Special Issue Article - Current Pediatric Research (2018) Volume 22, Issue 1
Interleukin 2 receptor alfa gene polymorphism in type 1 Egyptian diabetics.
Eman Ahmed Zaky1, Howida Hossny Elgebaly2, Amira A Adly1, Fadia M Atia3, Reem Z Ahmed21Department of Pediatrics, Faculty of Medicine, Ain Shams University, Egypt.
2Department of Medical Studies, Institute of Postgraduate Childhood Studies, Ain Shams University, Egypt.
3Department of Clinical Pathology, Faculty of Medicine, Suez Canal University, Egypt.
- *Corresponding Author:
- Eman Ahmed Zaky
Department of Pediatrics, Faculty of Medicine
Ain Shams University, Egypt
Tel: 00202-22589352
E-mail: emanzaky@med.asu.edu.eg
Accepted date: February 20, 2018
Abstract
Background: Genetic susceptibility has a crucial role in the development of type 1 diabetes mellitus (T1DM). Several genes were found to be involved; one of the most important genes is interleukin 2 receptor α (IL2RA) gene which was found to be associated with T1DM in several countries. Objective: Investigating the potential association of T1DM, its age of onset, and its complications with IL2RA receptor gene polymorphism in an Egyptian sample of type 1 diabetics. Methodology: A case control study was conducted enrolling 100 cases suffering from T1DM and 100 healthy controls of comparable age and sex. Assessment of random blood sugar, HbA1c, and genetic study of IL2RA rs706778 gene polymorphism were done for all studied subjects. Results: The AA and AG genotypes of IL2AR/CD25 rs706778 were significantly more prevalent among studied diabetics compared to controls. Furthermore, the mutant allele A was significantly more prevalent among diabetics while the G allele was significantly more prevalent among controls. Diabetics with AA genotype had significantly older onset of the disease compared to their peers with AG and GG genotypes. On the other hand, GG genotype was significantly more prevalent among diabetics with delayed puberty. Interestingly, AG genotype was significantly more prevalent among enrolled diabetics with neuropathy, retinopathy, micro albuminuria, hypertriglyceridemia, and hypercholesterolemia compared to studied diabetics without such complications. Conclusion: T1DM was significantly associated with IL2RArs706778 polymorphism in the current study, and it appears to be a risk factor for its age of onset and the development of its complications.
Keywords
Type 1 diabetes mellitus (T1DM), HbA1c, Interlukin 2 Receptor Alfa (IL2RA), CD25, Gene polymorphism, Single Nucleotide Polymorphism (SNP), Type 1 diabetes mellitus complications, allelic discrimination.
Introduction
Type 1 Diabetes Mellitus (T1DM) is the most common metabolic and endocrine disorder in children and young adults. Data from the large epidemiological studies all over the world show that on the basis of annual rates, all the increase in the incidence of T1DM is about 3% and about 79,000 children all over the world are estimated to develop it every year [1].
T1DM is an organ-specific, chronic disease [2]; causes of which are still unknown; nevertheless, there is a very strong evidence for the genetic predisposition to the disease and a clear, but still circumstantial, indicator for environmental factors that might lead to the auto-immune destruction of pancreatic B cells, a process in which auto reactive T cells play a pivotal role [3]. Such destruction leads to the development of a type of diabetes with complete dependence on insulin hormonal therapy; T1DM [4].
Many years of progressive auto-immune effects always lead to the clinical age of onset of T1DM. This long prediabetic phase is usually asymptomatic and rarely detected [5]. There is a growing body of evidence to suggest that in T1DM, such auto-immune destruction is the direct endresult of the failure of the immune regulatory system [6].
Rate of T1DM in siblings is about 6% and in monozygotic twins is about 35 to 50% indicating that both genetic and non-genetic environmental risk factors interplay leading to its occurrence. Therefore, T1DM is well known to be a multifactorial disease [7,8]. Genetic susceptibility might also affect body responses to environmental risk factors or physiological pathways (e.g., interferon induced helicase) [9].
Interleukin 2 receptor alpha (IL2RA) gene is a part of a high-affinity IL2 receptors complex system which is expressed on the regulatory T cells (T regs). Regulatory T cells are a subset of T cells that have a strong ability to suppress auto-reactive T cells while it is induced in other T cells [3].
Allelic differences in the region of IL2RA gene accounts for the genetic predisposition implicated in T1DM.The alpha chain of the IL2 receptor complex is a vital molecule that is expressed on T cells upon its activation and on natural T regs at baseline. T regs need IL2 for their growth and survival [10].
Because of such strong evidences of genetic susceptibility for the development of T1DM, the current study was designed to investigate the potential association of T1DM, its age of onset and the development of its complications with IL2RA receptor gene polymorphism in a sample of Egyptian type 1 diabetic children and adolescents compared to their age and gender matched non-diabetic counterparts.
Study Design and Research Methodology
The current case control study was carried out according to the code of ethics of the World Medical Association Declaration of Helsinki (1998) [11] for experiments involving humans. The study protocol was approved by the Ethical Committee of the Scientific Research, Institute of Postgraduate Childhood Studies, Ain Shams University. The study objectives, steps, and the potential benefits and side effects were discussed with caregivers of all included children as well as their children if older than 12 years of age. Consequently, written informed consent was obtained from the legal caregivers of all enrolled children. Confidentiality of all data and test results of all studied children were protected.
Participants
Two hundred children were enrolled in the current study; they were classified into 2 study groups as follows
Group I (Children with Type I DM)
It included 100 diabetic children who were chosen by simple random sampling technique [12] from those under regular follow up at Diabetes Specialized Outpatient Clinic, Children’s Hospital, Ain Shams University, Egypt during the period of the clinical and laboratory part of the study from June 2014 to June 2016. Their ages ranged between 5 and 18 years with a mean age of 13.7 ± 4.1 years. They were 53 females (53%) and 47 males (47%) with a female to male ratio of 1.13. All included cases were diagnosed as Type I DM using C peptide protein test and were under regular treatment with human insulin therapy (mean dose of which was 1.0 ± 0.29 Unit/Kg). The mean age at onset of the disease was 7.1 ± 3.9 years while the mean duration of having the disease was 5.2 ± 4.1 years. Any diabetic child with associated chronic physical illness, handicap, genetic syndrome, and or systemic infections was excluded from the study.
