- Biomedical Research (2012) Volume 23, Issue 3
Inhibition/prevention of primary liver tumors in mice given a daily dietary extract of North American ginseng (Panax quinquefolius) following a hepatoma-inducing agent
Punithavathi Durairaj, Sandra C. MillerDepartment of Anatomy & Cell Biology, McGill University, Montreal, QC, Canada
Accepted date: June 12 2012
Abstract
The present study assessed the ability of an extract of North American ginseng to prevent/ inhibit the development of hepatoma in mice, pre-injected with a known liver tumor inducer, dimethylnitrosamine (DEN). We hypothesized that a standardized, proprietary extract of this herb would inhibit the development of this tumor, given its success in abating leukemia and extending life span in mice. CVT-E002 was given 4 hours after DEN injection and administered daily via the diet until mice were 82 wk old. Control mice consumed identical chow without CVT-E002. At various intervals during the study, mice were euthanized and their spleen, bone marrow, and blood were taken for enumeration of natural killer (NK) cells, lymphocytes and “other” hemopoietic cells. There was a significant elevation in the absolute numbers of lymphocytes (p<0.0001: spleen; p<0.0001: BM) in CVT-E002-fed, DEN pre-injected mice vs control-diet mice. NK cells in the spleen and bone marrow (BM) were also significantly elevated at p<0.005 and p< 0.001, respectively. The proportions of blood-borne lymphocytes and NK cells reflected their increased absolute numbers in the spleen and BM. Finally, whereas 100% of control-diet mice had developed hepatoma and were euthanized by 82 wk, 100% of CVT-E002-fed mice remained alive, healthy and hepatoma- free at 82 wk, remaining healthy even after returning to control diet until euthanized as healthy animals at 98 wk. CVT-E002-driven immunoenhancement is believed to be the mechanism by which hepatoma was inhibited/prevented
Keywords
Immune cells, ginseng, hepatoma, pre-clinical, in vivo
Introduction
More that 5 decades ago, it was shown that dimethylnitrosamine (DEN) had the capacity to produce liver tumors in rats. Subsequent research revealed that approximately 90% of nitrosamine compounds were carcinogenic. In the 1970’s, there was an increased frequency of liver cancer found in Norwegian farm animals since they were fed on herring meal, which was preserved with sodium nitrite. The latter compound reacts with dimethylamine in fish and the resulting compound is den. Nitrosamines are produced from nitrites and secondary amines. Indeed, the biochemical mechanism responsible for Den-induced hepatocarcinogenesis has been identified [1 – 7].
Nitrosamine formation can occur under conditions of strong acidity such as exists in the human stomach. Nitrosamines are found in many foodstuffs, especially alcoholic beverages, meats and cheeses which are preserved with nitrite pickling salt. The USA and Canadian governments now order limits on the amount of nitrites used in the preservation of meat and other products in order to decrease cancer risk among consumers. Moreover, nitrosamines are found in abundance in auto exhaust, and in tobacco products as well as in the smoke these products generate, thereby placing even non-smokers at risk to nitrosamine exposure. Thus, nitrosamines are virtually ubiquitous in modern civilization and affect all age groups. Nevertheless, there is evidence that immune system fortification may be at least one of the methods by which hepatomas may be abated or prevented from developing [8 – 10].
Recently, we have experienced considerable successes in abating neoplasms, of hematologic origin in vivo [11, 12], by stimulating specific, anti-cancer cells of the immune system, i.e., NK cells, by means of daily dietary administration of a proprietary extract (CVT-E002) of North American ginseng (Panax quinquefolius). We hypothesized that this herbal derivative would also amelio rate/prevent DEN-induced hepatoma in a murine model and secondly, would do so by means of immunostimulation. Our results proved to be very successful in hepatoma prevention/inhibition and did indeed reveal immuno-enhancement as the possible mechanism.
Materials and Methods
Mice
Male pups of the C3H/HeN strain were raised in-house at the McGill University Animal Care Facility. Their mothers were purchased from Charles River Laboratories, St. Constant, QC, Canada. All mice (mothers and pups) were maintained under controlled environmental conditions of temperature and humidity, in microisolator cages under laminar flow conditions. All mice were placed on a 12 hr light/dark cycle. Veterinary and technical services in the facility ensured, moreover, that all edicts issued by the CCAC (Canadian Council on Animal Care) were strictly adhered to. All personnel involved in the handling of mice in this study have passed the required training courses in rodent husbandry. Sentinel mice in the facility, regularly demonstrated the absence of all common mouse pathogens.
