Research Article - Biomedical Research (2017) Volume 28, Issue 10
Initial experience of partial cystectomy with transurethral laparoscopic technique for bladder carcinoma
Congxiang Han*, Jinyu Li, Xiacong Lin, Li Zhao, Zhongying Yu, Wei Li and Weijie Xu
Department of Urology, 175th Hospital of PLA, Xiamen University Affiliated Southeast Hospital Zhangzhou, Fujian, PR China
- *Corresponding Author:
- Congxiang Han
Department of Urology
175th Hospital of PLA
Xiamen University Affiliated Southeast Hospital
PR China
Accepted on February 22, 2017
Abstract
Background: To establish a new technique for en bloc resection of bladder carcinoma using a transurethral laparoscopic technique.
Methods: Nine patients with primary bladder tumors underwent Partial Cystectomy with Transurethral Laparoscopic Technique (PCTLT). A 22 Fr Peel-Away sheath was inserted into the urethra for endoscopy, fluid discharge, and tumor removal. A 20.8 Fr nephroscope was used for observation. The operating channel of the nephroscope was utilized for appliance placement and perfusion. PKSTM Plasma-CutTM was used for tumor excision and haemostasis. Ultra-thin scissors were used for tumor separation and cutting. A laparoscope-like technique was utilized to perform en bloc resection of the tumor and the entire muscle layer at the base, as well as the surrounding tissues within 0.5 cm of the tumors. Intraoperative hemorrhage, operation time, obturator nerve reflex and postoperative complications were observed, and pathological stage and postoperative follow-up were performed.
Results: This technique was successful in all patients. No patients experienced bladder perforation or required open surgery. Perioperative blood loss was minimal and obturator nerve reflex was not obvious. Pathological stage of the tumors was Ta or T1. Patients were followed for 4 to 8 months postoperatively with no recurrence at the resection site and other site.
Conclusions: Preliminary clinical results have demonstrated that PCTLT is a safe and effective treatment of Non-Muscle Invasive Bladder Cancer (NMIBC), which contributes to the pathological staging and clinical treatment of the bladder carcinoma, whereas middle- and long-term clinical efficacy remains to be elucidated.
Keywords
Urinary bladder, Carcinoma, Cystectomy, Laparoscopic techniqueTransurethral operation.
Introduction
Bladder carcinoma is a common urinary system tumor that poses a serious threat to human health [1]. Approximately 75% to 85% of bladder carcinoma is restricted to the mucosal or sub-mucosal layer [2], and all guidelines recommend TURBT as the gold standard for the initial diagnosis and treatment of Non-Muscle Invasive Bladder Cancer (NMIBC) [3-5]. The purpose of TURBT is complete removal of all visible tumors and acquisition of tissue for pathologic evaluation. Achievement of the above purposes is no so easy for an inexperienced surgeon [6] and for NMIBC, the probability of recurrence after 1 year ranges from 15% to 70% [7]. Along with the development of endoscopic technique and equipment, surgical interventions for NMIBC have also advanced. As emerging surgical options, transurethral en bloc resection of bladder tumors and transurethral partial cystectomy are superior in the diagnosis, staging, and treatment of bladder tumors compared with other surgical interventions [8-12]. To the best of our knowledge, we established a new method of en bloc resection of bladder carcinoma including the entire detrusor muscular layers at the base and the surrounding tissue using a transurethral laparoscopic technique to complete partial cystectomy in 9 patients with newly diagnosed NMIBC.
Materials and Methods
Between August 2015 and December 2015, 9 patients newly diagnosed with primary NMIBC presented to our hospital and underwent Partial Cystectomy with Transurethral Laparoscopic Technique (PCTLT). The mean patient age was (58.9 ± 10.4 years), (range: 42 to 72 years), 7 male and 2 female. Preoperatively, all 9 patients underwent color Doppler ultrasound to examine the urinary system, as well as cystoscopy and pelvic CT or MRI. Color Doppler ultrasound detected no tumors in the upper urinary tract of any patients. Pelvic CT or MRI found no lymph node enlargement. According to imaging findings, all bladder tumors were single masses and classified within the T2 stage. Five tumors were behind the delta region and lateral wall, 1 was in the bladder delta region, 2 were in the anterior wall, and 1 was in the dome. Mean tumor diameter was 1.7 ± 0.7 cm (range: 1 to 3 cm).