Group II (Controls)
It enrolled 100 physically healthy children aged between 6 to 17 years with a mean age of 13.1 ± 4.3 years; they were 54 (54%) males and 46 females (46%). Controls were selected consecutively from children attending Outpatient Clinic, Children’s Hospital, Ain Shams University, Egypt for either growth monitoring or regular check-up during the period of the clinical and laboratory part of the study from June 2014 to June 2016.
Procedure
All participants were subjected to the following
1. Full history taking laying stress on age at onset of type I DM in enrolled cases, disease duration, details of insulin therapy (type, dose, and frequency), disease presentation and complications, family history, and past history of any concomitant disease, handicap, medications and or operations [13].
2. Thorough clinical examination with special emphasis on vital data, and all body systems examination [14], Weight in Kg, height in meters, and body mass index (BMI=weight in kg/height2 in meters) of all enrolled children and adolescents were assessed and interpreted according to the Egyptian growth charts [15] while staging of puberty was evaluated according to Tanner’s staging [16].
3. Biochemical assessment which included:
• Random blood glucose measurement using colorimetric method; its normal value is less than or equal TO 140 mg/dl [17].
• Determination of glycosylated hemoglobin (HbA1c) using quantitative colorimetric method glycol hemoglobin in whole blood sample; its normal range lies between 4.5 and 6% [18].
• Total blood cholesterol level measurement; its normal value is less than 150 mg/dl [19].
• Blood triglyceride level, its normal value is less than 200 mg/dl [19].
• Quantitative assessment of urinary microalbumin for detection of diabetic nephropathy complication. Normoalbuminuric is defined as 24 h urinary albumin excretion resamples had te of <20 μg/ min while microalbuminuria is defined as albumin excretion rate of 30-300 mg/dl/24 h [17].
4. Genetic study
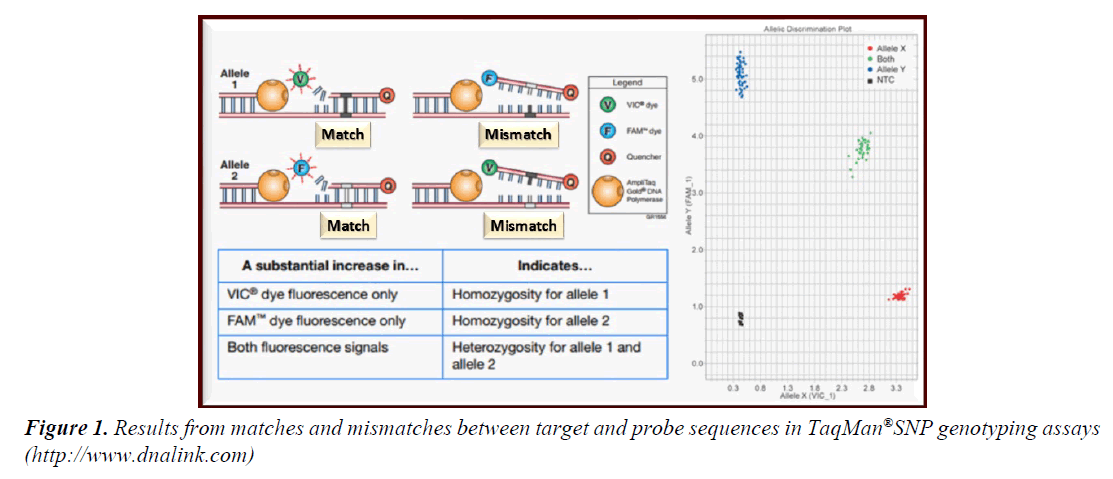
• Molecular identification of IL2AR/CD25 polymorphism was carried out using real time PCR allelic discrimination. The region of interest was intron 1 of IL2AR/CD25 allele on chromosome 10p15 (i.e., rs706778). Allelic discrimination assay is a multiplexed end-point assay which defines single nucleotide sequence variants using TaqMan probe based reagent configuration [20].
• For each sample in allelic discrimination assay, a specific pair of fluorescent dye detectors was used; two TaqMan® MGB (minor groove binder) probes that target a particular single nucleotide polymorphism (SNP) site. One fluorescent dye detector is a perfect match to the wild type (allele 1 in the current study was G allele) while the other fluorescent dye detector was a perfect match to the mutant allele (allele 2 in the current study was A allele) (Figure 1). Accordingly, allelic discrimination classified tested samples into homozygotes (samples with only allele 1 or 2; GG or AA) or heterozygotes (samples with both alleles 1 and 2; AG) [21].
Figure 1: Results from matches and mismatches between target and probe sequences in TaqMan®SNP genotyping assays (http://www.dnalink.com).
5. Data analysis was done using Statistical Package for Social Science (SPSS version 22) [22]. Means and SD were calculated for numerical variables while frequencies were calculated for categorical ones. Chi-square test or Fisher’s exact test were used to compare studied groups concerning categorical variables while Student’s “t” test was used to studied groups regarding measured numerical variables. Z test was used in comparing the frequency distribution of the tested alleles and genotypes between studied groups with calculation of confidence interval (CI) and odd ratio at confidence level (CL)=95%. The obtained results were considered statistically insignificant at “p” values >0.05, significant at “p” values <0.05 and highly significant at “p” values <0.01.
