis ointment | 10 | 12.10 ± 1.73 | 26.89 | 0.001* | |
| ivermectin | 10 | 11.80 ± 1.04 | 26.22 | 0.001* | |
| Propolis+ivermectin | 10 | 13.80 ± 2.25 | 30.67 | 0.001* | |
| Serum cortisole (ng/ml) |
(-ve) Control | 10 | 2.73 ± 0.56 | 100.00 | |
| (+ve) Control | 10 | 5.18 ± 0.95 | 190.02 | 0.001* | |
| 10% Propolis ointment | 10 | 1.92 ± 0.15 | 70.36 | 0.030* | |
| ivermectin | 10 | 3.92 ± 1.05 | 143.65 | 0.001* | |
| Propolis+ivermectin | 10 | 1.84 ± 0.17 | 67.57 | 0.015* | |
| *Significant level between the studied groups compared to healthy control when P ≤ 0.05. | |||||
Table 2. Mean ± S.D and independent t-test for total protein, albumin, globulin, ALP, AST, ALT and serum cortisol in control positive, 10% propolis ointment, ivermectin (400 μg/ kg, S/C) and propolis ointment in combination with ivermectin groups compared to healthy control.
| Parameters | Group | Area under the curve | Cut-off value | Sensitivity% | Specificity% |
|---|---|---|---|---|---|
| Total protein (g/dl) |
(+ve) Control | 0.620 | 5.500 | 80.0% | 40.0% |
| 10% Propolis ointment | 0.620 | 5.500 | 60.0% | 60.0% | |
| ivermectin | 0.880 | 6.500 | 60.0% | 100.0% | |
| Propolis+ivermectin | 0.700 | 5.500 | 80.0% | 60.0% | |
| Albumin (g/dl) |
(+ve) Control | 0.700 | 3.500 | 60.0% | 80.0% |
| 10% Propolis ointment | 0.600 | 3.500 | 40.0% | 80.0% | |
| ivermectin | 0.600 | 3.500 | 100.0% | 20.0% | |
| Propolis+ivermectin | 0.700 | 3.500 | 60.0% | 80.0% | |
| Globulin (g/dl) |
(+ve) Control | 0.820 | 2.500 | 40.0% | 100.0% |
| 10% Propolis ointment | 0.720 | 2.500 | 40.0% | 100.0% | |
| ivermectin | 0.820 | 2.500 | 40.0% | 100.0% | |
| Propolis+ivermectin | 0.560 | 3.000 | 20.0% | 100.0% | |
| Alkaline phosphatase (IU/L) |
(+ve) Control | 1.000 | 57.500 | 100.0% | 100.0% |
| 10% Propolis ointment | 1.000 | 56.000 | 100.0% | 100.0% | |
| ivermectin | 1.000 | 63.500 | 100.0% | 100.0% | |
| Propolis + ivermectin | 1.000 | 69.500 | 100.0% | 100.0% | |
| AST (IU/L) |
(+ve) Control | 1.000 | 55.620 | 100.0% | 100.0% |
| 10% Propolis ointment | 0.640 | 46.900 | 60.0% | 80.0% | |
| ivermectin | 1.000 | 45.295 | 100.0% | 100.0% | |
| Propolis+ivermectin | 0.760 | 45.950 | 60.0% | 100.0% | |
| ALT (IU/L) |
(+ve) Control | 0.920 | 48.405 | 80.0% | 100.0% |
| 10% Propolis ointment | 1.000 | 28.460 | 100.0% | 100.0% | |
| ivermectin | 1.000 | 27.870 | 100.0% | 100.0% | |
| Propolis+ivermectin | 1.000 | 29.510 | 100.0% | 100.0% | |
| Serum cortisole (ng/ml) |
(+ve) Control | 1.000 | 3.930 | 100.0% | 100.0% |
| 10% Propolis ointment | 1.000 | 2.195 | 100.0% | 100.0% | |
| ivermectin | 0.880 | 2.840 | 100.0% | 80.0% | |
| Propolis+ivermectin | 1.000 | 2.190 | 100.0% | 100.0% | |
| AUC: Area Under the Curve; AUC=0.5-0.65 (useless biomarker); AUC=0.7-0.85 (good biomarker); AUC=0.86-1 with satisfactory sensitivity and specificity (excellent biomarker). | |||||
Table 3. ROC-curve of all parameters in control positive, 10% Propolis ointment, Ivermectin (400 μg/kg, S/C) and propolis ointment in combination with ivermectin groups.
