Case Report - Journal of Clinical Ophthalmology (2023)
Updates in Ocular Therapeutics and surgery
Intracranial perineural spread from presumably regressed squamous cell carcinomas.
Bart K Chwalisz1,2, Konstantinos AA Douglas1, Vivian Paraskevi Douglas1*, Joseph F Rizzo1 Frederick A Jakobiec1, Paula Cortes Barrantes1, Michael K Yoon1, Otto Rapalino3
1Department of Ophthalmology, Massachusetts Eye and Ear Harvard Medical School, Boston, United States
2Department of Neurology, Massachusetts Eye and Ear Harvard Medical School, Boston, United States
3Department of Radiology, Massachusetts Eye and Ear Harvard Medical School, Boston, United States
- Corresponding Author:
- Dr. Vivian Paraskevi Douglas
Department of Ophthalmology,
Massachusetts Eye and Ear Harvard Medical School,
Boston,
United States
E-mail: vivianparaskevi_douglas@meei.harvard.edu
Received: 19-Mar-2020, Manuscript No. AACOVS-23-8405; Editor assigned: 24-Mar-2020, PreQC No. AACOVS-23-8405 (PQ); Reviewed: 07-Apr-2020, QC No. AACOVS-23-8405; Revised: 19-July-2023, Manuscript No. AACOVS-23-8405 (R); Published: 16-Aug-2023, DOI: 10.35841/aacovs.7.4.658-662
Citation:Chwalisz BK, Douglas KAA, Douglas VP, et al. Intracranial perineural spread from presumably regressed squamous cell 661 carcinomas. J Clin Ophthalmol 2023;7(3):658-662.
Abstract
Most cases of intracranial Squamous Cell Carcinomas (SCC) result from either metastatic or perineural spread from sinus or other primary head and neck malignancies and Primary Intracranial Squamous Cell Carcinoma (PISCC) occurs very rarely. We present two cases of female patients who were both diagnosed with an intracranial SCC of unknown origin. In both cases, the patients presented with cranial nerve findings and radiological evidence of involvement of the cavernous sinus and adjacent cranial nerves. SCC was confirmed by biopsy and a detailed analysis of pathological and immunohistochemical findings was consistent with perineural spread of a presumed cutaneous SCC despite no visible skin lesion, leading to the conclusion of a regressed superficial epithelial primary tumor. We propose that the explanation that best fits the clinical data of both cases is a vanishing malignant epithelial tumor. We believe that this is the first report of perineural spread of SCC with disappearance of two malignant squamous primary tumors.
Keywords
Squamous cell carcinoma, Intracranial, Unknown origin, Vanishing, Perineural spread.
Introduction
Intracranial SCC mostly occurs either via spread from extracranial sources, primarily in the head and neck. Spread occurs most commonly via a perineural route and only exceptionally by hematogenous metastasis. It can also rarely develop as a Primary Intracranial Squamous Cell Carcinoma (PISCC), mostly associated with an epidermoid or dermoid cyst and commonly found in close proximity to the precursor lesion. Intracranial SCC de novo without a preexisting tumor is an even less common tumor, with only seven cases reported in the literature. The symptoms and signs as well as the radiologic features of intracranial SCC are variable and correlate with the tumor’s size, its location and its distinct components. In all cases of intracranial SCC, there is a guarded prognosis due to the impossibility of a complete surgical resection [1].
We present two unusual cases without any exact parallel in the literature. Both patients developed progressive symptoms of unilateral cranial nerve dysfunction and facial pain. Radiographic findings demonstrated abnormalities in cavernous sinus and surrounding structures. The histopathological and immunohistochemical findings of the left frontal nerve suggested perineural spread of an undetected primary skin SCC at presentation. However, detailed analysis suggested that there was most likely a cutaneous SCC that had been eliminated by the host’s immune system (i.e., cancer immune surveillance). The second patient presented with atypical facial pain associated with decreased sensation in a trigeminal distribution (i.e., anesthesia dolorosa) and ipsilateral facial weakness. She was found to have evidence of intracranial extension of a tumor that appeared to be tracking perineurally from the soft tissues of the oral vestibule or oropharynx but without a visible superficial lesion. On pathologic evaluation, this tumor was of squamous cell origin with glandular features (adenosquamous variant) [2].
The patients were seen in routine clinical practice by one of us (BKC). Both patients gave consent for taking of photographs. Radiological imaging was obtained on a 3T MRI scanner with administration of gadolinium contrast. The following pathological stains were performed for case 1: H and E, von Kossa, Ki-67, S100, cytokeratins. Stains for case 2 included: H and E, cytokeratins, p63, S100, SOX10, Ki-67 [3].