Results
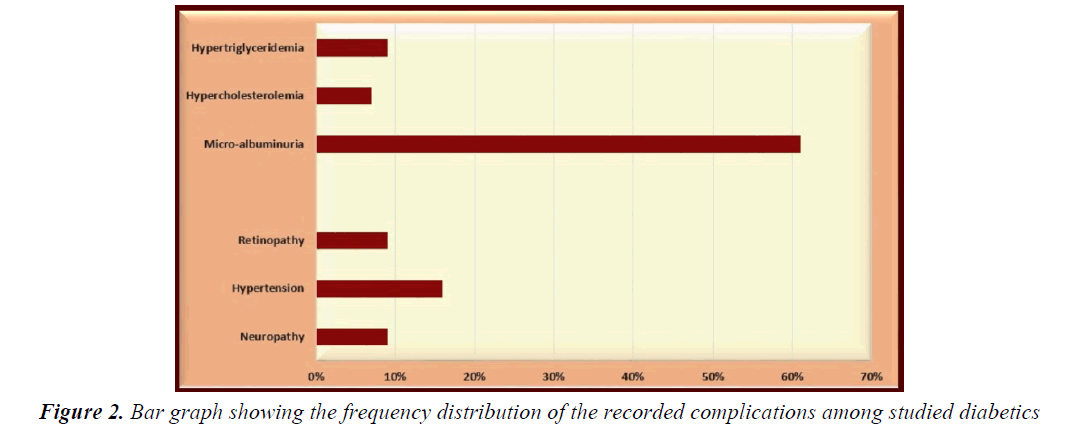
Cases and controls were well matched regarding their mean age (“t”=1.4634, p=0.1465) and gender distribution (X2=0.9801, p=0.3222). Positive family history of DM was significantly more encountered in enrolled diabetics (72%) compared to controls (35%); X2=27.5148, p<0.0001 as well as consanguinity; X2=21.4286, p<0.0001. Body mass index (BMI) was above the 95th percentile in 7% of enrolled diabetics, between the 5th and the 95th in 47%, and below the 5th in 46% of them while all controls had BMI between the 5th and the 95th percentiles (p=0.0071, <0.0001 and <0.0001, respectively) (Table 1). Microalbuminuria was the most frequently recorded complication in studied diabetics (61%) followed by hypertension (16%) while hypercholesterolemia was the least (7%). Other complications included neuropathy, retinopathy, and hypertriglyceridemia (9% for each) (Table 1 and Figure 2). The studied diabetic sample included 74 adolescents (74%) compared to 72 of controls; 22 of them showed delayed puberty (29.73%) compared to none of controls; X2=34.29, p<0.0001 (Table 1).
| Groups\Variables | Group I(Diabetics)No=100 | Group II(Controls)No=100 | X2 | P value |
|---|---|---|---|---|
| No (%) | No (%) | |||
| Gender distribution | ||||
| Males | 47 (47%) | 54 (54%) | 0.9801 | 0.3222 |
| Females | 53 (53%) | 46 (46%) | ||
| Positive family history of DM | 72 (72%) | 35 (35%) | 27.5148 | <0.0001** |
| Positive consanguinity | 45 (45%) | 15 (15%) | 21.4286 | <0.0001** |
| BMI percentile distribution | ||||
| <5th percentile | 46 (46%) | 0 (0%) | 72.1088 | <0.0001** |
| 5th-95th percentile | 47 (47%) | 100 (100%) | 72.1088 | <0.0001** |
| >95th percentile | 7 (7%) | 0 (0%) | 7.2539 | 0.0071** |
| Distribution of encountered complications | ||||
| Neuropathy | 9 (9%) | 0 (0%) | 9.4241 | 0.0021** |
| Hypertension | 16 (16%) | 0 (0%) | 17.3913 | <0.0001** |
| Retinopathy | 9 (9%) | 0 (0%) | 9.4241 | 0.0021** |
| Micro-albuminuria | 61 (61%) | 0 (0%) | 87.7698 | <0.0001** |
| Hypercholesterolemia | 7 (7%) | 0 (0%) | 7.2539 | 0.0071** |
| Hypertriglyceridemia | 9 (9%) | 0 (0%) | 9.4241 | 0.0021** |
| Delayed puberty in adolescents | 22/74 (29.73%) | 0/72 (0%) | 34.29 | <0.0001** |
Chi-square test (X2) was used for statistical comparison between cases and controls. P>0.05=statistically insignificant; p<0.01**=statistically highly significant
Table 1. Statistical comparison between studied diabetics and controls regarding frequency distribution of gender, positive family history of DM, body mass index and encountered complications.
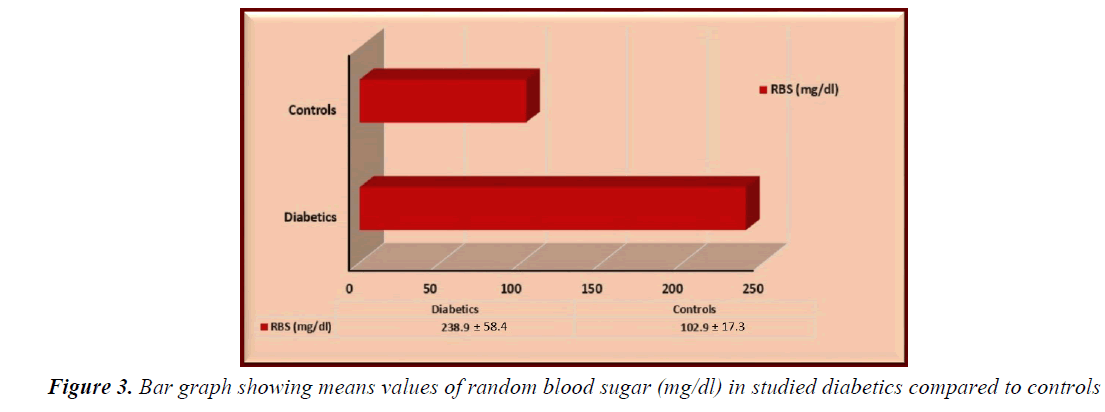
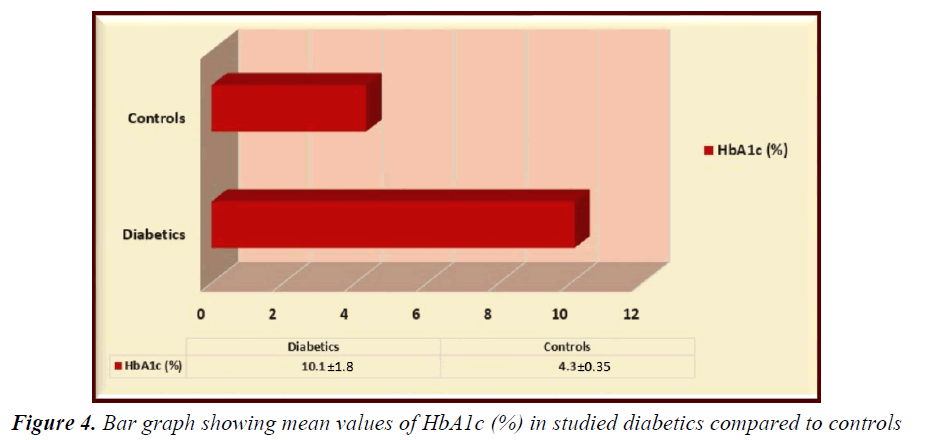
Random blood sugar and HbA1c were significantly higher in enrolled diabetics compared to controls (“t”=22.3286, p<0.0001, “t”=31.6298, p<0.0001, respectively) (Figures 3 and 4).
