Research Article - Journal of Translational Research (2018) Volume 2, Issue 1
Inhibition of cancer cell immune evasion by combined application of cytotoxic T-lymphocytes and natural killer cells
Yongxin Zhang1*, Ying Wang1, William K. Decker2, Zhenying Wang1, Monica Zimmerman1
1Zyxell Inc. Carrollton, TX 75007, USA
2Department of Pathology and Immunology, Baylor College of Medicine, Houston, TX 77030, USA
- *Corresponding Author:
- Yongxin Zhang
Zyxell In.,
Carrollton, TX 75007
USA
Phone: 972-975-8600
E-mail: zyx@zyxellinc.com
Accepted date: March 16, 2018
Citation: Zhang Y, Wang Y, Decker KW, et al. Inhibition of cancer cell immune evasion by combined application of cytotoxic T-lymphocytes and natural killer cells. J Transl Res. 2018;2(1):12-19.
Abstract
Development of cancer is often dependent upon subversion of immune surveillance which can include alteration of MHC class I antigen expression to avoid recognition by cytotoxic T lymphocytes (CTL) and natural killer (NK) cells. CTL and NK cells recognize and destroy MHC class I positive and negative cancer cells, respectively. To determine whether selection pressure from CTL and/or NK cells can modulate MHC class I expression levels on cancer cells as well as to determine if combined application of CTL and NK cells can inhibit immune evasion of cancer cells, MHC class I antigen on cancer cells was examined by flow cytometry and cancer cell killing was evaluated by in vitro and in vivo cytotoxic assays. These exper-iments demonstrated that: 1) MHC class I antigen expression on any individual cancer cell can be highly variable, 2) the strength of MHC class I antigen expression on cancer cells can change during culture, 3) high levels of MHC class I expression mediated greater sensitivity to CTL killing whereas lower levels of MHC class I imparted greater sensitivity to NK cell killing, 4) immune selection pressure can actively modulate surface MHC class I expression, and 5) combined application of CTLs and NK cells mediated much more effective cancer cell cytotoxicity. These results suggest that combined application of CTLs and NK cells could significantly increase the efficiency of cancer cell killing by inhibiting positive and negative selection pressures.
Keywords
Cancer, Cell therapy, T-cells, NK Cells, MHC, Immuno-editing, Immune surveillance.
Introduction
The occurrence of cancer closely parallels progressive immune evasion in which nascent cancer cells escape from active immune surveillance. Immune evasion in cancer involves a variety of sophisticated mechanisms including inhibition of homeostatic regulatory cell function, defective cancer cell antigen presentation, and secretion of soluble immune suppressive factors [1]. A better understanding of these mechanisms would be of great benefit to cancer therapy and prevention. The elimination of cancer cells in vivo relies at least partially on the role of immunocytes, and there exist a variety of innate and adaptive immune cell subsets that are currently in use for cell therapy of cancer [2-10]. Cytotoxic T-lymphocytes (CTLs) and natural Killer (NK) cells are the two most widely studied immunocytes in cancer immunity. Both of these immune cell subsets exhibit powerful cytoreductive killing function but through very different mechanisms of action. CTLs work through the T cell receptor (TCR) which recognizes MHC class I complexed with specific peptide antigens on the target cell surface [11-13]. In contrast, NK cells possess ligandbased activating and inhibitory receptors (KIRs) which inhibit NK cell killing when bound to cells expressing normal levels of MHC but actively kill bound target cells that exhibit loss of MHC and/or up-regulation of stress-induced ligands [14-16]. Thus, CTLs and NK cells work through opposing mechanisms to eliminate cancer cells in the body. In previous therapeutic studies, investigators have applied either CTLs (including CAR-T cells) or NK cells with promising preliminary results, yet complete eradication and remission is not often observed in the monotherapeutic setting [2,5,6,15].
In our recent studies involving primary cancer cell culture, we observed that cancer cells often express MHC molecules at variable levels and that the mean density of MHC expression, as detected by flow cytometry, is different at different stages of culture. This critical observation lead us to investigate if similar shifts in MHC class I antigen expression could also occur in response to cytoreductive selective pressures from CTL or NK cells. Adoptive transfer of combined NK and CTLs was also tested to validate in vitro experimental results in an in vivo model system.
Materials and Methods
Human cancer cell culture
Breast cancer (Btc002, tubular carcinoma), prostate cancer (Pac 001, adenocarcinoma), ovarian cancer (Oec 003, epithelial ovarian cancer), lung cancer (PLs008, small cell lung cancer), and liver cancer (Lhcc 006, hepatocellular carcinoma) primary tumor tissues were obtained from the ZYX Biotech Company (Carrollton, TX) and isolated as described previously [17]. Breast cancer cells were grown in Eagle's MEM supplemented with 10 μg/μL insulin. Prostate cancer cells were grown in Ham's medium/nutrient mixture F-12. Ovarian cancer cells were grown in DMEM/F-12, and liver cancer cells were cultured in DMEM. All media were supplemented with 10% fetal bovine serum except for liver cancer cells which were cultured in 20% FBS. Cells were seeded (4 cultures/cell line) in 10 ml cell culture bags with magnetic beads for ZYX bioreactor culture [13,17] at the following densities: breast cancer, prostate cancer and ovarian cancer cells at 1 × 105/ml, lung cancer cells at 2 × 104/ml, and liver cancer cells at 2.5 × 104/ml. Pre-, day 3 and day 6 post-culture, adherent cells were dissociated with enzyme-free cell dissociation buffer (Thermo-Fisher Scientific, Waltham, MA) and analyzed by flow cytometry for MHC class I antigen expression.