Hepatoma induction
Male mice were injected, once only at 7 weeks of age with DEN, i.e., N-diethylnitrosamine – (C2H3)2NNO, having been purchased from Sigma Aldrich Inc. This compound is carcinogenic to all animal species, targeting the organs of the respiratory and gastrointestinal tract, having a propensity for the liver (Sigma Aldrich, Inc., fact sheet). DEN was administered at a dose of 25mg/kg body weight or 0.025 mg/gm body weight. Mice of 7 weeks of age have a body weight of 24-25 gm and consequently 0.6 mg DEN was injected in a vehicle of 0.9% physiological saline, i.e., 0.036ml/mouse intraperitoneally. The mouse strain used in this study, the gender, the age at the time of DEN injection, and the dosage and frequency of den are in agreement with existing literature for hepatoma induction [13-15].
The dietary additive
CVT-E002 is a proprietary extract of North American ginseng (Panax quinquefolius), produced by Afexa Life Sciences, Inc., Edmonton, AB, Canada. CVT-E002 consists of specific polysaccharides (poly-furanosylpyranosyl- saccharides). Standard chemical and biological assays are applied in combination with consistent manufacturing processes to ensure that both the composition and the pharmacological activity of each lot of CVT-E002 are identical [16, 17], and free of microbial contaminants. The extract is already available to consumers, marketed as a powerful anti-viral agent under the label Cold fX ® and proven effective against respiratory viruses, i.e., colds and flu. The extract is a strong immune stimulant in vivo and in vitro, resulting in the augmented production of several lymphocyte-stimulating cytokines [17, 18]. It in is a very effective stimulant specifically of NK cells, elevating these numbers in vivo to significantly super-normal levels [19, 11, 12].
A homogenized mixture of powdered standard mouse chow (Labchow, Agribrands, Inc., Woodstock, ON, Canada) ± CVT-E002 was provided daily via the diet as 6 mg of powdered chow ± CVT-E002 (80mg/6gm chow/- day/mouse). Mice were housed 1/cage to avoid fighting and thus maintain absolute consistency in behaviour throughout the duration of this study, given that the steroid hormones produced during aggression, i.e., males fighting males, are known to be immuno-suppressive and would, thus, add a second confounding and nonquantifiable variable.
Mice received daily dietary CVT-E002 (80mg/24gm body weight) within 4 hours of having been injected with den. This dose has been previously defined in our lab as the one which was maximally effective in NK cell/- lymphocyte stimulation in adult mice [11, 12, 19]. Control DEN-injected mice, received untreated chow, but were otherwise placed under identical conditions of housing and husbandry as were those receiving the dietary additive. Any mice which showed clinical signs of hepatoma (palpable liver tumors) were euthanized, prior to their becoming moribund, and their spleen, bone marrow, and blood were extracted for cell identification and quantification of NK cells, lymphocytes and non-immune, hemopoietic cells.
Preparation of the organs for analysis of immune and hemopoietic cell content
Single cell suspensions of the spleen and bone marrow were prepared by our standard laboratory methods [20- 23]. Each spleen, upon removal from each mouse (CVTE002- consuming and control-diet) was pressed through a stainless steel mesh into medium (GIBCO, Invitrogen Corp, Burlington, ON, Canada) containing 10% heat inactivated newborn bovine serum (NBS) (GIBCO – as above). Both femurs (bone marrow source) were aseptically removed and transferred to ice cold medium containing 10% heat inactivated NBS. The bone marrow was expressed from both femurs/mouse by repeated flushing with medium. Free cell suspensions from both organs were obtained by gently repeated pipeting. Suspensions were then layered for 7 min onto 1.5 ml pure NBS to allow the sedimentation of any non-cellular debris into the NBS. The aggregate-free supernatants were then removed and centrifuged for 7 min (1100rpm 4ºc) and the resulting pellet was re-suspended in a fixed volume of fresh medium + NBS. The total number of nucleated cells was obtained by means of a hemocytometer (American Optical Co., Buffalo,, NY, USA). Thus, the total number of cells in each spleen and in both femurs, for every mouse (CVT-E002-consuming and control-diet) could be determined.