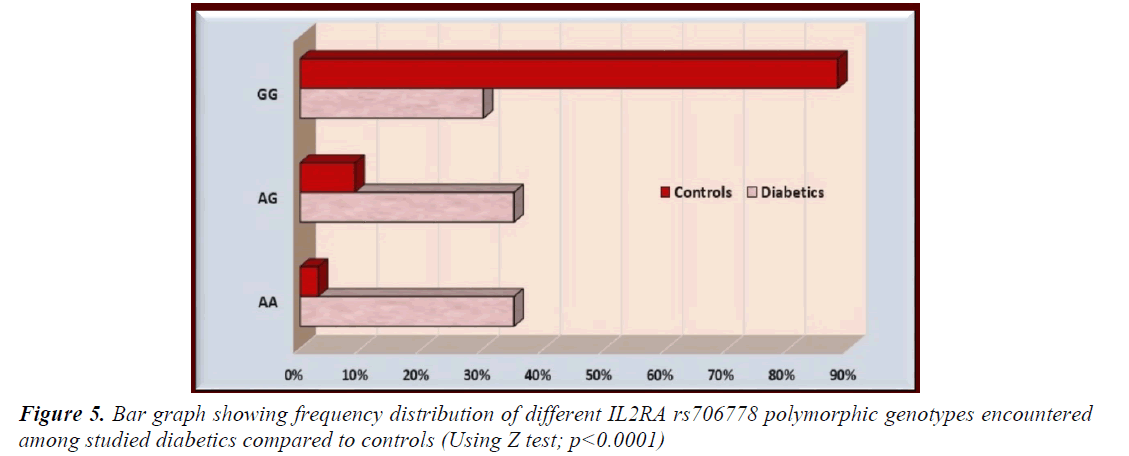
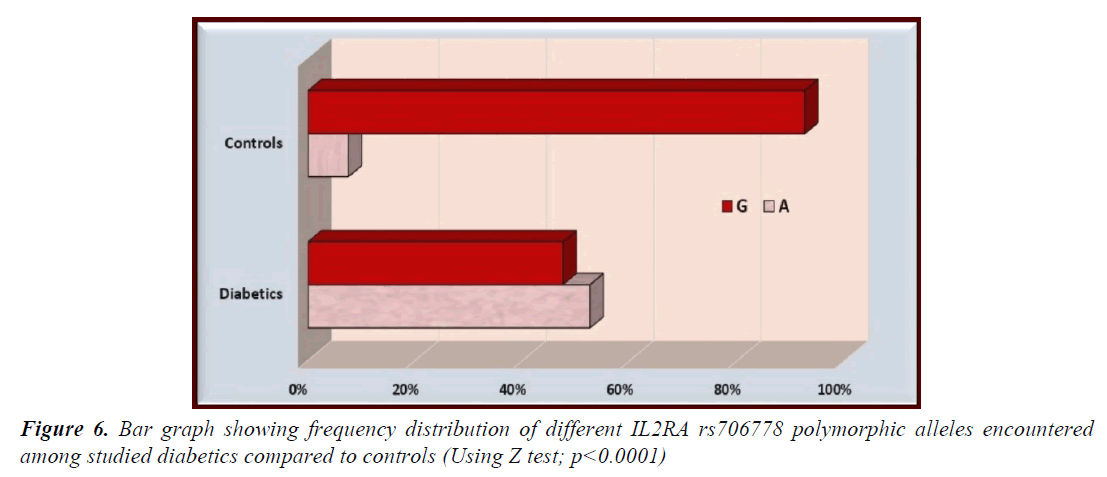
The AA and AG genotypes of IL2AR/CD25 rs706778 were significantly more prevalent among studied diabetics compared to controls while GG genotype was significantly less prevalent in diabetics compared to controls (p<0.0001 for all) (Table 2 and Figure 5). Furthermore, the mutant allele A was significantly more prevalent among diabetics while the G allele was significantly more prevalent among controls (p<0.0001) (Table 3 and Figure 6).
Groups \ Variables |
Group I (Diabetics) No=100 | Group II (Controls) No=100 | Z | CI at CL=95% | Odd Ratio | P value |
|---|---|---|---|---|---|---|
| No (%) | No (%) | |||||
| AA | 35 (35%) | 3 (3%) | 4.589 | 5.1388-58.9863 | 17.4103 | <0.0001** |
| AG | 35 (35%) | 9 (9%) | 4.159 | 2.4495-12.1011 | 5.4444 | <0.0001** |
| GG | 30 (30%) | 88 (88%) | 7.527 | 0.0279-0.1224 | 0.0584 | <0.0001** |
Z test was used for statistical analysis
OR: Odd Ratio; CI: Confidence Interval; CL: Confidence Level
p<0.01**=highly significant.