Cell enumeration and flow cytometry
Total cell counts were determined by hemacytometry following trypan blue staining and flow cytometry with propidium iodide (PI) staining. CTL enumeration was conducted using flow cytometry, and cell markers were stained (including intracellular stain) by means of established standard protocols [17-21]. Specifically, cancer cells were stained with FITCanti- human HLA-A,B,C antibody (Biolegend, San Diego, CA, Mouse IgG2a, κ) and PE-anti-human CD45 (Biolegend, Mouse IgG1, κ). Activated CTLs were stained with PerCP-antihuman CD8 (Biolegend, Mouse IgG1, κ) and PE-anti-human CD137 (Biolegend, Mouse IgG1, κ) [13,17-21]. NK cells were defined as CD3-CD56+ based on staining with PerCP-antihuman CD3 (Biolegend, Mouse IgG2a, κ), FITC-anti-human CD56 (Biolegend, Mouse IgG1, κ) and PE-anti-human CD69 (Biolegend, Mouse IgG1, κ) to determine activation status [22- 24]. Suitable isotype controls from the same manufacturer were applied. Cells were stained (including intracellular staining for the analysis of MHC class I antigen expression in cancer cells) and analyzed as described previously [13,17-21]. All cells from pre- and post- culture used for the staining were derived from the same lot (cells from the same tissue and processed in the same container at the same time). Flow-Check™ Fluorospheres (Beckman Coulter, Inc. Cat#6605359) were used to calibrate the flow cytometer prior to each use. In addition to the percentage of positive cells, mean fluorescence intensity (MFI) was used to evaluate the MHC class I expression.
Immune effector expansion and cytotoxic assays
Commercially available pairs of autologous human cancer cells (small cell lung cancer [PLs008]) [17] and peripheral blood mononuclear cells (PBMC, [Mnc013], ZYX Biotech) from a normal healthy donor (as allogenic NK cell targets) were used in this study. Cancer cells were approximately 80% MHC class I-positive and 100% CD45-negative. All mononuclear cell fractions contained at least 108 CD3+CD45+ cells and all were positive for CD45. Cytokines and other reagents for CTL and NK cell culture and detection have been described previously [17-21]. PLs008 cancer cells were used as stimulators for CTL expansions and as targets for CTL and NK cell lytic assays. Autologous PBMC were bioreactor-sorted18 to separate CD3+ and CD3- fractions which were then stimulated to generate CTL and NK cell effectors respectively. An established protocol [17-21] with slight modifications was used in this study. Briefly, (1) CD3+ and CD3- cells from the mononuclear fraction were separated in the bioreactor. The CD3+ cells were maintained in the original reaction chamber (1st RC) for CTL expansion whereas the CD3- cells were sorted into an auxiliary reaction chamber (2nd RC) for NK cell expansion. (2) For CTL expansions, cancer cells were grown and enriched in cell culture bags of ZYX Bioreactor for 6 days as described above. Irradiated (20 Gy) cancer cells were cultured with the corresponding autologous CD3+ cells in the ZYX Bioreactor [13,17] in 8 ng/mL IL-2 and 10 ng/mL IL-7 for 12 days, as determined by the ZYX bioreactor control algorithm [17]. (3) For NK cell expansions, autologous or allogenic CD3- cells were cultured in 8 ng/mL IL-2, 50 ng/ml IL-12 and 10 ng/ ml IL-18 for 12 days as determined by the ZYX btr control algorithm [13,17,22-24]. All cytokines were purchased from PeproTech (Rocky Hill, NJ). (4) Following cell expansion, CD8+ cells were isolated from the 1st RC (for CTL enrichment) and CD56+ cells were isolated from the 2nd RC (for NK cell enrichment) by the automated bioreactor-embedded cell sorter using anti-CD8 and anti-CD56 magnetic separation beads (Miltenyi Biotec, San Diego, CA) as described previously [17]. After isolation, purities of CD8+ cells and CD56+ cells were >95% and >92%, respectively, and CD8+CD137+ activated CTL [25-28] and CD56+CD69+ activated NK cell was >88% and >81%. Controls consisted of SEB-stimulated cells, unstimulated cells, and/or cells stimulated with autologous and allogeneic tumor cells. For allogenic effectors, only NK cell expansion and enrichment were conducted.
Analysis of cancer cell MHC class I expression
Cancer cell seeding density was 0.2 × 106/ml; CTL, NK cell, and autologous PBMC seeding density was 0.5 - 1.0 × 106/ml. IL-2 was added at a concentration of 8ng/ml during the cytolytic expansion. Cells were cultured for 10 days and cells were examined for their MHC class I expression by flow cytometry at the end of co-culture.
For testing the effects of CTLs and NK cells on cancer cell MHC class I expression, co-cultures were labeled as A (cancer cells alone), B (cancer cells+autologous NK cells), C (cancer cells+allogenic NK cells), D (cancer cells+CTLs), E (cancer cells+CTLs+allogenic NK cells), F (cancer cells+CTLs+allogenic NK cells), G (cancer cells+CTLs+allogenic NK cells+autologous PBMC), and H (cancer cells+CTLs+allogenic NK cells+autologous PBMC). PBMCs from a patient and health donor were processed for the activation and expansion of cancerspecific CTL, autologous NK and allogenic NK cells as aforementioned.
For testing the effects of lower Effector/Target cell ratio (E/T ratio) on the MHC class I expression of target cells, 106/ ml effector cells (CTLs or autologous NK cells), 0.5x106/ml effector cells (50%) with 0.5x106/ml autologous PBMC and no effector controls were also cultured.
in vitro cytotoxicity assay
in vitro cytotoxic assays were performed as described previously [13,17-21] using lung cancer cells as stimulators for the CTL assay and as targets for both the CTL and NK cell assays. The LDH Cytotoxicity detection Kit (Clontech, Mountainview, CA) was used for cytotoxicity analysis according to the manufacturer’s instructions with slight modification as described previously [17-21].
in vivo cytotoxicity assay
Cancer cells were enriched and injected subcutaneously into NOD/scid mice as described previously [17]; cancer-specific CTLs and NK cells were prepared as described above and adoptively transferred into mice by tail vein injection 24 hours after cancer cell implantation. Four weeks and six weeks after cancer cell inoculation, the size of subcutaneous cancer nodules was measured (length × width × thickness [mm3]) in a blinded fashion.