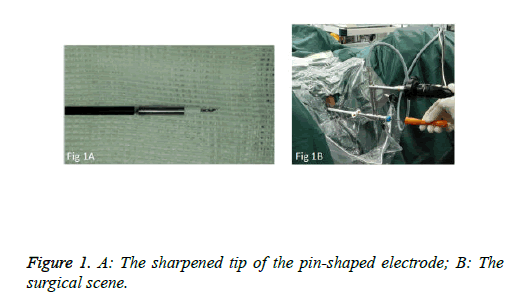
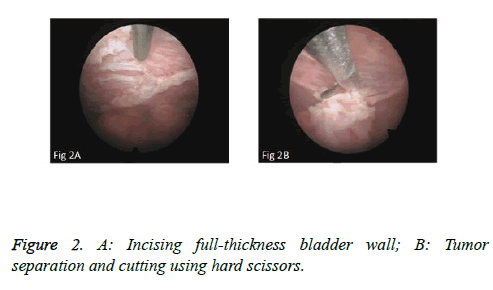
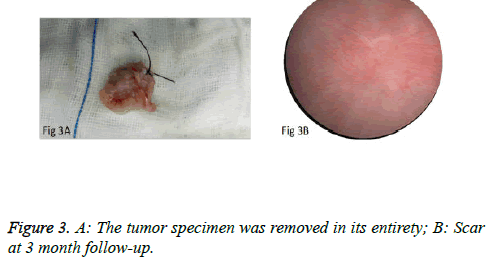
We used PKSTM Plasma-CutTM (REF 714510, 5 Fr Electrode/33 cm, GYRUS ACMI, Made in USA), a plasma needle electrode, as our electronic cutting device. The tip of the pin-shaped electrode was sharpened to match the electronic plasma cutting system (Figure 1A). Cutting power was set at 200 W and the electrocoagulation power was 100W. In addition, 22Fr fascia dilator, 22 Fr peel-away sheath, 20.8 Fr nephroscope (Wolf), 6 Fr scissor, and 6 Fr clamp were prepared. Continuous epidural anesthesia or general anesthesia was administered, and patients were in a lithotomy position. Contralateral lower extremity of the affected side was slightly abduced. Initially, the peel-away sheath was inserted near the bulbar urethra, guided by a fascia dilator and rinsed with normal saline. The nephroscope was placed to observe the presence of urethra under direct vision guided by a television surveillance system. Then the peel-away sheath was inserted into the bladder. Normal saline flowed in through the nephroscope operation channel and flowed out through the gap between the Peel-Away sheath and the nephroscope to maintain a clear visual field (Figure 1B). The site, size, number and morphology of the tumors and the relationship between the tumors and ureteral orifice were observed under nephroscope. A ring-shaped mark was created approximately 0.5 to 1.0 cm from the tumor root and the tumor blood vessels were blocked. The mucosa, sub-mucosa, and muscular layer were cut from the fibrous connective tissues in the bladder outer layer (Figure 2A). Hard scissors were used for tumor separation and cutting (Figure 2B). Cutting procedures were performed from the peripheral to the center. Peripheral blood vessels were cut off, and then the entire detrusor muscular layer at the base of the tumor was separated from the loose layers of the muscular layer and fibrous tissues. At the presence of blood vessels at the base, electrocoagulation was initially conducted, followed by electronic cutting. The visual field of interruption was distant from peripheral tissues until the entire tumor and muscular layer tissues at the base were completely resected. The tumor specimens were removed in one piece from the peel-away sheath using a 6 Fr clamp, and the tumors consisted of the entire muscular layer (Figure 3A). For patients with large tumors, which cannot be removed in its entirety from the peel-away sheath, tumors should be cut into several pieces prior to complete interruption. Under endoscope, the bladder cavity was irrigated with normal saline to eliminate the tumor cells exfoliated from the bladder, followed by repeated observation and thorough haemostasis. Intraoperatively, the Foley catheter was indwelt, and 50 ml of 5% glucose solution and 30 mg of pirarubicin (THP) were injected and maintained in the bladder for 30 minutes. Postoperatively, conventional irrigation of the bladder was performed for 6 consecutive hours; the catheter was indwelt for 7 days and removed after repeated intravesical chemotherapy.