Case Presentation
Clinical history
Case 1: A 54-year-old woman presented with an over 2-year course of progressive left-sided facial pain, diplopia and leftsided facial weakness. The first symptom was a sore spot in the area of the left eyebrow with no visible skin lesion. She subsequently developed numbness in the supraorbital area that progressively transformed into pain in the left V1 distribution that would occur intermittently for 15 minutes to a couple of hours, several times each day. About a year after onset, the left V1 area had become completely anesthetic (but painful).
Additionally, she had lost the ability to wrinkle her forehead on the left side. There was no weakness of eye closure, eye opening or mouth movements. A few months later she developed horizontal binocular diplopia. Magnetic Resonance Imaging (MRI) demonstrated mild fullness and asymmetrical enhancement of the left cavernous sinus. An extensive laboratory workup was unrevealing, including basic metabolic labs, autoimmune serologies, inflammatory markers and four Lumbar Punctures (LPs), which included cytology and flow cytometry analysis. A diagnosis of Tolosa-Hunt syndrome was made and treatment with corticosteroids was initiated. The horizontal diplopia resolved with an oral prednisone taper over several weeks. However, while the patient was still completing the corticosteroid taper, she developed vertical diplopia and left-sided ptosis and mydriasis. A course of IV corticosteroids resolved the ptosis and mydriasis but not the persistent facial pain or diplopia. In addition, the patient complained of superimposed “electric” shooting pains on the same side that spread to involve the left ear and became refractory to weekly IV corticosteroids [4].
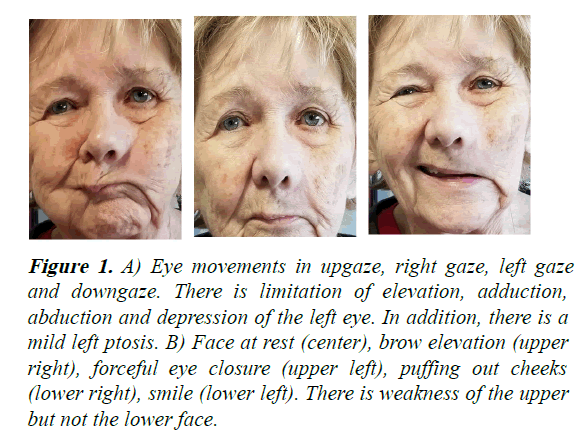
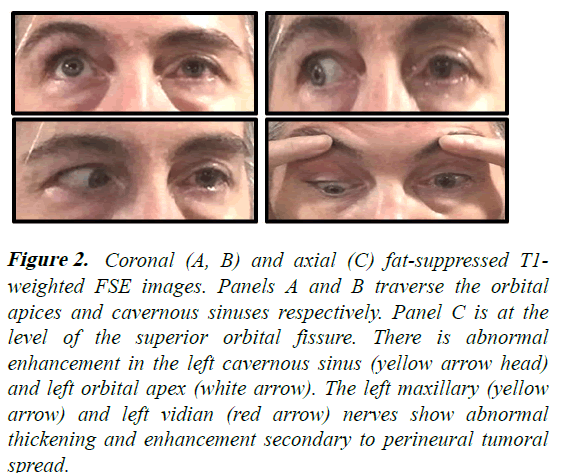
On examination, the left pupil was unreactive and fixed at 7 mm. There was limited elevation, adduction, abduction and depression of the left eye (Figure 1). There was mild ptosis, chemosis and conjunctival injection with corkscrew vessels OS. Fundi were normal. Decreased sensation to pain was present in the distribution of V1 and V2 on the left face. There was weakness of brow elevation, forehead wrinkling and eye closure on the left. A follow-up MRI now with orbital views demonstrated findings that were similar but more extensive than the prior study, with abnormal gadolinium enhancement that involved the left cavernous sinus, superior orbital fissure, orbital apex, V2 at the foramen rotundum, vidian canal and pterygopalatine fossa (Figure 2). PET-CT scan of the body was normal. Biopsy of the left frontal nerve was performed [5].
Figure 1: A) Eye movements in upgaze, right gaze, left gaze and downgaze. There is limitation of elevation, adduction, abduction and depression of the left eye. In addition, there is a mild left ptosis. B) Face at rest (center), brow elevation (upper right), forceful eye closure (upper left), puffing out cheeks (lower right), smile (lower left). There is weakness of the upper but not the lower face.