Mice and grouping
Animals were maintained at Zyxell Inc in accordance with an IRB approved protocol and according to the principles expressed in the National Institutes of Health, USPHS, Guide for the Care and Use of Laboratory Animals. Female 12-16 week-old NOD/ scid mice (Charles River Laboratories, Wilmington, MA) were housed in a specific pathogen-free environment in which cages covered with barrier filters were housed in laminar flow hoods. Twenty four hours before cancer cell inoculation, mice were given 3.5 Gy gamma-irradiation as previously described [17]. Each irradiated NOD/scid mouse (6 per cohort) received 1 × 106 lung cancer cells subcutaneously in the dorsolateral thorax and 2 × 106 expanded CD8+ T cells or NK cells by intravenous injection 24 hours later. Tumor size was measured in a blinded fashion every other week for 6 weeks. In the in vivo human CTL study, the human lung cancer cells from the same patient were used as stimulator cells (irradiated at 20 Gy) for CTL expansion and as target cells for subcutaneous inoculation, and the CTL and autologous NK cells were from the peripheral blood of the same patient and the allogenic NK cells were from a healthy donor.
Cohorts were A (cancer cells alone), B (cancer cells+autologous NK cells), C (cancer cells+allogenic NK cells), D (cancer cells+CTLs), E (cancer cells+CTLs+allogenic NK cells), F (cancer cells+CTLs+allogenic NK cells), G (cancer cells+CTLs+allogenic NK cells+autologous PBMC), and H (cancer cells+CTLs+allogenic NK cells+autologous PBMC).
Detection of early stage apoptosis
Annexin V binding assay was performed with an established protocol [17,20]. The percentage of Annexin V+/CD45+ (lymphocytes) cells and Annexin V+/CD45- cells (cancer cells) were determined.
Interferon gamma (IFN-γ) detection
An enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) was used to detect IFN-γ in co-culture supernatants using a previously established protocol [17-21].
Data analysis
Analysis of variance was used to compare the numbers of CTL and NK cell and the percent cell lysis between different groups. SAS and SAS Statview (SAS Institute, Cary, NC, USA) were used to perform analyses. A p-value <0.05 was considered to be significant. All experiments are the result of six repetitions unless stated otherwise.
Results and Discussion
MHC class I antigen shift of cancer cells in primary culture
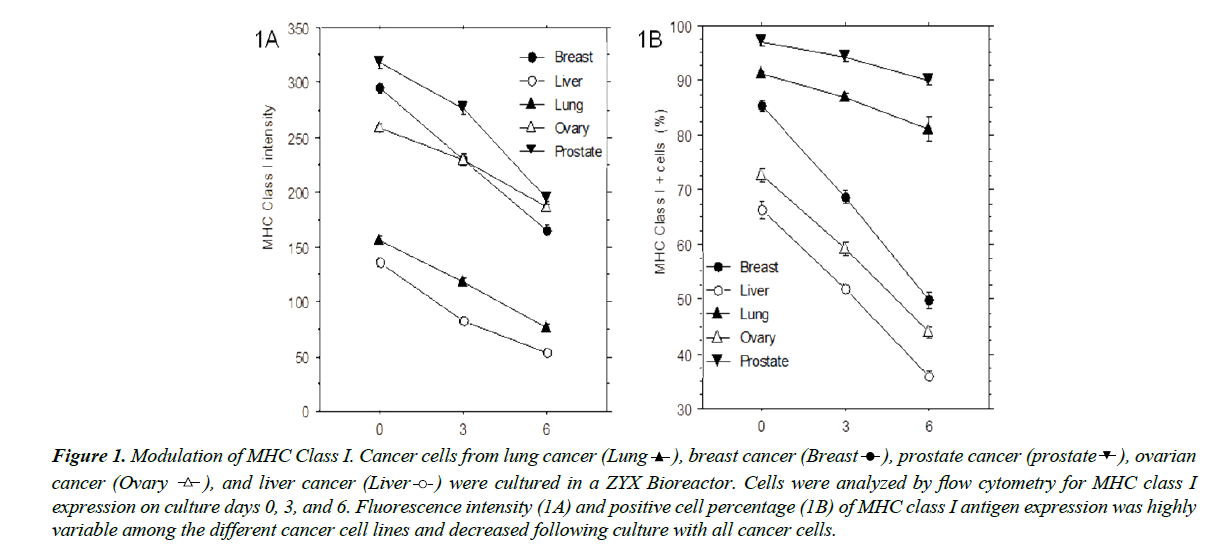
Cryopreserved cancer cells derived from lung cancer, breast cancer, prostate cancer, ovarian cancer, and liver cancer were cultured in the ZYX bioreactor, and MHC class I surface expression was analyzed by flow cytometry on culture days 0, 3, and 6. All five cell lines expressed highly variable levels of MHC class I as reflected by fluorescence intensity and total number of positive cells. As shown in Figure 1A and 1B, both of these declined significantly (p<0.05) during culture. Cells from different cancer tissues exhibited variable fluorescence intensity and total numbers of positive cells, indicating that the MHC class I expression on cancer cells can be unstable and/or inconsistent.
Figure 1: Modulation of MHC Class I. Cancer cells from lung cancer (Lung  ), breast cancer (Breast
), breast cancer (Breast  ), prostate cancer (prostate
), prostate cancer (prostate  ), ovarian
cancer (Ovary
), ovarian
cancer (Ovary  ), and liver cancer (Liver
), and liver cancer (Liver  ) were cultured in a ZYX Bioreactor. Cells were analyzed by flow cytometry for MHC class I
expression on culture days 0, 3, and 6. Fluorescence intensity (1A) and positive cell percentage (1B) of MHC class I antigen expression was highly
variable among the different cancer cell lines and decreased following culture with all cancer cells.