The postoperative intravesical instillation regimen started 1 week after surgery, and consisted of 30 mg of THP weekly for 8 weeks, followed by monthly maintenance for 1 year postoperatively. A surveillance cystoscopy was performed 1 month after surgery and then every 3 months for the first 2 years, and every 6 months thereafter.
Results
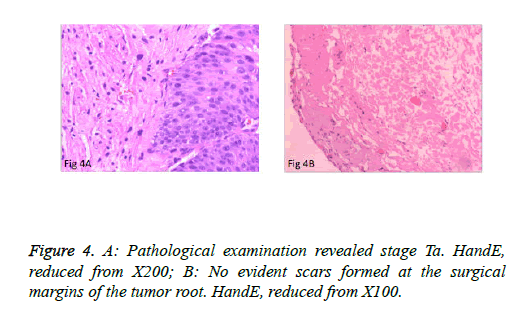
The surgeries of 5 patients with tumors behind the delta region and lateral wall were conducted under general anesthesia or under continuous epidural anesthesia. PCTLT was successful and uneventful in all 9 patients, and none changed to TURBT or open surgery. Obturator nerve reflex intraoperatively was not obvious, and no patients experienced bladder perforation or obvious fluid extravasation. Mean operation time was 15.2 ± 6.0 minutes (range: 8 to 25 minutes) per patient. Perioperative blood loss was minimal. The mean indwelling time of the urethral Foley catheter was 7 days. Surgical wounds were completely healed in all cases at postoperative 3 months and no incidence of vesical diverticulum was detected (Figure 3B). Pathological examination of the specimens revealed the muscular layer in 9 specimens and low-grade urothelial carcinoma, including stage T1 in 2 cases and stage Ta in 7 cases (Figure 4A). No obvious scars formed at the surgical margins of the base of the tumor (Figure 4B). Patients were monitored for 5.9 ± 1.4 months (4-8 months). There was no recurrence at the resection site or the other site during early follow-up.
Discussion
TURBT is one of the most commonly performed procedures by practicing urologists for the diagnosis, staging, and treatment of NMIBC [13]. TURBT is applied for histopathological diagnosis, identifying tumor staging, determining risk factors related to clinical prognosis, and complete resection of the tumors [2]. However, the fragmental excision adopted in conventional TURBT breaches the tumorfree principle. In addition, TURBT has certain limitations, such as hemorrhage, obturator nerve reflex, uncontrolled electrocutting depth, wound scars, high rate of residual tumors, inaccurate pathological staging, high short- and long-term recurrence rate, and so on [14-16]. Therefore, we have to reevaluate and choose the optimal surgical approach for bladder carcinoma. ReTUR (Repeat Transurethral Resection) has been considered a vital supplement to the treatment of bladder tumors. It not only provides accurate pathological evidence for clinical diagnosis [17], but it also excises the latent or remnant tumors after surgery [18]. Nevertheless, repeated operations are likely to increase surgical risk and expense. The optimal strategy is to completely remove the bladder tumor during the first operation and provide pathological specimens for accurate pathological diagnosis.
In recent years, along with the development of endoscopic techniques, certain progresses have been achieved in transurethral en bloc resection of bladder tumor and transurethral partial cystectomy. This technique can completely remove the tumor; decrease the risk of planting exfoliated tumor cells, and supply comparatively intact tumor basal muscular layer and tumor margin, thereby guaranteeing the accuracy of pathological staging [19]. Furthermore, there is a significant reduction in the recurrence rate [20], and the rate of progression was also relatively less with en-bloc TURBT [8,21]. It is a challenge to implement en bloc resection of bladder tumor by conventional TURBT. Some scholars attempted to improve the clinical efficacy of traditional TURBT by modifying surgical appliances, equipment, and approach. Kawada [22] reported a 2.5 cm papillary sessile bladder tumor was treated via a rotational resection technique with a new arched electrode to replace longitudinal resection using a conventional loop electrode. Ukai [23] used a short curved needle electrode to make a circular incision around and level incisions underneath the tumor, and for tumor retrieval. Yang [8] established a new technique to vaporize and incise the full-thickness bladder wall and peel off entire muscular layers with a 2 μm continuous wave laser to treat bladder carcinoma. These techniques have achieved high clinical efficacy in the pre-clinical settings, however, the requirements for surgical equipment and technique are relatively demanding.
