Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2019) Volume 9, Issue 68
Inhibitory Properties of Combined Ethanol Seed Extract of P. guineense and T. conophorum on -Glucosidase Enzyme
Dokubo A1*, Odinaka EM2, Chukwu JAO31Department of Biochemistry, Rivers State University, Port Harcourt, Nigeria
2College of Medicine and Health Sciences, Abia State University, Uturu, Nigeria
3Department of Human Physiology, Alex Ekwueme Federal University, Ndufu-Alike, Ikwo, Nigeria
- Corresponding Author:
- Dokubo A
Department of Biochemistry Rivers
State University Port Harcourt, Nigeria
E-mail: dokubo.awolayeofori@ust.edu.ng
Accepted date: July 30, 2019
DOI: 10.35841/2249-622X.67.19-256
Visit for more related articles at Asian Journal of Biomedical and Pharmaceutical SciencesAbstract
The study was aimed to evaluate the α-glucosidase inhibitory properties of combined ethanol seed extract of Piper guineense and Tetracarpidium conophorum (CEPGTC). In vitro inhibitory assay was conducted in accordance with standard methods. p-nitrophenyl--D-glucopyranoside (pNPG) was used as a substrate. Fixed amount of the enzyme was incubated with increasing concentrations of CEPGTC seed extract (20-100 μg/ml) at 37°C for 15 min. The continuous production of p-nitrophenol was determined by measuring the absorbances at 410 nm. Acarbose at varying concentration (20-100g/ml) was used as the positive control. The median Inhibitory Concentration (IC50) values were graphically determined as the half-maximal inhibitory from plots of percent inhibition against log inhibitor concentration and by non-linear regression analysis. The enzyme kinetics (pattern of Inhibition) was determined using the Michaelis Menten’s equation and Lineweaver-Burk plot. The result showed that there was inhibition in all concentrations evaluated. The IC50 values estimated were 2.9 μg/ml and 2.5 μg/ml for CEPGTC and acarbose respectively. However, The IC50 value for acarbose was significantly (p<0.05) different from CEPGTC which is an indication of acarbose as effective -glucosidase inhibitor thus, used as positive control. An enzyme kinetics studies from the Lineweaver-Burke plot showed that CEPGTC seed extract demonstrated an uncompetitive inhibition on α-glucosidase while acarbose exhibited non competive (mixed inhibition). The inhibitory response and mechanism of inhibition observed in this study showed that combined ethanol seed extract of Piper guineense and Tetracarpidium conophorum has the potential to reduce glucose turnover and improve Hyperglycemia.
Keywords
Tetracarpidium conophorum, Piper guineense, Inibitory, Kinetics, -glucosidase, Enzyme.
Introduction
Diabetes mellitus (T2DM) has been described as a metabolic disorder that results to hyperglycemia due to malfunctioning of insulin action and secretion [1]. Hyperglycemia can cause glucose toxicity leading to the production of free radicals via glucose autoxidation, advanced glycation end products (AGEs) [2]. Free radicals have been implicated in a number of degenerative diseases [3]. One of the mechanisms utilized in the management of hyperglycemia is inhibition of carbohydrate metabolizing enzymes such as α-glucosidase (EC 3.2.1.20) in the epithelial tissues of small intestine with the intention of delaying glucose absorption by the walls of the small intestine [4]. There are so many effective synthetic drugs used as α- glucosidase inhibitors. Some of these include e.g., acarbose voglibose however, they are frequently associated with adverse effects and toxicity [5]. Therefore, the search for newer α- glucosidase inhibitors from plant origin becomes imperative. Several classes of active phytochemicals from crude extracts of different medicinal plant products have been used to combat hyperglycemic conditions [6]. A good number of these medicinal plants have been reported to inhibit α-glucosidase activity [6-9]. In traditional medical systems, combination of different plant species (poly therapy) has been utilized in managing hyperglycemia.