) were cultured in a ZYX Bioreactor. Cells were analyzed by flow cytometry for MHC class I
expression on culture days 0, 3, and 6. Fluorescence intensity (1A) and positive cell percentage (1B) of MHC class I antigen expression was highly
variable among the different cancer cell lines and decreased following culture with all cancer cells.
Among these cancer cell lines, the cells from prostate cancer, ovarian cancer, and liver cancer did not exhibit significant responses to CTL toxicity, and the breast cancer had a limited cell number. Therefore, these cell lines could not be used for the studies on the effects of CTLs and NK cells on cancer cell immune evasion, and only lung cancer cells (PLs008) were applied to further studies [17].
Impact of CTL and NK cell co-culture on cancer cell MHC class I expression
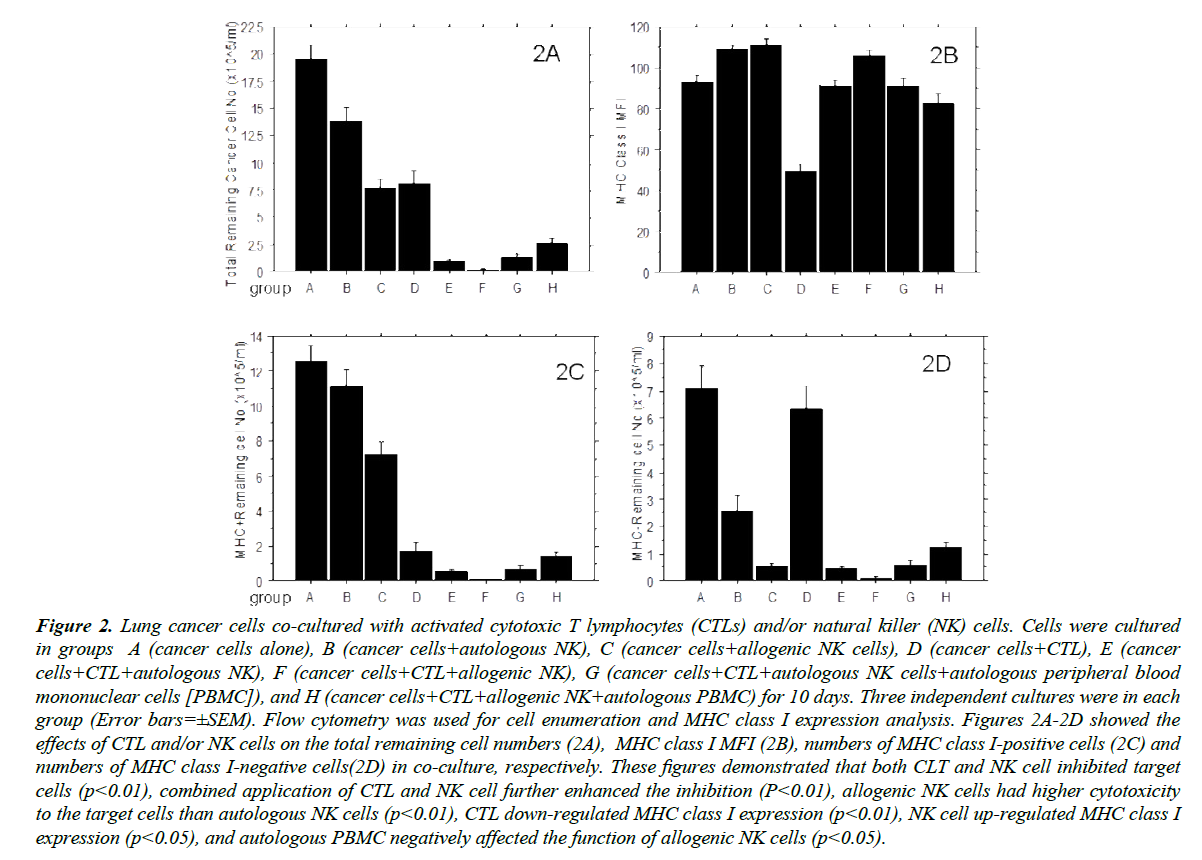
Lung cancer cells expressing MHC class I (HLA-A, B, C) at a pre-culture MFI of 162 (average of 6 detections with 83% of cells MHC class I-positive) were co-cultured in eight different ways: A (cancer cells alone), B (Cancer cells+autologous NK cells), C (cancer cells+allogenic NK cells), D (cancer cells+CTL), E (cancer cells+CTL+autologous NK cells), F (cancer cells+CTL+ allogenic NK cells), G (cancer cells+CTL+autologous NK cells+autologous PBMC) and H (cancer cells+CTL+allogenic NK cells+autologous PBMC). After 10 days of in vitro culture, cells were harvested and CD45- cells analyzed by flow cytometry. As indicated in Figure 2A, autologous NK cells significantly inhibited (p<0.01) the growth of the lung cancer cells and allogenic NK cells inhibited cancer cell growth to a substantially greater degree than the autologous NK cells (p<0.01), consistent with previously-reported findings [22-24]. The degree to which autologous CTL inhibited cancer cell growth was greater than that of autologous NK cells as anticipated due to high levels of MHC class I expression (>82%) present on the surface of pre-culture tumor cells and CTL could only recognize MHC Class I antigen-positive cells while NK cells target MHC Class I antigen-negative cells. When cancer cells were co-cultured with CTL and NK cells, the remaining cell numbers were further decreased in comparison to conditions A, B, C, and D, indicating that combined application of CTL and NK cells can more effectively kill cancer cells. The remaining cancer cell numbers in co-culture with allogenic NK cells was also lower than those cultured with autologous NK cells, suggesting autologous CTL and allogenic NK cells can synergistically lyse target cell populations. However, when nonselected autologous PBMC were added to cultures of CTL+NK cells, these results were reversed, suggesting that autologous PBMC may functionally inhibit the activity of allogenic NK cells. Further studies revealed that IFN-γ levels in group H cultures was nearly three-fold (2.72 ± 0.53, p<0.05) higher than that of group G. Further, there was a higher percentage of annexin V+ cells in co-cultures of allogenic NK cells and PBMC than in autologous NK cells and PBMC (23.6 ± 8.4% vs. 6.2±1.7%, p<0.01). PI staining results were consistent with Annexin V staining, but at relatively lower levels (12.2 ± 4.7% vs. 3.9 ± 1.2%, p<0.01). Moreover, >50% (58.4 ± 7.3%) of annexin V+ cells were also CD56+, suggesting that autologous PBMC interacted negatively with allogenic NK cells. Indeed, IL-2 in the culture media, which used to maintain the activity of NK cells and CTL, also unavoidably activated the immunocytes in the autologous PBMCs and further damaged the allogenic NK cells. As NK cells also take part in immune rejection (described by Benichou) [25], the allogenic NK cell reactivity to cancer cells was reduced in the presence of additional target cells such as autologous PBMCs applied in the study.