We used the peel-away sheath, indwelt inside the urethra, as the channel for the appliance, the nephroscope as observation access, the plasma band-form needle electrode as the electrocutting tool, and normal saline as the irrigating solution. Electro-cutting and electrocoagulation were performed through the operating channel of the nephroscope. During the procedure, hard scissors were used for tumor separation and resection through the operating channel of the nephroscope, which allowed us to properly expose the space between the bladder muscular layer and the connective tissues outside the muscular layer, which facilitated accurate electro-cutting. In addition, such procedures can increase the distance between the cutting plane and obturator nerve and decrease the incidence of obturator nerve reflex and bladder perforation. Eventually, the tumors were captured using the clamp and completely and conveniently removed via the peel-away sheath, which reduced the risk of tumor implantation induced by urethral contamination. It is a simple and precise way to perform tumor separation, cutting, and removal via the transurethral route, which is similar to the laparoscopic technique. The plasma electro-cutting system applied in this surgery has been widely adopted in PKRP and TURBT. The equipment is commonly utilized and the working principles and operating procedures are well known by physicians.
In this study, we improved and sharpened the needle tip of the plasma band-form needle electrode to adapt to thin layer cutting. Unlike 2 μm laser cutting, this method can deliver level cutting, effectively control the cutting depth, and facilitate rapid cutting. Precise operation is favorable for protecting the ureteral orifice, especially for the excision of tumors surrounding the ureteral orifice. Different tissue layers can be dissected explicitly during surgery. Moreover, no scars are left on the wound surface, and postoperative pathological diagnosis is not affected. The needle tip of the needle electrode is tiny, so the contact area with the tissue surface is limited, generating a small range of plasma during excitation. Therefore, electrical stimulation is relatively weak. In addition, the roots of tumors are initially separated by the scissors and subsequently subject to electro-cutting, which decreases the electrical stimulation to the tumor root, minimizes the risk of obturator nerve reflex, and significantly reduces the incidence of bladder perforation. Furthermore, resection of tumors located in the lateral bladder walls is typically conducted under general anesthesia combined with the use of muscle relaxants. No obturator nerve reflex was observed intra-operatively. For the excision of relatively large tumors, tumors should be cut into small masses using scissors and then simply removed. Intra-operatively, the apex of bladder and bladder anterior wall can be subjected to high pressure irrigation, theoretically, which can irrigate the exfoliated tumor cells and decrease the risk of tumor implantation. Ring-shaped partial excision of the bladder tumor can block the blood vessels nourishing the tumors and reduce the quantity of intraoperative hemorrhage. Moreover, in theory, a 2 μm wavelength laser can be utilized to replace the plasma band-form needle electrode as the cutting tool.
We used this new method to completely excise the tumor specimen, the surrounding tissue, and the whole muscle layer at the base, which is similar to the gross specimen obtained at open operation of partial cystectomy. Since this technique is similar to the laparoscopic approach in its procedures of tumor separation, cutting, and removal, this surgery is temporarily named PCTLT. We are also attempting to improve the surgical devices to achieve wound surface suture, accelerate early healing of the bladder wound, and decrease the risk of fluid effusion after surgery. During our study, there was minimal hemorrhage perioperatively, the histological structure was clear, the range of excision was precise, the incidence of obturator nerve reflex was low, and the operation was easy to control. At the same time, this technique can allow more accurate pathological staging for bladder tumors, because welloriented 1-piece specimens, including tumor and the whole detrusor muscle layer at the base and surrounding tissue, were obtained and could accurately reveal the microanatomic relationship and explicitly observe whether tumors infiltrate the muscular layer, and estimate the depth of tumor infiltration, which could contribute to accurate pathological staging, diagnosis, and prognosis, and effectively guide subsequent treatment. So this technique has two obvious advantages over conventional TURBT. 1. The tumor is removed in its entirety without fragmentation. 2. The margins (the lateral and base margins) can be precisely evaluated to ascertain the adequacy of surgical procedure. In TURBT, there are no true lateral and base margins although the muscularis propria is considered a surrogate for base margin status; therefore, there remains a high rate of under-staging. Since PCTLT is capable of completely removing the visible bladder tumors and supplying desirable specimens for pathological diagnosis and staging, which challenges the necessity of ReTUR.
Conclusions
As evidenced by the surgery conducted in this study, PCTLT can achieve en bloc resection of bladder tumors, including entire muscular layers and the tumor roots, which contributes to the complete excision of bladder tumors and precise pathological staging. Preliminary clinical application has demonstrated that PCTLT is convenient and feasible, yields few complications, has a low recurrence rate during early follow-up, and has high clinical efficacy.