Discussion
The drugs currently used for sarcoptic mange possess several limitations, such as the development of resistance [7], toxicity [8], and management difficulties increasing the need for safer and more effective drugs. Further studies on the treatment of sarcoptic mange with natural and herbal elements are needed. Topical application of 10% propolis ointment indicated that using this compound on S. scabiei effectively killed the mites. Complete recovery was observed 15 days after treatment. However, in ivermectin (400 μg/kg, S/C) treated rabbits recovery was observed at the end of the experiment. Our result of treatment success was formerly applied by [13,24,25] who registered the same factors in treatment of mange in rabbits but by using oils.
Pathological investigation revealed infiltration of inflammatory cells in epidermis and dermis and cross sections of embedded mites similar to those previously described in wild mammals [26], rabbits [3,27], and in sheep [28]. The histological structure of the epidermal and dermal layers was normal at the 2nd week of treatment with 10% propolis ointment. Skin sections from ivermectin-treated group showed slow improvement and somewhere few inflammatory cells and eggs were observed at the end of the experiment.
SEM of the skin samples taken from the 10% propolis-treated group revealed improvement in stratum cornium. This may be due the keratolytic effect of propolis. Similar result was recorded in ivermectin-treated group in addition to detection of S. scabiei eggs. Oppositely, the SEM image in the skin of positive control-group rabbits revealed adult mites and eggs in tunnels. These findings were similar to those reported by other investigators [29,30].
The results revealed that the level of total protein of rabbits in ivermectin-treated group was significantly (P ≤ 0.05) increased compared to values in the healthy control. This result is in conflict with a previous study [14], in which total protein level was significantly decreased in the ivermectin groups. The administration of ivermectin in rabbits revealed non-significant increase in serum albumin. Our findings are not consistent with another study in rats [13] and in rabbits [31] in which ivermectin led to significant decrease in serum albumin. The results revealed no significant differences between the treatment and control groups in the globulin levels [32]. In addition, our findings are not consistent with [13], in which serum globulin of rabbits in positive control and ivermectin groups was significantly (P ≤ 0.05) decreased compared to values in the negative control. On the other hand, the total protein and albumin are not significantly affected [32]. ALP levels in groups II, III, IV, and V were significantly decreased compared to the negative control group. Adverse effect of scabies in liver has been indicated by [33,34]. The mechanism of action behind the low ALP level has been partially attributed to weight loss in mangy rabbits which could be due to the “energy demands” of mange, such as production of parakeratotic scale and scratching, as well as to the lowered food intake [35]. AST and ALT levels were significantly increased in the positive control compared to values in the healthy control. AST was significantly decreased in ivermectin group which indicates the negative effects on hepatic factors. In addition, ALT was significantly decreased in ivermectin, Propolis, and propolis combined with ivermectin groups compared to the healthy control. This result is in conflict with a previous study of rabbits [13,36], rats [31], and rams and bucks [37] in which ivermectin-treated groups experienced inflammation of liver cells and impaired liver functions. The serum AST and ALT levels were significantly increased after 28 days of treatment with ivermectin in swine and cattle [38]. Cortisol is an important hormone released in response to stress [39]. Serum cortisol is often used in stress and welfare assessments [40,41]. The levels of cortisol in rabbits were significantly increased in the positive control and ivermectin-treated groups compared to values in the healthy control [20]. The levels of cortisol in propolis and propolis combined with ivermectin groups were significantly decreased compared to positive control and they are less significantly different compared to healthy control.
High values of specificity, sensitivity and Area Under the Curve (AUC) measured by ROC analysis suggested that the studied biomarkers could be used as predictive tools in testing the side effects of ivermectin and the potency of 10% propolis ointment in treating mange in rabbits. Among the measured parameters ALP, AST, ALT, and cortisol markers were the most predictive recording high specificity and sensitivity and AUC of almost 1.