Figure 2: Lung cancer cells co-cultured with activated cytotoxic T lymphocytes (CTLs) and/or natural killer (NK) cells. Cells were cultured in groups A (cancer cells alone), B (cancer cells+autologous NK), C (cancer cells+allogenic NK cells), D (cancer cells+CTL), E (cancer cells+CTL+autologous NK), F (cancer cells+CTL+allogenic NK), G (cancer cells+CTL+autologous NK cells+autologous peripheral blood mononuclear cells [PBMC]), and H (cancer cells+CTL+allogenic NK+autologous PBMC) for 10 days. Three independent cultures were in each group (Error bars=±SEM). Flow cytometry was used for cell enumeration and MHC class I expression analysis. Figures 2A-2D showed the effects of CTL and/or NK cells on the total remaining cell numbers (2A), MHC class I MFI (2B), numbers of MHC class I-positive cells (2C) and numbers of MHC class I-negative cells(2D) in co-culture, respectively. These figures demonstrated that both CLT and NK cell inhibited target cells (p<0.01), combined application of CTL and NK cell further enhanced the inhibition (P<0.01), allogenic NK cells had higher cytotoxicity to the target cells than autologous NK cells (p<0.01), CTL down-regulated MHC class I expression (p<0.01), NK cell up-regulated MHC class I expression (p<0.05), and autologous PBMC negatively affected the function of allogenic NK cells (p<0.05).
Figure 2B indicates MFI of MHC class I in the different groups. The MHC class I antigen in group A (cancer cells alone) decreased significantly compared to pre-culture (286.72), consistent with Figure 1A and suggesting that MHC class I expression on cancer cells shifted during culture. Although the co-cultures with NK cells exhibited a decrease in the total number of remaining cancer cells (Figure 2A) and a decrease in the number of remaining MHCneg cells (Figure 2D), groups B and C exhibited increased MHC class I intensity compared to group A, suggesting that NK cells killed or inhibited cancer cells lacking MHC class I or with diminished levels of MHC class I expression, but could not inhibit cancer cells with higher levels MHC class I expression. In contrast, CTLs in group D inhibited MHC class I positive cancer cells. These killed or inhibited cancer cells that expressed higher levels MHC class I but could not inhibit cancer cells with reduced MHC class I expression levels as indicated by the significantly diminished level of MHC class I expression on the remaining cells in the CTL treated group compared to control. The combination of CTL and NK cells exhibited the greatest cancer cell killing effect but only in co-culture with allogenic NK cells (group F); the remaining cells exhibited increased MHC class I antigen intensity. Mean MHC class I antigen intensity in the remaining cells (group H) was not significantly affected when the allogenic NK cells were inhibited by autologous PBMC, suggesting a negative effect of autologous PBMC on the functions of allogenic NK.
Figure 2C and 2D further show the impact of CTLs and NK cells on expression of MHC class I in the remaining cancer cells. When compared to control, MHC class I-negative cancer cells (Figure 2D) decreased markedly in the culture with autologous and allogenic NK cells, whereas MHC class I-positive cancer cells (Figure 2C) did not change significantly with autologous NK cells and only moderately decreased with allogenic NK cells. CTLs significantly reduced the MHC class I-positive cancer cells but did not have a significant effect on the MHC class I-negative cancer cells. MHC class I-positive and -negative cancer cells were all further reduced when both NK cells and CTLs were seeded in the culture, and the inhibitory effects of autologous PBMC on the allogenic NK cells were the same for MHC class I-positive and -negative cancer cells. In this experiment, co-culture of cancer-specific CTLs with cancer cells resulted in downregulation of MHC class I expression, and a high percentage of these cancer cells no longer express MHC class I antigen. In contrast, when NK cells were cultured with the cancer cells, the remaining cancer cells in the culture with NK cells strongly upregulated MHC class I and nearly all of the cancer cells became MHC class I-positive.