However, our sample size is relatively small and the duration of follow-up is relatively short. Middle- and long-term randomized control studies with larger sample sizes are urgently required. Meanwhile, due to the limitations of visual field and surgical instruments, PCTLT is not applicable to the resection of tumors adjacent to the bladder neck. Therefore, the surgical equipment and appliances should be further modified.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics 2016. CA Cancer J Clin 2016; 66: 7-30.
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 2011; 59: 997-1008.
- American Urological Association: Guideline for the management of non-muscle invasive bladdercancer (stages Ta, T1, and Tis): 2007 update. Linthicum, Maryland: American Urological Association 2007.
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007; 178: 2314-2330.
- Persad R, Lamm D, Brausi M. Current approaches to the management of non-muscle invasive bladder cancer: comparison of current guidelines and recommendations. Eur Urol 2008; 7: 637.
- Kyung JO, Yoo DC, Ho SC. A novel transurethral resection technique for superficial flat bladder tumor: Grasp and bite technique. Korean J Urol 2015; 56: 227-232.
- Legrand G, Soliman H, Dubosq F, Vérine J, Desgrandchamps F. Prevalence and spectrum of microsatellite alterations in non-muscle invasive bladder cancers. Am J Cancer Res 2011; 1: 595-603.
- Yong Y, Zhi TW, Xu Z. Transurethral partial cystectomy with continuous wave laser for bladder carcinoma. J Urol 2009; 182: 66-69.
- Kramer MW, Rassweiler JJ, Klein J. En bloc resection of urothelium carcinoma of the bladder (EBRUC): a European multicenter study to compare safety, efficacy, and outcome of laser and electrical en bloc transurethral resection of bladder tumor. World J Urol 2015; 33: 1937-1943.
- Chen X, Liao J, Chen L. En bloc transurethral resection with 2-micron continuous-wave laser for primary non-muscle-invasive bladder cancer: a randomized controlled trial. World J Urol 2015; 33: 989-995.
- Kramer MW, Abdelkawi IF, Wolters M. Current evidence for transurethral en bloc resection of non-muscle-invasive bladder cancer. Minim Invasive Ther Allied Technol 2014; 23: 206-213.
- Wu Y, Lin T, Chen S. Comparison of the efficacy and feasibility of en bloc transurethral resection of bladder tumor versus conventional transurethral resection of bladder tumor. Medicine 2016; 95: 1-8.
- Richards KA, Smith ND, Steinberg GD. The importance of transurethral resection of bladder tumor in the management of nonmuscle invasive bladder cancer: a systematic review of novel technologies. J Urol 2014; 191: 1655-1664.
- Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005; 66: 4-34.
- Brausi M, Collette L, Kurth K. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 2002; 41: 523-531.
- Herr HW, Reuter VE. Progression of T1 bladder tumors: better staging or better biology? Cancer 1999; 86: 908-912.
- Soloway MS, Lee CT, Steinberg GD, Ghandi AA, Jewett MA. Difficult decisions in urologic oncology: management of high-grade T1 transitional cell carcinoma of the bladder. Urol Oncol 2007; 25: 338-340.
- Divrik T, Yildirim U, Eroglu AS. Is a second transurethral resection necessary for newly diagnosed pT1 bladder cancer? J Urol 2006; 175: 1258-1261.
- Kramer MW, Wolters M, Herrmann TR. En bloc resection of bladder tumors: ready for prime time? Eur Urol 2016; 69: 967-968.
- Hurle R, Lazzeri M, Colombo P. En bloc resection of nonmuscle invasive bladder cancer: a prospective single-center study. Urology 2016; 90: 126-130.
- Sureka SK, Agarwal V, Agnihotri S. Is en-bloc transurethral resection of bladder tumor for non-muscle invasive bladder carcinoma better than conventional technique in terms of recurrence and progression? A prospective study. Indian J Urol 2014; 30: 144-149.
- Kawada T, Ebihara K, Suzuki T. A new technique for transurethral resection of bladder tumors: rotational tumor resection using a new arched electrode. J Urol 1997; 157: 2225-2226.
- Ukai R, Kawashita E, Ikeda H. A new technique for transurethral resection of superficial bladder tumor in 1 piece. J Urol 2000; 163: 878-879.