Collectively, the results of this study demonstrated that propolis displays an antisarcoptic effect under in vivo conditions. Various concentrations and application methods of propolis are required to further examine the effectiveness of propolis in the treatment and healing of sarcoptic mange lesions. We recommend future molecular studies to further examine the apoptotic pathway of this molecule.
Acknowledgments
This research project was supported by a grant from the research center of the Center for Female Scientific and Medical Colleges in King Saud University.
References
- Scott DW, Miller WH, Griffin CE. Dermatoses of pet rodents, rabbits and ferrets. Muller Kirke Anim Dermatol 2001: 1415-1458.
- Dagleish MP, Ali Q, Powell RK, Butz D, Woodford MH. Fatal Sarcoptes scabiei infection of blue sheep (Pseudois nayaur) in Pakistan. J Wildl Dis 2007; 43: 512-517.
- Radi ZA. Outbreak of sarcoptic mange and malasseziasis in rabbits (Oryctolagus cuniculus). Comp Med 2004; 54: 434-437.
- Aiello SE. The merck veterinary manual 1998.
- Merck R.The Merck Veterinary manual. Merck Whitehouse Stat (9th edn.) 2005.
- Ahmad L, Khan A, Khan MZ, Hussain I, Mahmood F, Sleemi MK, Lodhi LA, Abdullah I. Toxico-pathological effects of cypermethrin upon male reproductive system in rabbits. Pest Biochem Physiol 2012; 103: 194-201.
- Currie BJ, Harumal P, McKinnon M, Walton SF. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis 2004; 39: e8-12.
- Halley BA, VandenHeuvel WJ, Wislocki PG. Environmental effects of the usage of avermectins in livestock. Vet Parasitol 1993; 48: 109-125.
- OBrien DJ. Treatment of psoroptic mange with reference to epidemiology and history. Vet Parasitol 1999; 83: 177-185.
- Khater HF. Ecosmart biorational insecticides: alternative insect control strategies. Interch Op Acc Publ 2012.
- Khater HF. Prospects of botanical biopesticides in insect pest management. Pharmacolog 2012; 3: 641-56.
- Khater HF. Bioactivity of essential oils as green biopesticides-recent global scenario. Essentials oils II. Rec Prog Med Plants 2013; 37: 151-218.
- Seddiek SA, Khater HF, El-Shorbagy MM, Ali AM. The acaricidal efficacy of aqueous neem extract and ivermectin against S. scabiei var. cuniculi in experimentally infested rabbits. Parasitol Res 2013; 112: 2319-2330.
- Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 2011; 133: 253-260.
- Wagh VD. Propolis: A wonder bees product and its pharmacological potentials. Adv Pharmacol Sci 2013; 2013: 308249.
- Sanchez TA, Garcia PA, Zamora CI, Martinez MA, Valencia VP, Orozco AL. Use of propolis for topical treatment of dermatophytosis in dog. Op J Veter Med 2014; 4: 239.
- Damiani N, Fernandez NJ, Maldonado LM, Alvarez AR, Eguaras MJ, Marcangeli JA. Bioactivity of propolis from different geographical origins on Varroa destructor (Acari: Varroidae). Parasitol Res 2010; 107: 31-37.
- Lekimme M, Mignon B, Tombeux S, Focant C, Marechal F, Losson B. In vitro entomopathogenic activity of Beauveria bassiana against Psoroptes spp. (Acari: Psoroptidae). Veter Parasitol 2006; 139: 196-202.
- Galdhar CN, Khangal PS, Pawar ML, Rasal TD, Digraskar SU. Clinico-biochemical and therapeutic studies on notoedric mange in pet rabbits. J Parasit Dis 2015; 39: 113-116.
- Hallal-Calleros C, Morales-Montor J, Vazquez-Montiel JA, Hoffman KL, Nieto-Rodriguez A, Flores-Perez FI. Hormonal and behavioural changes induced by acute and chronic experimental infestation with Psoroptes cuniculi in the domestic rabbit Oryctolagus cuniculus. Paras Vect 2013; 6: 1-10.