Table 2. Frequency distribution of different IL2RA rs706778 polymorphic genotypes encountered among studied diabetics compared to controls
AA genotype of IL2AR/CD25 rs706778 polymorphism had significantly older onset of the disease compared to their peers with AG and GG genotypes. On the other hand, GG genotype was significantly more prevalent among diabetics with delayed puberty compared to their counterparts with normal puberty. Disease duration did not show a significant association with any of the encountered genotypes while positive family history of DM and higher BMI were significantly more encountered in diabetics with AA and AG genotypes (Table 4).
| Groups Variables |
Group I (Diabetics) No=200 (100 × 2 alleles) | Group II (Controls) No=200 (100 × 2 alleles) | Z | CI at CL=95% |
O R | “p” |
|---|---|---|---|---|---|---|
| No (%) | No (%) | |||||
| A | 105 (52.5%) | 15 (7.5%) | 8.607 | 7.5195-24.7118 | 13.6316 | <0.0001** |
| G | 95 (47.5%) | 185 (92.5%) |
Z test was used for statistical analysis
OR: Odd Ratio; CI: Confidence Interval;
CL: Confidence Level
p<0.01**=highly significant
Table 3. Frequency distribution of different IL2RA rs706778 allelic polymorphism encountered among studied diabetics compared to controls
| IL2RA rs706778 Polymorphism |
Age at onset <5 years | Age at onset between 5-10 years | Age at onset>10 years | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No=33 | No=46 | No=21 | |||||||||||||||||
| No | % | No | % | No | % | ||||||||||||||
| AA | 10 | 30.30 | 9 | 19.57 | 16 | 76.19 | |||||||||||||
| AG | 12 | 36.36 | 18 | 39.13 | 5 | 23.81 | |||||||||||||
| GG | 11 | 33.33 | 19 | 41.30 | 0 | 0 | |||||||||||||
| Test | Chi2 test=84.7535 | ||||||||||||||||||
| P value | <0.0001** | ||||||||||||||||||
| IL2RA rs706778 Polymorphism |
Puberty | Family History | Disease Duration | ||||||||||||||||
| Normal No=52 |
Delayed No=22 |
Positive No=72 |
Negative No=28 |
<5 years No=47 |
>5 years No=53 |
||||||||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | ||||||||
| AA | 23 | 44.23 | 8 | 36.36 | 30 | 41.67 | 5 | 17.86 | 17 | 36.17 | 19 | 35.85 | |||||||
| AG | 20 | 38.46 | 7 | 31.82 | 28 | 38.89 | 7 | 25.00 | 18 | 38.30 | 16 | 30.19 | |||||||
| GG | 9 | 17.30 | 7 | 31.82 | 14 | 19.44 | 16 | 57.14 | 12 | 25.53 | 18 | 33.96 | |||||||
| Test | Fisher’s exact test | ||||||||||||||||||
| P value | 0.0491* | <0.0001** | 0.3772 | ||||||||||||||||
| IL2RA rs706778 Polymorphism |
BMI<5th percentile No=46 |
BMI=5th-95th percentile No=47 |
BMI>95th percentile No=7 |
||||||||||||||||
| No | % | No | % | No | % | ||||||||||||||
| AA | 7 | 15.22 | 21 | 44.68 | 7 | 100 | |||||||||||||
| AG | 21 | 45.65 | 14 | 29.79 | 0 | 0 | |||||||||||||
| GG | 18 | 39.13 | 12 | 25.53 | 0 | 0 | |||||||||||||
| Test | Chi2 test=149.3339 | ||||||||||||||||||
| P value | <0.0001** | ||||||||||||||||||
Chi-square test (X2)/Fisher’s exact test were used for statistical comparison between different studied diabetic groups
P>0.05=statistically insignificant; p<0.05*=statistically significant, p<0.01**=statistically highly significant .
Table 4. Frequency distribution of the studied polymorphism in different recorded categorical clinical variables among studied diabetics
Interestingly, AG genotype of the studied IL2AR/ CD25 rs706778 polymorphism was significantly more prevalent among enrolled diabetics with neuropathy, retinopathy, micro albuminuria, hypertriglyceridemia, and hypercholesterolemia compared to studied diabetics without such complications. On the other hand, hypertension did not show a significant association with any of the encountered genotypes (Table 5).
| IL2RA rs706778 Polymorphism |
Neuropathy | Retinopathy | Hypertension | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive No=9 |
Negative No=91 |
Positive No=9 |
Negative No=91 |
Positive No=16 |
Negative No=84 |
||||||||||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | ||||||||||
| AA | 3 | 33.33 | 33 | 36.26 | 2 | 22.22 | 33 | 36.26 | 5 | 31.25 | 30 | 35.71 | |||||||||
| AG | 6 | 66.67 | 28 | 30.77 | 5 | 55.56 | 30 | 32.97 | 7 | 43.75 | 28 | 33.33 | |||||||||
| GG | 0 | 0 | 30 | 32.97 | 2 | 22.22 | 28 | 30.77 | 4 | 25.00 | 26 | 30.95 | |||||||||
| Test | Fisher’s exact test | ||||||||||||||||||||
| P value | <0.0001** | 0.0045** | 0.2879 | ||||||||||||||||||
|
IL2RA rs706778 Polymorphism |
Micro-albuminuria | Hypertriglyceridemia | Hypercholesterolemia | ||||||||||||||||||
| Positive No=61 |
Negative No=39 |
Positive No=9 |
Negative No=91 |
Positive No=7 |
Negative No=93 |
||||||||||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | ||||||||||
| AA | 14 | 22.95 | 21 | 53.85 | 0 | 0 | 35 | 38.46 | 2 | 28.57 | 32 | 34.41 | |||||||||
| AG | 26 | 42.62 | 9 | 23.08 | 5 | 55.56 | 30 | 32.97 | 5 | 71.43 | 31 | 33.33 | |||||||||
| GG | 21 | 34.43 | 9 | 23.08 | 4 | 44.44 | 26 | 28.57 | 0 | 0 | 30 | 32.26 | |||||||||
| Test | Fisher’s exact test | ||||||||||||||||||||
| P value | <0.0001** | <0.0001** | <0.0001** | ||||||||||||||||||
Fisher’s exact test was used for statistical comparison between different studied diabetic groups
P>0.05=statistically insignificant, p<0.01**=statistically highly significant.