Subsequently, from each organ of every mouse, smears were made from the cell suspensions, onto Superfrost plus® microscope slides (Fisher Scientific, Whitby, ON, Canada). Immediately prior to euthanasia in each mouse, blood was extracted from the lateral tail vein and smears were directly prepared from the extracted blood. All smears (spleen, bone marrow, blood) were then stained for morphological identification of NK cells and lymphocytes, using Macneal’s tetrachrome hematologic stain (Sigma-Aldrich, Oakville, ON, Canada). This stain provides a 4-color discrimination of the key cellular components (DNA, RNA, protein), as well as providing a clear distinction of cells size, shape and anatomical components, i.e., cytoplasmic granules, Golgi zone, stage of mitosis, etc. Each smear was then “read” for its proportions of lymphocytes, NK cells and a third category, “others” which included all the remaining non-NK, nonlymphocytic, hemopoietic cells.
From 500 total blood cells/smear, 1000 total spleen cells/smear and 2000 total bone marrow cells/smear, the proportions (%) of cells in each of these categories were obtained in every smear from all 3 organs of every mouse. NK cells were identified by virtue of their being small to medium sized cells (7-9 microns in nuclear diameter and of lymphocytic morphology, but bearing 2-5 stained cytoplasmic granules [24-26], clearly visible at x100 in the light microscope. In fact, the presence of cytoplasmic granules was one of the first methods of identifying NK cells [27]. Other lymphocytes, i.e., T and B, are morphologically identical to NK cells, and exist in the same cell size range, but never bear granules.
Using this method of NK cell identification, we have accurately monitored the influence of CVT-E002 on NK cells [28].
Finally, the absolute numbers of cells in each of these categories of cells were obtained for the spleen and bone marrow (both femurs together) for each mouse, by converting the percentage values in each case, via the known total cell content of each organ, the latter having been recorded from the hemocytometer at the time of organ extraction at euthanasia. In the case of the blood analysis for every mouse, the percentage values of these 3 categories of cells (lymphocytes, NK cells, others), were recorded as relative (%) values only.
Results
Fig 1, 2 show the results of CVT-E002 on the absolute numbers of lymphocytes, NK cells and other hemopoietic cells in 3 organs of DEN-injected mice, consuming either control chow or CVT-E002-containing chow. Mice in the control-diet group began to develop tumors and were euthanized between 52 and 66 wk of age, having been injected with the hepatoma inducer, DEN, at 7 wk of age. All control-diet, DEN-injected mice had palpable hepatoma at the time of euthanasia and these mice provided the control-diet data.
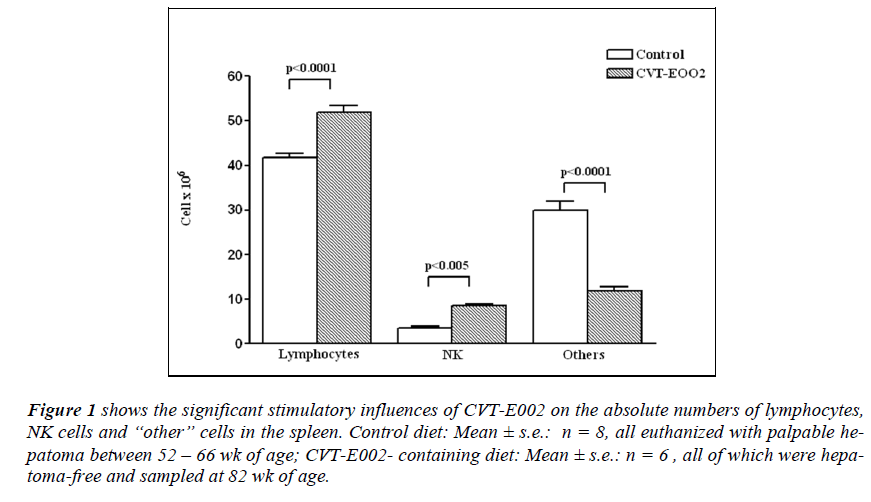
Figure 1: shows the significant stimulatory influences of CVT-E002 on the absolute numbers of lymphocytes, NK cells and “other” cells in the spleen. Control diet: Mean ± s.e.: n = 8, all euthanized with palpable hepatoma between 52 – 66 wk of age; CVT-E002- containing diet: Mean ± s.e.: n = 6 , all of which were hepatoma- free and sampled at 82 wk of age.