Piper guineense belongs to the Piperaceae family. It is commonly known as guinea pepper, black pepper in English, ‘Uziza’ in Igbo and ‘Iyere’ in Yoruba [3]. The leaves have been reported to exhibit anti-microbial, antifungal and antioxidant properties [10,11]. Tetracarpidium conophorum belongs to the Euphorbiaceae family. It is known as African walnut, ‘Ukpa’ in Igbo. The leaves bark and fruits of T.conophorum have been reported for antihyperglycemia potentials [12,3]. This study is aimed at assessing α-glucosidae inhibitory properties of combined ethanol seed extract of Piper guineense (P. guineense) and Tetracarpidium conophorum (T. conophorum) and mechanism of inhibition pattern.
Materials and Methods
The Seeds Tetracarpidium conophorum and Piper guineense were collected from and Mile 1 and Mile 3 Markets Port Harcourt, Rivers State and were authenticated at the Plant Science and Biotechnology Department, Rivers State University, Port Harcourt, Nigeria. The seeds were sorted, cleaned and ground to fine powder using a blender. A known weight, 100 g of each powder was combined and maceration with ethanol and water (8:2) at room temperature. The mixture was intermittently shaken to facilitate extraction for 3 days. The extracts were filtered using a Buchner funnel and Whatman no. 1 filter paper. The resulting filtrate was evaporated to dryness under water bath and later oven dried at a temperature of 50°C. A stock solution of 200 mg/ml of the extract was prepared using dimethyl sulfoxide. Concentrations ranging from 20 μg/ml to 100 μg/ml were prepared for the assay.
Chemicals and reagents
pNPG (p-nitrophenyl--D-glucopyranoside), -glucosidase from Saccharomyces cerevisiae were purchased from Sigma Aldrich, USA. Other chemicals used were of analytical grade and obtained from the Department of Biochemistry, Rivers State, Nigeria and other reputable firms.
-Glucosidase Inhibitory assay
The effect of the combined ethanolic seed extracts of Piper guineense and Tetracarpidium conophorum on -glucosidase activity was determined according to the method with slight modification. -glucosidase from Saccharomyces cerevisiae was used as the enzyme source. The substrate solution pnitrophenyl-- D-glucopyranoside (pNPG) was prepared in 50 mM phosphate buffer, pH 6.9. 100 L of -glucosidase (1.0 IU/ml) was preincubated with 50 L of the different concentrations of combined ethanolic extracts of P.guineense and T. conophorum (20-100g/ml) for 10 min. Then 50 L of 3.0 mM pNPG was then added to start the reaction. The reaction mixture was incubated at 37°C for 20 min and stopped by adding 2 ml of 0.1 M Na2CO3. The -glucosidase activity was determined by measuring the yellow-colored para nitrophenol released from pNPG at 410 nm. Acarbose at varying concentration (20-100g/ml) was used as the positive control. The percentage inhibition of α-glucosidase activity was calculated via the following equation:
%Inhibition=[1 – AE/Ac ] × 100
AE=Absorbance of the Extract, Ac=Absorbance of Control
The concentration of the tested samples giving 50% inhibition of the enzyme activity(IC50) values were determined from plots of percent inhibition against log inhibitor concentration and by non-linear regression analysis. All samples were assayed in triplicate.
Kinetics of inhibition
The mode of enzyme inhibition of the combined ethanolic extract of P.guineense and T.conophorum was determined according to the method described by Ozuogwu and Akuba, 2018 with slight modifications [9]. In one set of reating tubes, 50 l of the extract (IC50 concentration) was preincubated with 100 L of -glucosidase solution for 10 min at 25°C. In another set of tubes -glucosidase was preincubated with 50 L of phosphate buffer (50 mM, pH 6.9). Then 50 L of PNPG at increasing concentrations in mM (0, 1.25, 2.5, 5.0, 10.0 and 20.0) was added to both sets to start the reaction. The mixture was then incubated for 10 min at 25°C, and 500 L of Na2CO3 was added to stop the reaction. Acarbose was used as a positive control. The amount of reducing sugars released was determined spectrophotometrically using a paranitrophenol standard curve and converted to reaction velocities. A double reciprocal plot (1/V versus 1/[ ]) where V is reaction velocity and [ ] is substrate concentration was plotted. The type (mode) of inhibition of the extract on -glucosidase activity was determined by analysis of the double reciprocal (Lineweaver- Burk) plot and Michaelis Menten kinetics.