Table 5. Frequency distribution of the studied polymorphism in different recorded clinical and biochemical complications among studied diabetics
Discussion
The exact etiology of T1DM is still not well defined but there is an agreement that it is a multifactorial disorder where both strong hereditary susceptibility and potentially hazardous environmental factors interact together with subsequent development of the disease [3,23]. The pathogenesis of T1DM entails autoimmune destruction of pancreatic beta cells, the insulin producers, with for life dependency on insulin replacement [24].
Because of the strong evidences of genetic susceptibility for the development of T1DM [25], the current study was designed to investigate the potential association of T1DM, its age of onset, and related complications with IL2AR receptor gene polymorphism in a sample of Egyptian type 1 diabetics.
The mean age of the enrolled diabetics was 13.7 ± 4.1 years while the mean age at onset of the disease was 7.1 ± 3.9 years and that of illness duration was 5.2 ± 4.1 years. In a wide retrospective study done at Mansoura city in Nile Delta, Egypt, the mean age of diabetics were 12 and 10 years in females and males respectively [26] while in Habeeb et al. [27] study, it was 15.3 ± 2.9 years.
In the current study, 33% of enrolled diabetics had a disease onset before the age of 5 years. This frequency of younger age of onset was higher than that recorded by Gyürüs [28] among Hungarian children in the first four years of life which was 22.5%. The recorded high frequency of the early onset of the disease in our study might be due to the interaction between strong genetic susceptibility (72% positive family history) and potential exposure to environmental risk factors that might initiate an auto-immune response or enhance and exaggerate already existing B cell destruction [4].
Female to male ratio in the present study was 1.13 (females=53% and males=47%). Such finding was similar to that of El-Ziny et al. [26] who found that females represented about 55.7% of their total studied diabetics (1600 children with T1DM during the period from 1994 to 2011). In general, there is no consensus about gender distribution in DM. Wandell et al. [29] reported no gender difference in diabetic children from 0 to14 years of age but in subjects aged from 15 to 39 years males were more up to 2 folds than females.
Positive family history of the disease is considered as a risk factor for the development of T1DM [23]. Family history of DM was recorded in 72% of enrolled diabetics in the present study that was significantly higher compared to controls p<0.0001. Such finding was also much higher than that obtained by Gyürüs [28] which was 12.5% and that reported by Tawfik et al. [30] in their study in one of the delta governorates in Egypt (32.5%). On the other hand, consanguinity is a long standing habit in Egypt and is considered as a risk factor for the development of multifactorial disorders including DM [31]. Consanguinity rate in diabetics enrolled in the current study (45%) was also significantly higher compared to controls (15%); p<0.0001.
Mean random blood sugar (RBS) of our studied diabetics was 238.9 ± 58.4 mg/dl while the mean HbA1c was 10.1 ± 1.8%. Those means are higher than those recorded by other investigators in other countries. Abourazzak et al. [32] found that the mean RBS in a French sample that included 378 diabetic children was 171 mg/dl while Nansel et al. [33] found that the mean HbA1c was about 8.4% among 340 American diabetic children. On the other hand, the mean HbA1c in another Egyptian study conducted by Tawfik et al. [30] and included 80 diabetic children at Menoufya governorate was closer to our study (9.4%). Such variation in the level of control of DM in different countries might reflect a spectrum of socio-cultural and environmental factors that might interfere with the patient compliance to dietary control and insulin therapy as well as the lack of awareness of how serious the complications of poorly controlled diabetes.
BMI is related mainly to the degree of control of DM. Bad control of metabolism could result in ketoacidosis and its complications, hypoglycemia, and poor growth [4]. In spite of that T1DM is an insulin-deficient disorder, features of insulin resistance are increasingly common [34]. Weight gain that occurs due to intensive insulin therapy in patients with T1DM could be attributed to decreased glycosuria and improved energy utilization and better dietary flexibility [33].
In the current study, body mass index (BMI) was above the 95th percentile in 7% of enrolled diabetics, between the 5th and the 95th in 47% and below the 5th in 46% of them while all controls had BMI between the 5th and the 95th percentiles (p=0.0071, <0.0001 and <0.0001, respectively). This result is different from that obtained by Nansel et al [33] who found overweight in 35% of their diabetic children and Habeeb et al. [27] who reported BMI˃85% in 40% of their studied sample. The high frequency of low BMI among our cases could be explained by poor control of diabetes and less compliance to insulin therapy as proved by the recorded high mean values of RBS and HbA1c.
Microalbuminuria was the most frequently recorded complication in our studied diabetics (61%) followed by hypertension (16%) while hypercholesterolemia was the least (7%). Other complications included neuropathy, retinopathy, and hypertriglyceridemia (9% for each). Demirel et al. [35] conducted a cross-sectional study on children with T1DM who were over 11 years of age or had diabetes duration of two years and found that the frequency of neuropathy and micro albuminuria to be about 0.6% and 16.1%. None of their patients had diabetic retinopathy. Hypertension and dyslipidemia rates were 12.3% and 30.3%, respectively. The differences between our findings and those of Demirel et al. [35] could be due to the different mean age and disease duration of their study compared to ours.
In general, nephropathy is the commonest well known micro vascular complication in adolescents with T1DM and the one with the earliest onset. Cho et al. [36] and Harjutsalo et al. [37] reported micro albuminuria rates of 3% and 25.4%, respectively which were found to be dependent on disease duration. The reported rate of the same complication was much higher in our study (61%) and could be attributed to poorer control of the disease in our enrolled diabetics compared to theirs.