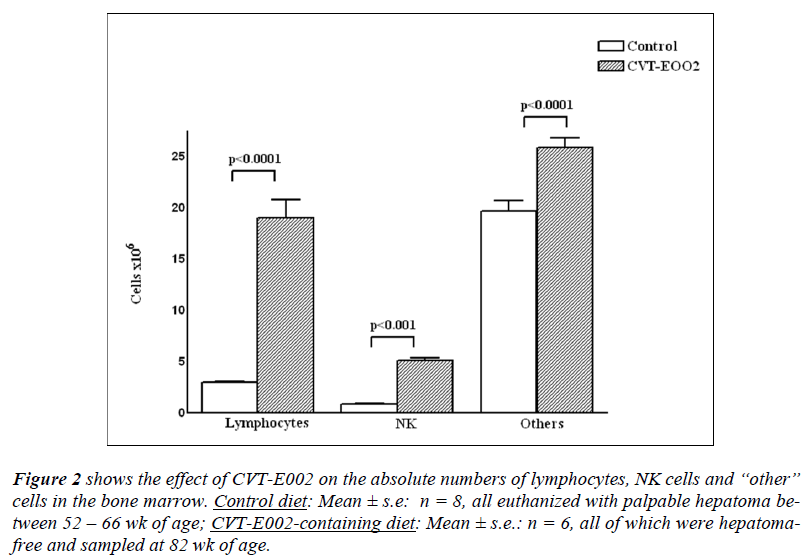
Figure 2: shows the effect of CVT-E002 on the absolute numbers of lymphocytes, NK cells and “other” cells in the bone marrow. Control diet: Mean ± s.e: n = 8, all euthanized with palpable hepatoma between 52 – 66 wk of age; CVT-E002-containing diet: Mean ± s.e.: n = 6, all of which were hepatomafree and sampled at 82 wk of age.
Fig. 1 indicates that the absolute number of lymphocytes in the spleens of mice in the control group (consuming untreated chow) were significantly lower than that of their cage-mates consuming daily dietary CVT-E002. The absolute number of NK cells in the control group was also significantly lower than that of mice consuming CVTE002.
The remaining, grouped hemopoietic cells were, by contrast, significantly higher in control-diet mice relative to those of CVT-E002-consuming mice.
In the bone marrow of DEN-injected mice (fig. 2), again a significant disparity was found between mice on the control diet and those on the CVT-E002- containing diet. The absolute numbers of lymphocytes, NK cells, and grouped hemopoietic cells were all significantly lower in controldiet mice vs those in CVT-E002-consuming mice. Given that the bone marrow is the central generating site for all the above cells (NK cells, B lymphocytes, pro-T lymphocytes and the “other” hemopoietic cells), the increased absolute numbers of cells in the 3 main cell categories enumerated in this organ, necessary means increased production in the precursor cells of these lineages in the presence of CVT-E002.
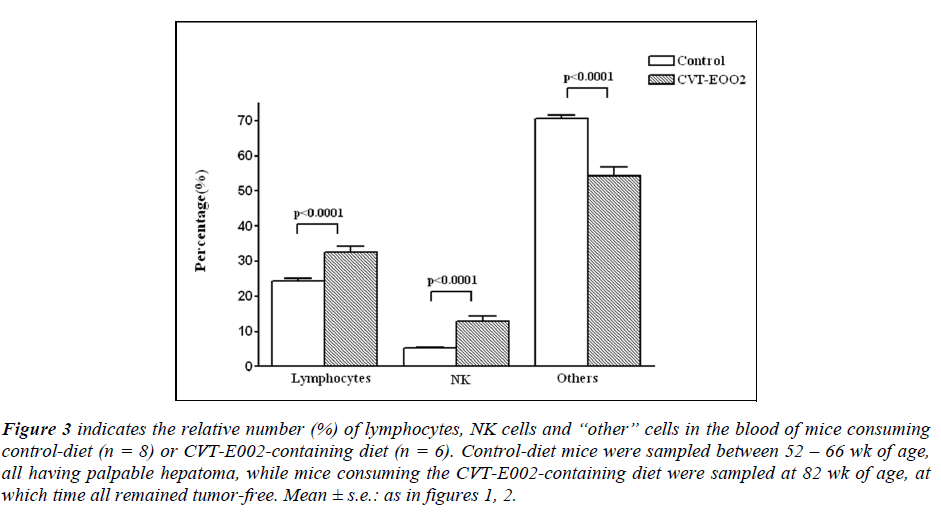
In the blood of CVT-E002-consuming mice (fig. 3), the relative numbers (%) all 3 cell categories were influenced by the dietary presence of the extract. The proportions (%) of lymphocytes and NK cells were significantly in creased in the blood of all CVT-E002-fed mice vs controldiet mice. However, the remaining hemopoietic cells (grouped as “others”), reflected our observations in the spleen, i.e., the proportion (%) of “others” in CVT-E002- fed mice was lower than that of control-diet mice. The spleen, being on the blood circulation pathway, mirrors the content of the blood. Indeed, that is what is found when comparing the relative levels of all 3 of these cell categories in the blood with their absolute numbers in the spleen (fig. 1).