Statistical analysis
The data obtained were expressed as the mean ± SD of three replicates. Analysis was performed using Quest Graph™ IC50 Calculator, Graphpad prism 8 Software and Excel 2010. Oneway analysis of variance (ANOVA) and Duncan test were used to evaluate the possible differences among the means. P values ≤ 0.05 were considered as significant differences.
Results and Discussion
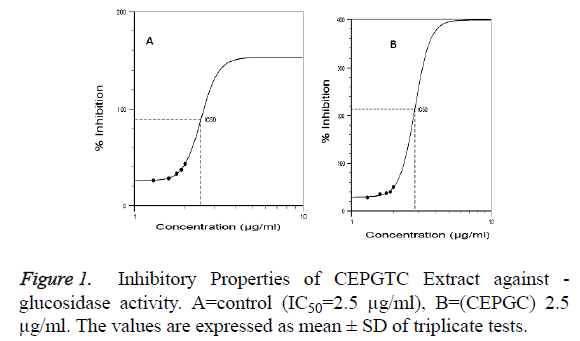
Glucosidases are enzymes that participate in carbohydrate metabolism. Thus, α-glucosidase inhibitors can play important role in glucose toxicity [13]. The -glucosidase inhibitory potential of combined ethanolic extract of P. guineense and T.conophorum was evaluated using S. cerevisiae as the source of enzyme. The substrate for the enzyme was paranitrophenyl-- D-glucopyranoside (PNPG). The inhibitory potentials on α-glucosidase activity at increasing concentrations of combined ethanolic extract of P. guineense and T. conophorum was compared to acarbose, a standard - glucosidase inhibitor (Positive control) as shown in Table 1 and Figure 1. The results showed that there was inhibition in all concentrations evaluated. However, the percentage inhibition of the combined ethanolic extract of P. guineense and T. conophorum (CEPGTC) on α-glucosidase was less than that of acarbose. The median Inhibitory Concentration (IC50), which is the concentrations that produce 50% inhibition under the specific experimental conditions for the α-glucosidase activity was estimated to be 2.9 μg/ml while that of the standard, acarbose was 2.5 μg/ml. The IC50 value for acarbose was significantly (p<0.05) different from the combined ethanolic extract of P. guineense and T. conophorum (CEPGTC) which is an indication of acarbose as effective - glucosidase inhibitor thus, used as positive control. Acarbose has structural resemblance to oligosaccharides. It alters enzymatic hydrolysis and absorption of glucose from dietary sources by the small intestine [14]. The inhibitory response observed in this study showed that increasing the concentrations of the extracts (inhibitor) has the potential to reduce glucose turnover.
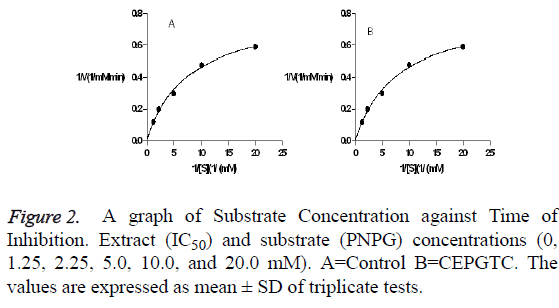
The inhibition mode was elucidated using the Michaelis Menten ’ s equation and Lineweaver – Burk plot. Results indicated a mixed and non-competitive type of inhibition as shown in Figure 2. Increasing concentrations of the inhibitors will decrease the maximum velocity of the reaction (Vmax) and affinity between the enzyme and the substrate may.
| Substrate concentration | % Inhibition | |
|---|---|---|
| [S]µg/ml | Acarbose (Positive Control) | CEPGTC |
| 20 | 28.04 | 26.11 |
| 40 | 35.11 | 28.25 |
| 60 | 37.04 | 33.12 |
| 80 | 40.01 | 37.12 |
| 100 | 50.35 | 43.18 |
Table 1: The Percentage Inhibition of ��-glucosidase by Acarbose and Combined Ethanolic Extract of P. guineense and T. conophorum (ratio1:1).