in vivo cytotoxicity
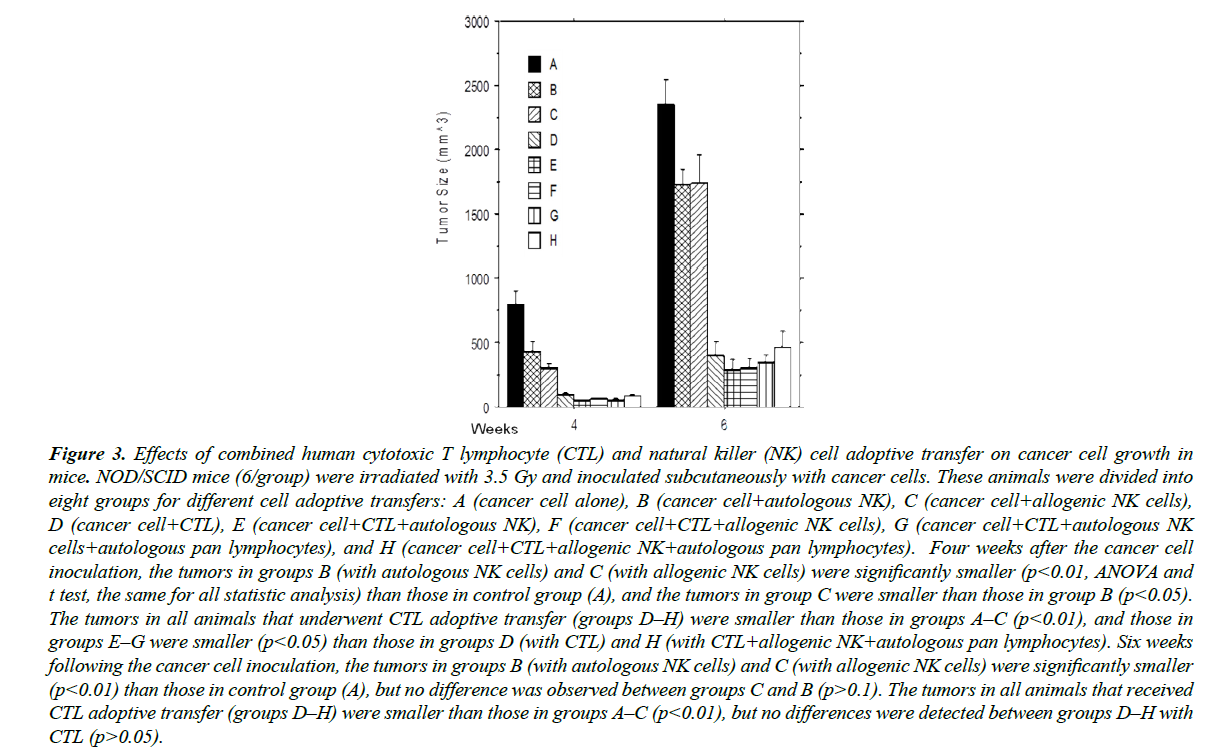
CTLs and NK cells were adoptively transferred into mice 24 hours after cancer cell inoculation, and tumor size was measured at 4 and 6 weeks following inoculation (Figure 3). The results at 4 weeks were consistent with the aforementioned in vitro studies. Autologous NK cells inhibited cancer development, yet allogenic NK cells (C) were even more efficient than autologous NK cells at inhibiting cancer cell growth (B), consistent with clinical observations [22-24]. The inhibition of tumor development by CTLs (D) was significantly stronger than that of autologous NK cells (B), a phenomenon likely related to the greater number of MHC class I-positive cells in the target cell population, as CTLs normally only recognize MHC class I antigen-positive cells. When CTL (E) and NK cells (F) were injected into the mice in tandem, tumor growth was most-potently inhibited in comparison to A, B, C, and D, indicating the combined administration of CTL and NK cells more effectively killed cancer cells. Tumors treated with allogenic NK cells (F) were also smaller than those treated with autologous NK cells (E), further underscoring the concept that autologous CTLs and KIR-mismatched allogenic NK cells can synergistically act on target cells. However, the results were reversed when autologous PBMCs were mixed with NK cells to produce groups G and H, suggesting that some components in autologous PBMCs negatively affected the killing function of allogenic NK cells. Consistent with other studies [22-24,28-30], our data indicate that allogeneic NK cells were more cytotoxic to cancer cells than autologous NK cells, although cytokineactivated autologous NK cells gained multi-log-fold killing activity against target cells after ex vivo expansion. However, our current studies on co-culture of cancer cells, allogeneic NK cells, and total autologous PBMCs also demonstrate that autologous PBMCs inhibit target activity of allogeneic NK cells to cancer cells, a phenomenon caused, in part, by a natural host versus graft reaction. Considering many patients undergoing immunocyte therapy are fully immunocompetent, adoptively transferred allogeneic NK cells may be rejected, particularly upon repeated administration, significantly compromising their anticancer function. Therefore, it could be important to keep it as an option to use autologous NK cell adoptive transfer, particularly in combination with CTL adoptive transfer, in clinical cancer immunotherapy.
Figure 3: Effects of combined human cytotoxic T lymphocyte (CTL) and natural killer (NK) cell adoptive transfer on cancer cell growth in mice. NOD/SCID mice (6/group) were irradiated with 3.5 Gy and inoculated subcutaneously with cancer cells. These animals were divided into eight groups for different cell adoptive transfers: A (cancer cell alone), B (cancer cell+autologous NK), C (cancer cell+allogenic NK cells), D (cancer cell+CTL), E (cancer cell+CTL+autologous NK), F (cancer cell+CTL+allogenic NK cells), G (cancer cell+CTL+autologous NK cells+autologous pan lymphocytes), and H (cancer cell+CTL+allogenic NK+autologous pan lymphocytes). Four weeks after the cancer cell inoculation, the tumors in groups B (with autologous NK cells) and C (with allogenic NK cells) were significantly smaller (p<0.01, ANOVA and t test, the same for all statistic analysis) than those in control group (A), and the tumors in group C were smaller than those in group B (p<0.05). The tumors in all animals that underwent CTL adoptive transfer (groups D–H) were smaller than those in groups A–C (p<0.01), and those in groups E–G were smaller (p<0.05) than those in groups D (with CTL) and H (with CTL+allogenic NK+autologous pan lymphocytes). Six weeks following the cancer cell inoculation, the tumors in groups B (with autologous NK cells) and C (with allogenic NK cells) were significantly smaller (p<0.01) than those in control group (A), but no difference was observed between groups C and B (p>0.1). The tumors in all animals that received CTL adoptive transfer (groups D–H) were smaller than those in groups A–C (p<0.01), but no differences were detected between groups D–H with CTL (p>0.05).