Figure 3: indicates the relative number (%) of lymphocytes, NK cells and “other” cells in the blood of mice consuming control-diet (n = 8) or CVT-E002-containing diet (n = 6). Control-diet mice were sampled between 52 – 66 wk of age, all having palpable hepatoma, while mice consuming the CVT-E002-containing diet were sampled at 82 wk of age, at which time all remained tumor-free. Mean ± s.e.: as in figures 1, 2.
Because the blood is a conveyor organ, not only of newly generated, immune cells and assorted hemopoietic cells produced in the bone marrow, but also of mature, preexisting, circulating and re-circulating immune and hemopoietic cells produced elsewhere in the body (lymph nodes, gut-associated and lung-associated tissues and subcutaneous sites), it is difficult to assign a specific reason for the levels observed in the blood for any of the 3 cell categories presented in fig. 3.
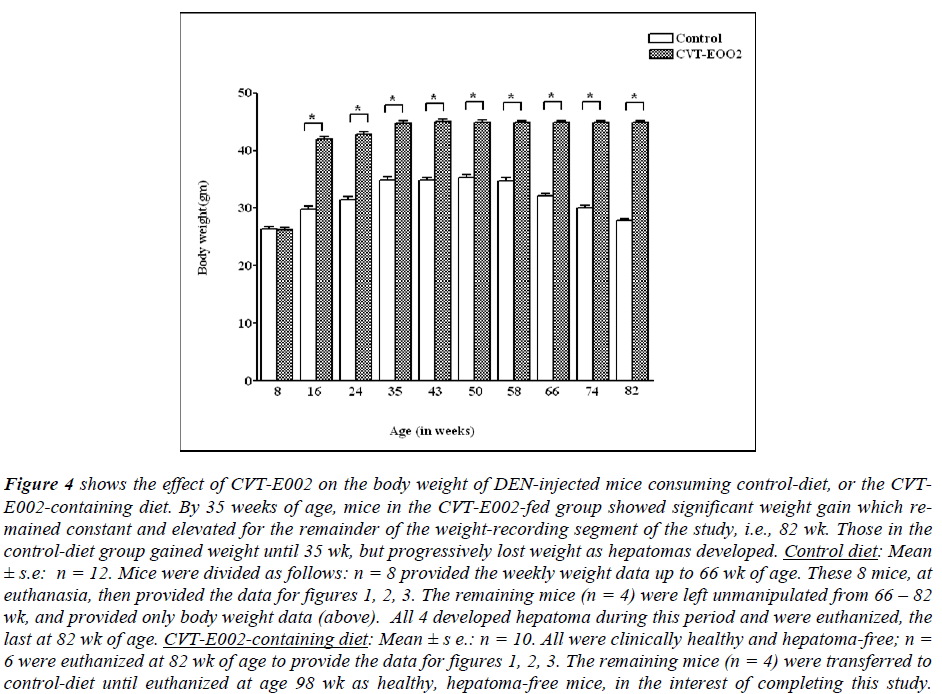
Fig. 4 indicates the progressive changes in body weights throughout the study beginning 1 wk after DEN injection +/- the daily dietary additive, CVT-E002. No control comparisons could be made beyond 82 wk of age since control-diet mice had either been already euthanized for cell analysis (fig. 1 - 3), or were left with no further manipulation to assess life span (n = 4). However, between 66 - 82 wk of age, these 4 remaining mice developed palpable hepatoma and were progressively euthanized - the last one euthanized was at 82 wk of age.