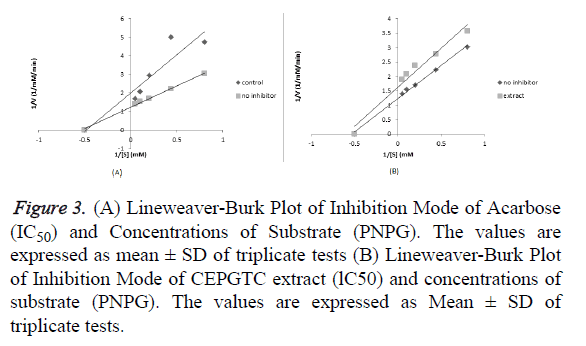
The enzyme kinetics showed the mechanism of inhibition according to Michaelis Menten ’ s and Lineweaver-Burk equation. It gives a visual representation of the types of enzyme inhibition. The features of mixed inhibition include the lines for different concentrations of inhibitors in the Lineweaver Burk plots intersecting to the left of the 1/vo axis. Furthermore increasing concentrations of the inhibitors result to decrease in the maximum velocity (Vmax) while the Km which is a measure of binding affinity between the enzyme and the substrate may decrease or increase. The inhibitors bind to both the free enzyme (E) where substrates also bind and enzyme-substrate complex (ES) forming enzyme-substrateinhibitor complexes (ESI) that alters catalysis and product formation. This type of inhibition was observed for acarbose with decrease in Vmax (0.816-0.502 mM/min) and Increase in Km (1.87-2.08 mM) as shown in Figure 2. This is an indication that there was no completition with the substrate for binding to the active site but binds to a separate site (allosteric site) on the enzyme to inhibit the conversion glucose. Other studies conducted in in vitro inhibition of carbohydrate metabolizing enzymes and in vivo anti-hyperglycaemic potential of methanol extract of Desmodium velutinum leaves reported similar inhibition pattern [9]. This mechanism may be used by the extract to suppress the production of glucose during hydrolysis of dietary carbohydrates. The features of uncompetitive inhibitors results to both decrease in Vmax and Km [15,16]. in this type inhibition, the inhibitor binds to the enzyme substrate complex only forming an inactive enzyme substrate inhibitor complex. In this case, increase in availalibity of substrate facilitates binding of the inhibitor to ES complex [17,15]. This mode of inhibition was observed for the extract with decrease in Vmax (0.816-0. 616 mM/min)And decrease in Km (1.87-1. 67 mM) as shown in Figure 3. This is an indication that the extract had the potential to alter catalysis with substrate binding decreasing production of glucose. The type of inhibition pattern is an indication that both the extract and acarbose are not structural analog with the substrate [9]. Their presence decreased the affinity of the substrate for the enzyme and reduced glucose turn over. Furthermore, increased concentration of substrate enhance this type of inhibition, This could be a reason of the increased percentage inhibition observed for α-glucosidase activity as the concentration of extract increase, delaying glucose production. This study is in agreement with previous reports which have shown that plant products exhibit strong inhibition against -glucosidase [18-20].
Figure 3: (A) Lineweaver-Burk Plot of Inhibition Mode of Acarbose (IC50) and Concentrations of Substrate (PNPG). The values are expressed as mean ± SD of triplicate tests (B) Lineweaver-Burk Plot of Inhibition Mode of CEPGTC extract (lC50) and concentrations of substrate (PNPG). The values are expressed as Mean ± SD of triplicate tests.