On the other hand, the current studied diabetic sample included 74 adolescents (74%) compared to 72 of controls; 22 of them showed delayed puberty (29.73%) compared to none of controls; X2=34.29, p<0.0001.The same results were obtained also by Elamin et al. [38] and Rohrer et al. [39] who reported similar delay in the mean age of puberty in adolescents with T1DM in Sudan and Germany. Elamin et al. [38] related that delay in puberty in T1DM to the long disease duration and high concentration of HbA1c; similar explanation is also applicable in our study.
The relation between T1DM and IL2RA locus was identified by Vella et al. [20] using a multi-locus genetically associated tests. They employed a tag single nucleotide polymorphism (SNP) approach in a large sample of T1DM (7,457 patients and controls and 725 multiplex families) and documented a strong statistical association of a locus in IL2RA region and the disease. Such finding was replicated by Qu et al. [40] who found a highly significant association between 2 intronic SNPs (rs706778 and rs3118470) in the 5´ terminal of IL2RA gene and T1DM while other 10 studied SNPs failed to show similar association. Similarly, Kawasaki et al. [41] had found a significant association of rs706778 and also rs3118470 with an increased risk of the T1DM unlike 2 other studied SNPs. T1D Genetics Consortium (T1DGC), in a trial to confirm and finally map the reported associations between the different SNPs and T1DM collected DNA samples from affected sib pair (ASP) families and genotyped polymorphic markers. Such mapping documented the significant association betweenrs706778 polymorphism and T1DM although it was not the most associated SNP with the disease [42].
In the current study, the AA and AG genotypes of IL2AR/ CD25 rs706778 were significantly more prevalent among studied diabetics compared to controls (OR=17.4103, 5.4444, respectively); P<0.0001 for both. Furthermore, the mutant allele A was significantly more prevalent among diabetics while the G allele was significantly more prevalent among controls (OR=13.6316; P<0.0001). Different studies in Europe found similar relations of different degrees between T1DM and IL2RA polymorphisms including Belgium [43], Finland [44] and Poland [45] with ORs =1.59, 1.5 and 1.3, respectively. Furthermore, Kawasaki et al. [41] found that “A” allele of IL2AR rs706778 to be significantly higher in patients with T1DM with an odd Ratio of 1.32. Relation of these alleles is secondary to the association of HLA-DR alleles of the HLA class II with T1DM in many ethnic groups [7].
In Egypt, two studies evaluated the distribution of CTLA- 4 (+49 A/G) gene variants and their association with the clinical and laboratory findings in Egyptian diabetic children. Tawfik et al. [30] found AA, AG and GG genotypes of CTLA-4 polymorphism in 42.5%, 52.5% and 5%, respectively of their sample with T1DM with insignificant differences compared to controls. On the other hand, Arafa et al. [46] found AA genotype in 20%, AG in 65% and GG in 15% with significant association between AG polymorphism and T1DM. To the best knowledge of the authors of the present study, this is the first study in the Middle East that investigated the potential association of T1DM, its age of onset, and related complications with IL2ARrs706778 polymorphism.
Diabetics with AA genotype in the current study had significantly older onset of the disease compared to their peers with AG and GG genotypes. Those findings are different from the results obtained by Kawasaki et al. [41] and Fichna et al. [45] who excluded any association between age at onset of T1DM and IL2RA polymorphism. On the other hand, Aminkeng et al. [43] found that children with polymorphism in the IL2RA gene have younger age of onset than other diabetic patients but without any statistical significance; taking in consideration the fact that Aminkeng et al. [43] considered the early onset to be before 15 years of age. IL2RA gene is expressed in different cell types including β cells of pancreatic islets where its role as an IFN-γ down-regulator is protective against cytokine mediated apoptosis. Such protective function of the gene if weakened or lost could affect the age of onset of T1DM [47].
On the other hand, GG genotype was significantly more prevalent among our diabetics with delayed puberty while positive family history of DM was significantly more encountered in diabetics with AA and AG genotypes. It is well known that genetic factors has a pivotal key role in disease development, for instance, the risk for diabetes in patient siblings is much higher than its risk in the general population [48]. There is only one available study that evaluated and disproved the association of IL2RA gene polymorphism with family history of T1DM [43].
Łuczy´nski et al. [49] found that overweight and obesity to be associated with gene SNP in both control and diabetic children but the effect was less in diabetic children. In contrast, Aminkeng et al. [43] reported insignificant association between genotypes and BMI. Furthermore, Nansel et al. [33] found similar prevalence of overweight and obesity in the general United States youths and diabetics. In the current study, higher BMI was significantly more encountered in enrolled diabetics with AA and AG genotypes.
In general, DM is associated with high risk of cardiovascular disorders (CVD) due to shared pathological pathways of inflammation and endothelial dysfunction [50] but in T1DM, it is not common to develop CVD without kidney impairment [34]. On the other hand, some researchers found significant association between retinopathy or nephropathy and HLA class I or II but other researchers have failed to show such association. Meanwhile, MHC Class II genes are considered as significant protective elements against one or more of the micro vascular complications of DM [51]. Diabetic nephropathy runs in families and retinopathy is another damaging micro vascular complication that has inherited causes [52].
Finally, Monti et al. [53] found that DM complications (neuropathy, retinopathy, and nephropathy) were more common in patients with family history of DM compared to those without such history suggesting a genetic basis for these complications. Interestingly, AG genotype was significantly more prevalent among our studied diabetics with neuropathy, retinopathy, microalbuminuria, hypertriglyceridemia and hypercholesterolemia compared to studied diabetics without such complications.
Conclusion
T1DM was significantly associated with IL2RArs706778 polymorphism in the current study, and it appears to be a risk factor for its age of onset and the development of its complications.