Figure 2: Coronal (A, B) and axial (C) fat-suppressed T1- weighted FSE images. Panels A and B traverse the orbital apices and cavernous sinuses respectively. Panel C is at the level of the superior orbital fissure. There is abnormal enhancement in the left cavernous sinus (yellow arrow head) and left orbital apex (white arrow). The left maxillary (yellow arrow) and left vidian (red arrow) nerves show abnormal thickening and enhancement secondary to perineural tumoral spread.
Case 2: A 75-year-old woman was referred for assessment of left facial pain. About a year prior to presentation, the patient developed left-sided facial aching. Six to eight weeks later, she also developed shooting pains in that area, numbness and drooping of the left side of her face. The left side of the face and inside of the mouth then became pruritic. An MRI with and without contrast did not show a trigeminal nerve lesion; mild-moderate subcortical white matter hyperintensities were attributed to microvascular changes. A diagnosis of trigeminal neuralgia was made. Due to intolerance to medical therapy, the patient had trigeminal gamma knife surgery. Afterwards, the superimposed neuralgiform attacks became less frequent but more severe. The constant background pain did not improve and in fact continued to worsen over time, as did the facial weakness [6].
We found mild-moderate left facial weakness, decreased sensation in left V2 and left supraorbital distributions, diminished left corneal reflex and mild jaw deviation to the left. Afferent visual function and extraocular movements were normal. Repeat MRI of the brain and face with contrast demonstrated asymmetrical enhancement of the left cavernous sinus, left maxillary nerve and left pterygopalatine fossa as well as abnormal enhancement and thickening of the left infraorbital nerve extending as far as the anterior oral vestibule. CT-PET imaging from the skull base to the thigh showed increased avidity of lower esophagus, which on biopsy was found to be ulceration with associated inflammation but no metaplasia. Biopsy of the nerve branches underlying the oral vestibule was performed [7].
Histopathologic and immunohistochemical findings
Case 1: The anterior orbital biopsy taken superonasally just behind the orbital rim displayed a partially necrotic tumor with focal calcifications (i.e., calcospherites) surrounded by chronic inflammation. The largest tumor cell aggregate in the anterior orbit was elongated and appeared to represent infiltration of a pre-existent nerve. In some areas, tumor cells were deeply eosinophilic, exhibited pleomorphic nuclei and also showed areas of squamous whorls with central keratinization. Smaller stellate neoplastic squamous units were observed with tapering extensions. The von Kossa stain confirmed the presence of calcification. There was a high Ki-67 staining pattern indicating significant mitotic activity. Deeper in the orbit the tumor broke up into small individual cell-units that were widely distributed in a desmoplastic stroma. The S100 stain was mildly positive in the large cellular cluster, suggesting antigenic remnants of a pre-existent nerve. This feature was supported by the observation of viable peripheral tumor with perineural infiltrating cells. Cytokeratin (CK) staining of the cells disclosed positivity for HMW-903 (heavy molecular weight cytokeratin) in the large aggregate and the small cords. This finding pointed toward the tumor’s origin from the epidermis, which exhibits high molecular weight cytokeratins. CK7 of intermediate molecular weight was negative, which would have been expected to be positive if the tumor had arisen from the conjunctival epithelium, a gland or the sinus mucosa. The deep orbital small cords of cells were also CK7 negative [8].
Case 2: The biopsy was dominated by sclerotic stroma with diffuse and dispersed chronic inflammation and scattered lymphoid aggregates without follicular organization. A partially fibrotic nerve with infiltrating small clusters of neoplastic perineural cells also was discovered. On higher power microscopy, some of the clusters appeared to have a small round or slit-like lumina with eosinophilic centers, which could not be clearly ascertained to be mucosubstances or distorted cytoplasm due to crush artifact. CK7 immunostained small infiltrating neoplastic units in the desmoplastic stroma. p63 positivity was identified in the nuclei of all the tumor cells regardless of location, thereby establishing a squamous lineage. Positive S100 and SOX 10 staining highlighted the involvement of the peripheral nerve. The small infiltrating cords of tumor cells in the desmoplastic stroma and those located perineurally were positive for intermediate and light weight cytokeratin 7, CK14. Focal high molecular weight cytokeratin positivity was consistent with the previously noted focal squamous pearls with central keratinization. Ki-67 was markedly positive in the tumor cell nuclei infiltrating the desmoplastic stroma and perineural tissues [9].