Conclusion
The inhibitory response observed in this study show that increasing the concentrations of the extracts (inhibitor) has the potential to reduce glucose turnover. The type of inhibition pattern is an indication that both the extract and acarbose are not structural analog with the substrate. The presence of the extract can affect decrease in the affinity of the enzyme for the substrate which may cause increase or decrease in Km but always decrease Vmax due to the formation of inactive enzymesubstrate- inhibitor complex. The potency of this type of inhibition cannot be affected by increase in substrate concentration. Thus, these spices can be utilized as effective inhibitors of α-glucosidase in managing NIDDM.
References
- Fernández-Mejía C. Oxidative stress and chronic degenerative diseases-a role for antioxidants. USA, 2013.
- Campos C. Chronic Hyperglycemia and Glucose Toxicity: Pathology and Clinical Sequelae. Clinical Features 2012; 6:1-8.
- Obomanu FG, Dokubo A. Studies on oxidative stress markers of alloxan –induced diabetic wistar rats treated with Tetracarpidium conophorum and Piper guineense (1:1 mixture). Int J Adv Res 2018; 5: 571-579.
- Shai LJ, Masoko P, Mokgotho MP, et al. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. South African J Botany 2010; 76: 465-470.
- Verspohl E. Novel pharmacological approaches to the treatment of type 2 diabetes. Pharmacol Rev 2012; 64: 188–237.
- Odugbemi T. Outlines and pictures of medicinal plants from Nigeria Edition. University of Lagos Press, 2006 Lagos, Nigeria.
- Kazeem MI, Raimi OG, Balogun RM, et al. Comparative study on the α-amylase and α-glucosidase inhibitory potential of different extracts of Blighia sapida Koenig. Amer J Res Commun 2013; 1: 178-192.
- Rouzbehan S, Moein S, Homaei A, et al. Kinetics of α-glucosidase inhibition by different fractions of three species of Labiatae extracts: a new diabetes treatment model. Pharmaceut boil; 55: 1483-1488.
- Ozougwu VEO, Akuba BO. In vitro Carbohydrate metabolizing enzyme and in vivo anti hyperglycemic potential of methanol extract of Desmodium velutinum leaves. Res J Med Plants 2018; 12: 48-50.
- Kiin-Kabari DB, Barimalaa IS, Achinewhu SC, et al. Effects of extracts from three indigenous spices on the chemical stability of smoke-dried catfish (Clarias lezera) during storage. African J Food, Agriculture, Nutrition and Development 2011; 11.
- Ojinaka MC, Odimegwu EN, Chidiebere FE. Comaparative study of the nutrient and antinutrient composition of the seeds and leaves of Uziza (Piper guineense). J Envi Sci, Toxic Food Tech 2016; 10: 42-48.
- Akomolafe SF, Oboh G, Afolabi, Akindahunsi AA, et al. Antiperoxidative Activity of Tetracarpidium conophorum Leaf Extract in Reproductive Organs of Male Rats. Evid-Based Complem Alt Med 2015; 1-8.
- Tiwari AK, Madhusudanarao J. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Current Sci 2002; 109: 12
- Dhingra D, Michael M, Rajput H, Patil RT. Dietary fibre in foods: A review. J Food Sci Tech 2012; 49: 255-266.
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry (6th edition) 2013.
- Ahern K, Rajagopal I, Tan T. Biochemistry, North Carolina 2017 1: 367–368.
- Nahorski SR, Ragan CI, Challiss RJ et al. Lithium and the phosphoinositide cycle: an example of uncompetitive inhibition and its pharmacological consequences. Trends Pharmacol Sci 1991; 12: 297-303.
- Ogunwande IA, Matsui T, Fujise T, et al. α-Glucosidase inhibitory profile of Nigerian medicinal plants in immobilized assay system. Food Sci Tech Res 2007; 13: 169–172
- AAT Bioquest 2019 Quest Graph TM IC50 Calculator.
- Adam Z, Khamis S, Ismail A, Hamid M. Inhibitory Properties of Ficus deltoidea on α-Glucosidase Activity. Res Journal Med Plants 2010; 4: 61-75.