A marked decrease in expression of MHC class I antigens was found in many cancer cells and cancer cell lines, with which the existence of a relevant mechanism for evading the host immune response to the tumor was suggested in previous studies [31- 33]. Our data further reveal that the expression of MHC class I antigens shifted under certain conditions, particularly under the immune selection pressure from CTLs or NK cells, in which cancer cells evaded the immune attack of immunocytes. Therefore, the combined application of CTLs and NK cells is more ideal to eliminate cancer cells, as shown in our current study.
Cytotoxicities of CTLs and NK cells
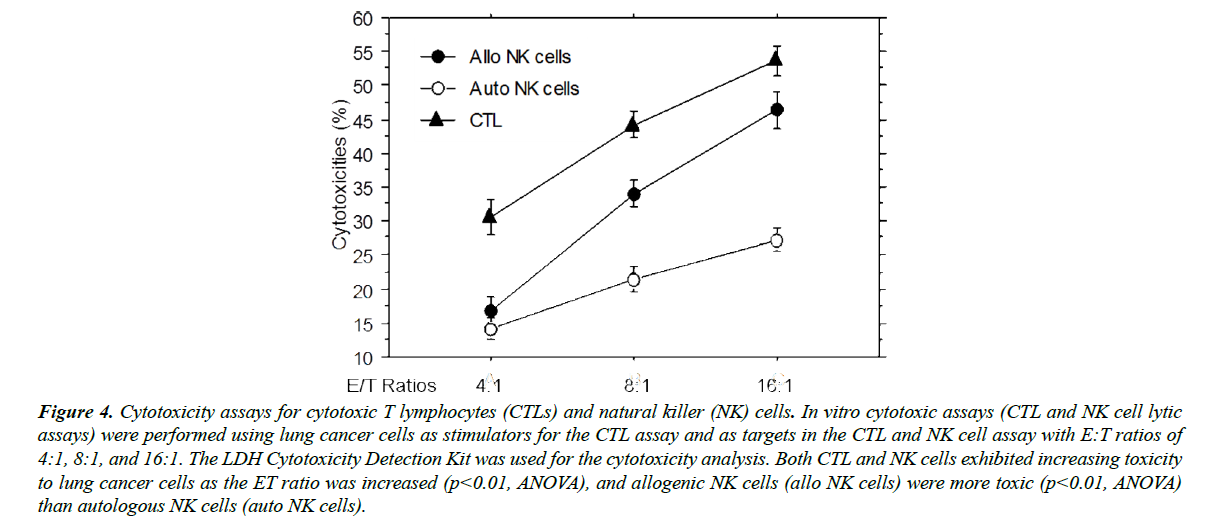
Cytotoxicity assays were conducted to confirm bona fide cytotoxic activities of the CTLs and NK cells used in this study. As shown in Figure 4, all CTLs, allogenic NK cells, and autologous NK cells exhibited potent target cell lytic activity. The cytotoxicity of the CTLs was higher than that of NK cells due to the greater number of MHC class I-positive target cells (>80%). Consistent with other studies [22-24], KIRmismatched allogenic NK cells lysed target cancer cells more efficiently than autologous NK cells, especially at higher E:T ratios. Considering that NK cells are also related to immune rejection [25] in HLA-mismatched organ transplantation, these data support that allogenic NK cells are also toxic to recipients’ cells that express the MHC class I antigen at the normal level.
Figure 4: Cytotoxicity assays for cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. In vitro cytotoxic assays (CTL and NK cell lytic assays) were performed using lung cancer cells as stimulators for the CTL assay and as targets in the CTL and NK cell assay with E:T ratios of 4:1, 8:1, and 16:1. The LDH Cytotoxicity Detection Kit was used for the cytotoxicity analysis. Both CTL and NK cells exhibited increasing toxicity to lung cancer cells as the ET ratio was increased (p<0.01, ANOVA), and allogenic NK cells (allo NK cells) were more toxic (p<0.01, ANOVA) than autologous NK cells (auto NK cells).
In this study, the ZYX Bioreactor was used for cell expansion and cellular biologic reactions. The ZYX Bioreactor is an automatic cell expansion system with significant advantages that permit elegant and precise cell culture-based experimental procedures [13,17,34]. (1) The bioreactor’s kinetic and static alternating culture programs allow cells to receive adequate metabolic support but minimal media flow shear-stress, permitting faster growth than conventional static cultures. (2) The bioreactor is configured to conduct cell culture and cell sorting in the same cell culture chamber. (3) The bioreactor can be programmed to maintain adequate contact among cells, critical for cytotoxicity tests with CTL and NK cells. (4) Sensitive instrumentation automatically determines peak cell harvest time during cell expansion. There also exists a live tissue-mimicking function (the gradient osmosis perfusion system); however, this function was not utilized here. Therefore, the data from the animal experiments of this study necessarily provided a solid support to conclusion obtained from ex vivo cell culture.
Our results indicated significant modulation in expression of MHC class I in many cancer cells and cancer cell lines in response to co-culture with immune effectors as well as the existence of a potential mechanism for evading the host immune response as suggested by previous studies [31-33]. The data further reveal that the expression of MHC class I antigens modulated under certain conditions, particularly under the stress of CTL or NK cell attack, leading to cancer cell immune evasion and proliferation of resistant cells. Therefore, the combined application of CTLs and NK cells is more ideal to eliminate cancer cells, as shown in our current study.