Results and Discussion
Our two cases are best classified as an intracranial squamous cell carcinoma of unknown origin. The vast majority of intracranial Squamous Cell Carcinomas (SCC) result from either metastatic or perineural spread from primary head and neck malignancies. In both cases, it appears that trigeminal nerve branches were affected first, causing a clinical picture of anesthesia dolorosa, i.e., numbness and pain mapping to the same trigeminal nerve distribution, a finding that should always raise the suspicion of perineural spread of malignancy. In both cases, there was subsequent spread to other nerves of the cavernous sinus (case 1) and the facial nerve likely via trigemino-facial nerve anastomoses (cases 1 and 2). The most likely such anastomosis in our cases is in the vidian nerve, which combines facial nerve parasympathetics in the greater petrosal nerve with sympathetics from the cavernous internal carotid artery in the lesser petrosal nerve; radiological involvement of the vidian nerve could indeed be demonstrated in case 1 [10].
The clinical picture and radiologic findings of the first case did not resolve whether this tumor originated de novo intracranially or developed initially as SCC of the epidermis or of head and neck mucosa with subsequent disappearance of tumor at its primary site of origin. A similar clinical question arises with respect to the second case, in which the clinical picture and imaging findings demonstrated intracranial cranial nerve involvement but the pathology suggested a mucosal origin probably from the oropharynx. A more distant metastatic source was ruled out by negative PET-CT of body in both cases [11].
Hypothesis 1: Primary intracranial SCC
Primary Intracranial Squamous Cell Carcinoma (PISCC) is a very rare neoplasm that is primarily associated with malignant transformation of epidermoid or dermoid cysts that are lined by squamous epithelium. It can also develop in craniopharyngiomas or other cystic lesions. Most such cysts are found in the cerebellopontine angle and parasellar regions. Intracranial SCC de novo without a preexisting tumor is even less common and to our knowledge, only seven cases have been described in the literature. However, careful consideration of the clinical, radiologic and pathologic features in our cases suggests that these earlier reported cases do not resemble our two cases [12].
Hypothesis 2: Vanishing primary tumor
Disappearance of SCC of the skin occurs occasionally after skin biopsy. Spontaneous disappearance of a cutaneous SCC, as occurred in our first case, has not been reported previously. Our patient’s first symptom was a persistently sore spot just above the left eyebrow, which suggests this localization as the tumor’s primary site. Dermatological examination of the area over the patient’s left eyebrow was negative, but the pathological data demonstrated centripetal perineural spread along a large nerve branch-probably the supraorbital nerve-into the anterior orbit before breaking up into minute compressed cords that elicited a desmoplastic response in the stroma in the posterior orbit [13].
The immunohistochemical finding of positive heavy molecular weight cytokeratin staining and negative intermediate weight Cytokeratin (CK) 7 support our conclusion of a cutaneous origin, since the epidermis is the tissue layer that uniformly expresses the former but not the latter. It is not plausible that the tumor might have arisen in the deep orbit or a surrounding structure or compartment like a sinus or in proximity to the cavernous sinus, since this scenario would have required the unlikely coalescence of the small posterior orbital cords into the large anterior orbital unit that we found, which was reminiscent of a branch of the anterior ophthalmic portion of the trigeminal nerve structure. Furthermore, a mucosal origin would have entailed expression of lower weight cytokeratins rather than heavy weight ones [14].
We similarly hypothesize that in case 2 immune events led to regression of the surface primary epithelial tumor, while survival and progressive growth of a portion of the deeper invasive tumor led to perineural spread into the cranium. Moderately and poorly differentiated squamous cell carcinomas and lymphoepitheliomas are well known to originate in the tonsillar/vestibular region of the oropharynx and in the nasolacrimal duct and lacrimal sac mucosae. These tumors can be either well differentiated or poorly differentiated squamous cell carcinomas. Some lymphoepithelial carcinomas have a rich lymphoid stroma with scattered individual squamous cells (Schmincke variant) or more obvious neoplastic squamous clusters (Regaud variant) that are cytokeratin positive. They may create few local oropharyngeal symptoms or even be overlooked during otolaryngologic and ophthalmic examinations. The tumor cells in our second case were CK7 positive suggesting a mucosal origin; focal higher molecular weight cytokeratin positivity correlated with the occasional formation of squamous pearls with central keratinization. The p63 positivity corroborates the squamous lineage of the tumor and ruled out an adenocarcinoma. There were questionable small lumina could be either evidence of adenosquamous carcinoma or equally likely the result of cellular distortions created by crush artifact.