- Bancroft JD, Stevan A, Turner DR. Theory and practice of histological techniques, Churchill Livingstone New York (3rd edn.) 1990; 258.
- Felgenhauer BE. Techniques for preparing crustaceans for scanning electron microscopy. J Crustacean Biol 1987; 7: 71-76.
- Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 1979; 76: 5269-5273.
- Abd Aal El AM. Effect of propolis on mange in rabbits. J Egyptian Veter Med Assoc Parasitol 2005; 1: 293-304.
- Schmahl G, Al-Rasheid KA, Abdel-Ghaffar F, Klimpel S, Mehlhorn H. The efficacy of neem seed extracts. Parasitol Res 2010; 107: 261-269.
- Aboelhadid SM, Mahrous LN, Hashem SA, Abdel-Kafy EM, Miller RJ. In vitro and in vivo effect of Citrus limon essential oil against sarcoptic mange in rabbits. Parasitol Res 2016; 115: 3013-3020.
- Pence DB, Ueckermann E. Sarcoptic manage in wildlife. Rev Sci Tech 2002; 21: 385-398.
- Moharram SA. Experimental pathological studies on pesticides and parasitic infection in rabbits. MVSc Thesis Path Fac Vet Med Zagazig Univ 1996.
- Dimri U, Sharma MC. Effects of sarcoptic mange and its control with oil of Cedrus deodara, Pongamia glabra, Jatropha curcas and benzyl benzoate, both with and without ascorbic acid on growing sheep: epidemiology; assessment of clinical, haematological, cell mediated humoral immune responses and pathology. J Veter Med 2004; 51: 71-78.
- Arlian LG, Runyan RA, Estes SA.Cross infestivity of Sarcoptes scabiei. Journal of the Am Acad Dermatol1984; 10: 979-986.
- Robinson R. Fight the mite, and ditch the itch. Parasitol Today 1985; 1: 140-142.
- Arise RO, Malomo SO. Effects of ivermectin and albendazole on some liver and kidney functions indices in rats. Afr J Biochem Res 2009; 3: 190-197.
- Adu OA, Ladipo MK, Adebiyi OA, Akinfemi A, Igbasan FA. Performance and blood characteristics of pre-pubertal rabbits fed varied levels of dietary rare earth element (REE). World Appl Sci J 2009; 6: 1489-1494.
- Thamsborg SM, Hauge EM. Osteopenia and reduced serum alkaline phosphatase activity in grazing lambs naturally infected with gastrointestinal nematodes. J Compar Pathol 2001; 125: 192-203.
- Jain NC. Schalms veterinary hematology. Lea Febiger 1986.
- Skerratt LF, Middleton D, Beveridge I. Distribution of life cycle stages of Sarcoptes scabiei var wombati and effects of severe mange on common wombats in Victoria. J Wildl Dis 1999; 35: 633-646.
- Eman EE, Abdella OE. Effect of ivermectin and moxidectin on fertility and some biochemical parameters in male rabbits. Egypt J Agric Res 2000; 78: 293-301.
- Magda MM, Fatma ES. Effect of abamectin on some biochemical parameters in rams and bucks infected with mange. Egypt J Agric 2003; 81: 293-303.
- Slanina P, Kuivinen J, Ohlsen C, Ekstrom LG. Ivermectin residues in the edible tissues of swine and cattle: effect of cooking and toxicological evaluation. Food Additives Contaminants 1989; 6: 475-481.
- Muehlenbein MP, Watts DP. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Bio Psycho Soc Med 2010; 4: 1.
- Molony V, Kent JE. Assessment of acute pain in farm animals using behavioral and physiological measurements. J Anim Sci 1997; 75: 266-272.
- Orihuela A, Aguirre V, Hernandez C, Flores-Perez I, Vazquez R. Breaking down the effect of electro-ejaculation on the serum cortisol response, heart and respiratory rates in hair sheep (Ovis aries). J Anim Vet Adv 2009; 8: 1968-1972.