Study Limitations and Future Scope
The current study enrolled 100 type 1 diabetics and 100 controls of comparable age and sex which might be considered as inadequate sample to generalize its findings. Also the value of the statistical significance of the reported ORs of the studied genotypes and alleles in our diabetics compared to controls is compromised by the relatively wide confidence interval. Accordingly, it is recommended to conduct a future nationwide screening of IL2RArs706778 polymorphism in Egyptian diabetics as well as healthy controls of comparable age and sex focusing on those with positive family history of the disease to explore if the results of our novel study could be generalized or not. Finally, it is wise to apply strict monitoring of HbA1c with implementation of rigid dietary control and proper adjustment of insulin dosage for our diabetics to attain a better degree of disease control and reduce the prevalence of its serious complications among them.
Acknowledgement
The authors are grateful for the enrolled children and their caregivers; without their participation, this study would not have been accomplished.
References
- IDF. IDF Diabetes Atlas. 6th edn, International Diabetes Federation 2013; 19: 22-26.
- Sohrabin, Shekari KM, Mansoori DS. Evaluation of association between HLA class II DR4-DQ8 haplotype and type I diabetes mellitus in children of east Azerbaijan State of Iran. Adv Pharm Bull 2015; 5: 137-140.
- Belot MP, Fradin D, Mai N, et al. CpG methylation changes within the IL2RA promotor in type I diabetes of childhood onset. PLoS One 2013; 8: e68093.
- Patterson C, Guariguata L, Dahlquist G, et al. Diabetes in the young - A global view and worldwide estimates of numbers of children with type I diabetes. Diabetes Res Clin Pract 2014; 103: 161-175.
- Yamunadevi A, Basandi PS, Madhushankari GS, et al. Morphological alterations in the dentition of type I diabetes mellitus patients. J Pharm. Bioallied Sci 2014; 6: S122-126.
- Garg G, Tyler JR, Yang JH, et al. Type I diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+regulatory T cell function. J Immunol 2012; 188: 4644-4653.
- Sayad A, Akbari MT, Pajouhi M, et al. Investigation of the role of gender on the HLA-DRB1 and DQB1. Association with type I diabetes mellitus in Iranian patients. Cell J 2013; 15: 108-115.
- Park Y. Type 1 diabetes genetic susceptibility markers and their functional implications. J Genet Med 2014; 11: 1-10.
- Winkler C, Lauber C, Adler K, et al. An interferon induced helicase (IFIHI) gene polymorphism associates with different rates of progression from autoimmunity to type I diabetes. Diabetes 2011; 60: 685-690.
- Van-Belle TL, Coppieters KT, von Herrath MG. Type I diabetes: Etiology, immunology and therapeutic strategies. Physiol Rev 2011; 91: 79-118.
- The World Medical Association Declaration of Helsinki. The WMA declaration of Helsinki 1960 with recommendations on biomedical research on human subjects. Chirurgia (Bucur) 1998; 93: 138-140.
- Starnes DS, Yates DS, Moore DS. The Practice of Statistics for AP, 4th edn, Freeman WH and Company, NY. 2011.
- Buder G, Kirk J. Handbook of Clinical Pediatric Endocrinology, 2nd edn: Brook CGD, Dattani MT, Wiley-Blackwell, UK. 2012.
- Lissauer T, Clayden G. Illustrated Textbook of Paediatrics, 3rd edn, Mosby, US. 2007; 9-22.
- Ghalli I, Salah N, Hussien F, et al., (2008) Egyptian growth curves for infants, children, and adolescents. In: Crecere nel Mondo. (Eds) Satorio A, Buckler JMH, and Marazzi N. Quoted from Proceedings of the 1st National Congress for Egyptian Growth Curves, Cairo University, 2003. Ferring Publisher, Italy.
- Tanner JW, Davis PW. Clinical longitudinal standards for height velocity for North American children. J Pediatr 1978; 107: 317.
- Sacks DB, Bruns DE, Goldstein DE, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002; 48: 436-472.
- Al-Ansary L, Farmer A, Hirst J, et al. Point of care testing for Hb A1c in the management of diabetes: A systematic review and metanalysis. Clin Chem 2011; 57: 568-576.
- Vakkilainen J, Steiner G, Ansquer JC, et al. DAIS Group: Relationships between low density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: The diabetes atherosclerosis intervention study (DAIS). Circulation 2003; 107: 1733-1737.
- Vella A, Cooper JD, Lowe CE, et al. Localization of a type I diabetes locus in the IL2RA/CD25 region by use of tag single nucleotide polymorphisms. Am J Hum Genet 2005; 76666: 773-779.
- Osgood-McWeeney D, Galluzzi JR, Ordovas JM. Allelic discrimination for single nucleotide polymorphisms in the human scavenger receptor class B type 1 gene locus using fluorescent probes. Clin Chem 2000; 46: 118-119.
- Statistical Package for Social Science. SPSS, program version 22. SPSS for windows. SPSS Inc. Chicago. 2013.
- Chiang JL, Kirkman MS, Laffel LMB, et al. Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care 2014; 37: 2034-2054.
- Gero L. Type 1 diabetes mellitus: pathogenesis, symptoms and therapy. Orv Hetil 2010; 151: 533-539.
- Anjos S, Polychronakos C. Mechanisms of genetic susceptibility to type 1 diabetes: Beyond HLA. Mol Genet Metab 2004; 81:187-195.
- El-Ziny MA, Salem NA, El-Hawary AK, et al. Epidemiology of childhood type 1 diabetes mellitus in Nile Delta, northern Egypt - A retrospective study. J Clin Res Pediatr Endocrinol 2014; 6: 9-15.
- Habeeb NM, Youssef OI, Saab AA, et al. Adiponectin as a marker of complications in type 1 diabetes. Indian Pediatr 2012; 49: 277-280.
- Gyürüs EK. Epidemiology of type 1 diabetes in children in Hungary. PhD Thesis. Department of Paediatrics. Faculty of Medicine. University of Pécs 2011; 26-50.
- Wandell PE, Carlsson AC. Time trends and gender differences in incidence and prevalence of type 1 diabetes in Sweden. Curr Diabetes Rev 2013; 9: 342-349.
- Tawfik MA, Abou El-Ella SS, Abou Zona ZS. Genetic study of diabetic children. Master Thesis, Faculty of Medicine, M