Cancer immune surveillance and regression
Cancer immune surveillance plays a pivotal role in maintaining cellular homeostasis and inhibiting carcinogenesis. This notion is based on Paul Ehrlich’s proposal, first introduced in 1909, that the host immune system can recognize and destroy transformed cells before they become clinically apparent. The first experimental evidence of immune surveillance was published in 1957 by Frank Macfarlane Burnet, but the concept only gained attention, then acceptance, at the end of 20th century. The immune system uses several effector cells from both its innate and adaptive arms and various cytokines to fight cancerous cells.
Conclusion
An extension of this concept is often referred to as “cancer immunoediting”, which is conceptualized as consisting of three phases: Elimination, equilibrium and escape of neoplastic cells. Immune-mediated elimination of tumors cells is known to occur, but perhaps more typically the elimination of the tumor is only partial, with an “equilibrium” being established between tumor cells and the immune effector cells, which can limit tumor growth. Alternatively, tumor cells may genetically transform to adapt to a changing immunological milieu and create new tumor cell clones that may be less immunogenic and hence more resistant to host immune defense mechanisms, which can facilitate tumor growth and “escape” from immune surveillance.
Summary
This is the first report of two cases of perineural intracranial spread presumed to be from an epithelial SCC that subsequently regressed.
Funding
Department of ophthalmology research fund, Massachusetts Eye and Ear Infirmary. This research did not receive any specific grant from funding agencies in the public, commercial or non-profit sectors.
Competing Interests
The authors have no financial or proprietary interests in the materials mentioned herein. No conflicting relations exist for any author.
Ethics
The study followed the tenets of the declaration of Helsinki.
References
- Fowler BZ, Crocker IR, Johnstone PAS. Perineural spread of cutaneous malignancy to the brain: A review of the literature and five patients treated with stereotactic radiotherapy. Cancer. 2005;103(10):2143-53.
[Crossref] [Google Scholar] [PubMed]
- Oertel J, Piek J, Muller JU, et al. Posterior fossa squamous cell carcinoma due to dedifferentiation of a dermoid cyst in Klippelm feil syndrome. J Neurosurg. 2002;97(5):1244.
[Crossref] [Google Scholar] [PubMed]
- Gao S, Shi X, Wang Y, et al. Malignant transformation of craniopharyngioma: Case report and review of the literature. J Neurooncol. 2011;12:15.
[Crossref] [Google Scholar] [PubMed]
- Garcia CA, McGarry PA, Rodriguez F. Primary intracranial squamous cell carcinoma of the right cerebellopontine angle. J Neurosurg. 1981;54(6):824-8.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Chen Z, Dong Y, et al. Primary intracranial squamous cell carcinoma arising de novo: A case report and review of the literature. World Neurol. 2018;120:372-81.
[Crossref] [Google Scholar] [PubMed]
- Swetter SM, Boldrick JC, Pierre P, et al. Effects of biopsy-induced wound healing on residual basal cell and squamous cell carcinomas: Rate of tumor regression in excisional specimens. J Cutan Pathol. 2003;30(2):139-46.
[Crossref] [Google Scholar] [PubMed]
- Dubey P, Ha CS, Ang KK, et al. Nonnasopharyngeal lymphoepithelioma of the head and neck. Cancer. 1998;82(8):1556-62.
[Crossref] [Google Scholar] [PubMed]
- Hsu WM, Wang AG. Nasopharyngeal carcinoma with orbital invasion. Eye. 2004;18(8):833-8.
[Crossref] [Google Scholar] [PubMed]
- Bernardini FP, Croxatto JO, Orcioni GF, et al. Visual loss secondary to orbital apex invasion as the first manifestation of recurrent nasopharyngeal carcinoma. Ophthal Plast Reconstr Surg. 2009;25(3):248-50.
[Crossref] [Google Scholar] [PubMed]
- Jung H, Park SK, Heo KW, et al. Lymphoepithelial carcinoma of the maxillary sinus with orbital invasion. Auris Nasus Larynx. 2009;36(4):487-90.
[Crossref] [Google Scholar] [PubMed]
- Jakobiec FA, Stagner AM, Rubin PAD. Lymphoepithelial carcinoma of the nasolacrimal duct: Clinical, radiologic and immunopathologic features. Ophthal Plast Reconstr Surg. 2017;33(3S Suppl 1):S18-S21.
[Crossref] [Google Scholar] [PubMed]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137-46.
[Crossref] [Google Scholar] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991-8.
[Crossref] [Google Scholar] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three ES of cancer immunoediting. Annu Rev Immunol. 2004;22:329-60.
[Crossref] [Google Scholar] [PubMed]