Figure 4: shows the effect of CVT-E002 on the body weight of DEN-injected mice consuming control-diet, or the CVTE002- containing diet. By 35 weeks of age, mice in the CVT-E002-fed group showed significant weight gain which remained constant and elevated for the remainder of the weight-recording segment of the study, i.e., 82 wk. Those in the control-diet group gained weight until 35 wk, but progressively lost weight as hepatomas developed. Control diet: Mean ± s.e: n = 12. Mice were divided as follows: n = 8 provided the weekly weight data up to 66 wk of age. These 8 mice, at euthanasia, then provided the data for figures 1, 2, 3. The remaining mice (n = 4) were left unmanipulated from 66 – 82 wk, and provided only body weight data (above). All 4 developed hepatoma during this period and were euthanized, the last at 82 wk of age. CVT-E002-containing diet: Mean ± s e.: n = 10. All were clinically healthy and hepatoma-free; n = 6 were euthanized at 82 wk of age to provide the data for figures 1, 2, 3. The remaining mice (n = 4) were transferred to control-diet until euthanized at age 98 wk as healthy, hepatoma-free mice, in the interest of completing this study.
Fig. 4 reveals that soon after beginning to consume daily CVT-E002, the body weights of DEN-injected, mice progressively increased to statistically significant levels relative to their age-matched cage-mates, and remained elevated until 82 wk at which time all body weight measurements were concluded. The maximum increase in body weight occurred at 35 wk of age in both control-diet and CVT-E002-fed mice. However, unlike the progressive decline in body weight beginning at 43 wk of age in control- diet mice, the body weights of mice consuming daily CVT-E002 maintained a steady, elevated level from their maximum at 35 wk.
Finally, as an observation on actual life span extension mediated by consuming CVT-E002, we found that in striking contrast to the 100% development of hepatoma and ultimate euthanasia of all the control-diet mice by 82 wk (see above), a separate group of mice (n = 4), consuming daily CVT-E002 continued to live on, reaching 88 wk of age, still showing clinical health, normal range body weight, and no palpable tumor. At 88 wk of age, these 4 mice were removed from the CVT-E002 diet and placed on the control diet for the next 10 wk. These mice still continued to show no signs of palpable tumor and remained in clinical good health up to 98 wk of age, i.e., 2 ½ mo after removing dietary CVT-E002. At that time these 4 mice were purposely euthanized in the interest of completing this study.
Discussion
The concepts of “tumor prevention” and “tumor inhibition” are terms which, at the very earliest stages of neoplasm development, are virtually synonymous since there is no clinical appearance of tumor. The role of a healthy, strong NK cell-mediated arm of the immune system is to act as the “first line of defense”, targeting and contact killing (cytolytis) the earliest neoplastic cells, and consequently, preventing any clinical signs that a neoplasia had ever existed.
Our results in the present study, parallel our previous findings in mice bearing another type of neoplasm, i.e., leukemia, and receiving daily, dietary CVT-E002 [11, 12]. The similarities between these recent studies and our present study attest to the wide-ranging ability of this agent, CVT-E002, to significantly enhance tumortargeting NK cells. Indeed, it was with the intention of expanding our previous observations that we undertook the present study wherein we: (i) chemically induced a tumor of (ii) non-hemopoietic origin vs our recent work wherein we injected live tumor cells of hemopoietic origin, i.e., leukemia cells (11, 12). The similarity of our collective observations, then and now, supports the usefulness of CVT-E002 in prevention/inhibition of assorted tumors apparently by means of immuno-stimulation. Our present observations that CVT-E002 has produced such a profound increase in all the cells mediating immunity, parallels and supports earlier studies in vitro [17, 18].
The small group of mice which continued to consume daily, dietary CVT-E002 beyond 82 wk (oldest age used for generating the data of figs. 1, 2, 3) did indeed continue to live hepatoma-free for the next 6 wk at which time (age: 88 wk) they were converted to the control diet. This was done for the purpose of determining whether or not they would remain hepatoma-free or progressively begin to develop hepatoma in the absence of CVT-E002 in their diet. By 98 wk of age, i.e., 2 ½ months later, however, these mice remained tumor-free indicating that this extract had a residual influence on immune cells, specifically NK cells. Indeed, we have already demonstrated this phenomenon, i.e., long-lasting influence of CVT-E002 in sustaining elevated absolute numbers of NK cells many months after withdrawal of the extract, in both normal, pre-pubertal and normal, adult mice [19, 28]. The mechanism for this phenomenon is believed to reside in the bone marrow stromal cell microenvironment, well established as the governing influence over the generation of new NK cells [20, 29, 30, 31]. Bone marrow stromal cells are themselves under regulatory influence and are stimulated by the cytokines Il-1, Il-6 and TNF-α and these are the same cytokines whose levels are elevated in the presence of CVT-E002 [17, 18]. Consequently, it is reasonable to assume that the mechanism by which CVT-E002 results in NK cell elevation is an indirect one, acting by stimulating the NK cell-governing, microenvironmental stromal cell network, predominantly located in the bone marrow.
The unexpected result of progressive and sustained increase in body weight in CVT-E002-consuming, DENinjected mice, all of which had no hepatoma, suggests the possibility of CVT-E002 interaction with lipid physiology. CVT-E002-fed mice had increased their body weight by one-third up to 43 wk. The early increase in body weight in both groups simply results from body growth during their youth since both groups were only juveniles (7 wk old) when the study began. The progressive decline after 43 wk in body weight observed in the control-diet group could readily be explained by the developing hepatoma, because weight loss is a characteristic symptom of developing tumors in mammals including humans.
There is a theoretical, but improbable, mechanism for the early onset and rapid weight gain experienced by the CVT-E002 consuming mice. For instance, it is known that estrogen is related to lipid deposition in vivo, and like many herbs, Panax quinquefolius which contains CVTE002, has phytoestrogenic properties [32, 33]. However, CVT-E002 itself is a standardized extract containing only specific polysaccharides (poly-furanosyl-pyranosylsaccharides), and these molecules have no known estrogenic properties.
The answer to this peculiar but consistent observation of sustained body weight increase in these healthy, CVTE002- consuming mice may ultimately be revealed by biochemical and physiological studies. Once the mechanism of action of CVT-E002 is defined, CVT-E002 could prove to be of clinical significance for individuals suffering from cachexia which is so often observed during chemotherapy for tumors including hepatoma.
Acknowledgements
The authors would like to thank Afexa Life Sciences, Inc. for providing full financial support for this project.
Conflict of interest
The authors declare no conflict of interest with other any person, institution or commercial establishment in the execution of this project.
References
- Verna L, Whysner J, Williams GM. Nnitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 1996; 71:57-81.
- Corcos D, Defer, N, Raymondjean M, Paris B, Corral M, Tichonicky L, Kruh J, Glaise D, Saulmer A, Guguen- Guillouzo C. Correlated increase of the expression of the c-ras genes in chemically induced hepatocarcinomas. Biochem Biophys Res Commun 1984; 122:259-264.
- Beer DG, Schwarz M, Sawada N, Pitot HC. Expression of h-ras and c-myc protooncogenes in isolated gammaglutamyl transpeptidase-positive rat hepatocytes and in hepatocellular carcinomas induced by diethylnitrosamine. Cancer Res 1986; 46:2435-2441.
- Smith KL, Yeleswarapu L, Locker J, Lombardi B. Expression of p53 mutant protein(s) in diethylnitrosamine- induced foci o f enzyme-altered hepatocytes in male Fischer-344 rats. Carcinogenesis 1991; 12:1137- 1141.
- Johnson SJ, Burr AW, Toole K, Dack CL, Matthew J, Burt AD. Macrophage and hepatic stellate cell responses during experimental hepatocarcinogenesis. J Gastroenterol Hepatol 1998; 13:145-151.
- Ahn B, Han BS, Kim DJ, Ohshima H. Immunohistochemical localization of inducible nitric oxide synthase and 3-nitrotyrosine in rat liver tumors induced by nnitrosodiethylamine. Carcinogenesis 1999; 20: 1377- 1344.
- Matsuda M, Nakamoto Y, Suziki S, Kurata T, Kaneko S. Interferon-γ-mediated hepatocarcinogenesis in mice treated with diethylnitrosamine. Lab Invest 2005; 85: 655-663.
- Lavrovsky VA, Viksler VKH. Enhanced immune reactivity to hepatoma 22a cells in mtv-infected compared with mtv-free C3H mice. Immunology 1981; 44 (4):671-676.
- Cheent K, Khakoo SI. Natural killer cells and hepatitis c: action and reaction. Gut 2011: 60: 268-278.
- Subleski, JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of NK and NKT cells in the liver. Cancer res 2006; 66(22):11005-11012.
- Miller SC, Delorme D, Shan JJ. CVT-E002 stimulates the immune system and extends the life span of mice bearing a tumor of viral origin. Jour. Soc. Integ Oncol 2009; 7(4): 127-136.
- Miller SC, Delorme D, Shan JJ. Extract of North American ginseng (Panax quinquefolius) administered to leukemic, juvenile mice extends their life span. Jour Comp Integ Med 2011: doi: 10.2202/jcim1553- 3840.1315.
- Dragani TA, Manenti G, Gariboldi M, De Gregorio L, Pierotti MA. Genetics of liver tumor susceptibility in mice. Toxicol Letters 1995; 82/83: 613-619.
- Chuang SE, Kuo KL, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL. Curcumin-containing diet inhibits diethylnitrosoamine-induced murine hepatocarcinogenesis. Carcinogenesis, 2000; 21(2): 331-335.
- Wands J. Hepatocellular carcinoma and sex. New Engl J Med 2007; 357:19: 1974-1976.
- Shan JJ, Rodgers K, Lai C-T, Sutherland SK. Challenges in natural health product research: the importance of standardization. Proc West Pharmacol Soc 2007; 50:24-30.
- Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S, Pang P, Shan JJ. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolius). Jour Pharm Pharmacol 2001; 22:1515-1523.
- Wang M, Guilbert LJ, Li J, Wu Y, Pang P, Basu TK, Shan JJ. A proprietary extract from North American ginseng (Panax quinquefolius) enhances Il-2 and IFN-γ production in murine spleen cells induced by con-a. Int. Immunopharm 2004; 4: 311-315.
- Miller SC, Delorme D. An extract from North American ginseng stimulates spontaneous immunity in infant mice: sustained, augmented immunity in adulthood long after withdrawal of the extract. Jour Comp Integ Med 2008; doi: 10.2202/jcim1553-3840.1117.
- Miller SC. Production and renewal of murine natural killer cells in the spleen and bone marrow. Jour. Immunol 1982; 129(5): 2282-2286.
- Miller SC, Kearney SL. Effect of in vitro administration of trans retinoic acid on the hemopoietic cell populations of spleen and bone marrow: profound strain differences between A/J and C57bl/6J mice. Lab Animal Sci 1997; 48(1): 74-78.
- Maloney MX, Currier NL, Miller SC. Natural killer cell levels in older adult mice are gender-dependent: thyroxin is a gender-independent, natural killer cell stimulant. Nat Immun 1998; 16: 165-174.
- Sun L Z-Y, Currier NL, Miller SC. The American cone flower: a prophylactic role involving non-specific immunity. J Alt Comp Med 1999; 4(5): 437-448.
- Babcock GF, Phillips JH. Human NK cells: light and electron microscopic characteristics. Immunol Res. 1983; 2(1): 88-101.
- Ames IH, Gates CE, Garcia AM, John PA, Hennig AK, Tomar RH. Lysis of fresh murine mammary tumor cells by syngeneic natural killer cells and lymphokineactivated killer cells. Cancer Immunol Immunother 1987; 26: 161-168.
- Ames IH, Gagne GM, Garcia AM, John PA, Scatorchia GM, Tomar RH, McAfee JG. Preferential homing of tumor-infiltrating lymphocytes in tumor-bearing mice. Cancer Immunol Immunother 1989; 29: 93-100.
- Saksela E, Tarkkanen J, Carpen O, Virtanan I. Morphological and functional characteristics of the human NK system. In: natural killer cells, Serrou B, Rosenfeld C, Herberman, RB (eds), Elsevier Biomedical Press, Amsterdam, New York, Oxford, 1982; pp 81-93.
- Miller SC, Ti L, Shan JJ. Dietary supplementation with an extract of North American ginseng in adult and juvenile mice increases natural killer cells. Immunol Invest 2012;41(2) 157-170. doi: 10.3109/ii08820139.2011.599087.
- Pollack B, Rosse C. The primary role of murine bone marrow in the production of natural killer cells: a cytokinetic study. Jour Immuol 1987; 139: 2149-2156.
- Rosmaraki EE, Douagi I, Roth C, Colucci F, Clumano A, Disanto JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur Jour Immunol 2001; 31(6): 1900-1909.
- Vecchini F, Delfino D, Patrene KD, Deleo A, Lu L, Herberman RE, Boggs SS. Generation of natural killer cells from long-term cultures of mouse bone marrow. Nat Immun 1993; 12:1-16.
- King ML, Adler SR, Murphy LL. Extraction-dependent effects of American ginseng (Panax quinquefolium) on human breast cancer cell proliferation and estrogen receptor activation. Int Cancer Ther 2006; 5(3): 236-243.
- Duda RB, Zhong Y, Navas V, Li MZC, Toy BR, Alavarez IG. American ginseng and breast cancer therapeutic agents synergistically inhibit mcf-7 breast cancer cell growth. Jour Surg Oncol 1999; 72: 230-239.