We also found significant evidence that the mechanism by which tumor cells modulate surface MHC expression in response to cytolytic attack is an active process. This apparent ability of cancer cells to adapt in response to selection pressure from immunocyte effectors could be an important mechanism by which cancer cells evade immune surveillance and a critical reason for failures of previous anticancer cell therapies. When we co-cultured cancer cells with cancer-specific CTLs and NK cells together, the number of remaining cancer cells was significantly reduced in comparison to co-cultures with CTLs or co-cultures with NK cells alone. These data suggest that applying both effector cell types in tandem could have significantly enhanced antitumor effects.
In summary, our study demonstrated that (1) MHC class I antigen expression in cancer cells can vary and shift under certain conditions. (2) CTLs mainly impact MHC class I-positive cancer cells, whereas NK cells mainly impact MHC class I-negative cancer cells. (3) Allogenic (KIR-mismatched) NK cells were more cytolytic to cancer cells than autologous NK cells. (4) Immune selection pressures exerted by CTLs and NK cells on cancer cells could have resulted in MHC class I antigenic shift in cancer cells, further impacting immune evasion. (5) Combined application of CTLs and NK cells could significantly increase the cytotoxic effect by reducing the ability to evade cytolysis by MHC class I antigen shift. Additional in vitro and in vivo studies will be necessary to validate these findings and to translate them into clinical practice.
References
- Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. In Seminars in cancer biology. 2015;35:185-98.
- Raval RR, Sharabi AB, Walker AJ, et al. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer. Journal for immunotherapy of cancer. 2014;2(1):14.
- Kwong MLM, Neyns B, Yang. Adoptive T-cell transfer therapy and oncogene-targeted therapy for melanoma: the search for synergy. 2013;5292-99.
- Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Frontiers in oncology. 2013;3.
- Brenner MK. Will T-cell therapy for cancer ever be a standard of care? Cancer gene therapy. 2012;19(12):818-21.
- Rezvani K, Brody JD, Kohrt HE, et al. Cancer vaccines and T cell therapy. Biology of Blood and Marrow Transplantation. 2013;19(1):97-101.
- Shi H, Liu L, Wang Z. Improving the efficacy and safety of engineered T cell therapy for cancer. Cancer letters. 2013;328(2):191-7.
- Franks HA, Wang Q, Patel PM. New anticancer immunotherapies. Anticancer research. 2012;32(7):2439-53.
- Bernatchez C, Radvanyi LG, Hwu P. Advances in the treatment of metastatic melanoma: adoptive T-cell therapy. In Seminars in oncology. 2012;39(2):215-26.
- Pedrazzoli P, Comoli P, Montagna D, et al. Is adoptive T-cell therapy for solid tumors coming of age? Bone marrow transplantation. 2013;47(8):1013-9.
- Stiles AR, Liu CZ. Hairy root culture: bioreactor design and process intensification. In Biotechnology of hairy root systems. 2013;91-114.
- Wehler TC, Nonn M, Brandt B, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109(1):365-373.
- Zhang Y, Wang Y, Zhang M, et al. Restoration of retarded influenza virus-specific immunoglobulin class switch in aged mice. Journal of clinical & cellular immunology. 2015;7(2).
- Hivroz C, Chemin K, Tourret M, et al. Crosstalk between T lymphocytes and dendritic cells. Critical Review in Immunology. 2012;32(2).
- Hoyer S, Prommersberger S, Pfeiffer IA, et al. Concurrent interaction of DCs with CD4+ and CD8+ T cells improves secondary CTL expansion: It takes three to tango. European journal of immunology. 2014;44(12):3543-59.
- Becker PS, Suck G, Nowakowska P, et al. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunology, Immunotherapy. 2016;65(4):477-484.
- Zhang Y, Wang Y, Wang Z, et al. Cancer Specific CTL Expansion with ZYX Bioreactor. J Clin Cell Immunol. 2016;7:398.
- Mbawuike IN, Zhang Y, Couch RB. Control of mucosal virus infection by influenza nucleoprotein-specific CD8+ cytotoxic T lymphocytes. Respiratory research. 2007;8(1):44.
- Zhang Y, Qiu J, Zhou Y, et al. Plasmid-based vaccination with candidate anthrax vaccine antigens induces durable type 1 and type 2 T-helper immune responses. Vaccine. 2008; 26(5):614-22.
- Zhang Y, Wang Y, Gilmore X, et al. Apoptosis and reduced influenza A virus specific CD8+ T cells in aging mice. Cell death and differentiation. 2002;9(6):651.
- Zheng B, Zhang Y, He H, et al. Rectification of age-associated deficiency in cytotoxic T cell response to influenza A virus by immunization with immune complexes. The Journal of Immunology. 2007;179(9):6153-9.
- Cata JP, Conrad C, Rezvani K. Potential Use of Natural Killer Cell Transfer Therapy in the Perioperative Period to Improve Oncologic Outcomes. Scientifica. 2015.
- Sakamoto N, Ishikawa T, Kokura S, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. Journal of translational medicine. 2015;13(1):277.
- Ishikawa E, Tsuboi K, Saijo K, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer research. 2004;24(3):1861-1871.
- Benichou G, Yamada Y, Aoyama A, et al. Natural killer cells in rejection and tolerance of solid organ allografts. Current opinion in organ transplantation. 2011;16(1):47.
- Wolfl M, Kuball J, Ho WY, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110(1):201-210.
- Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. Journal of immunotoxicology. 2012;9(3):241-247.
- Li SY, Liu Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clinical pharmacology: advances and applications. 2013;5(1):47.
- Copier J, Bodman-Smith M, Dalgleish A. Current status and future applications of cellular therapies for cancer. Immunotherapy. 2011;3(4):507-516.
- Grupp SA. Adoptive cellular therapy. Curr Top Microbiol Immunol. 2011;344:149-72.
- Doyle AUSTIN, Martin WJ, Funa KEIKO, et al. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. Journal of Experimental Medicine. 1985;161(5):1135-51.
- Kaczmarek M, Frydrychowicz M, Rubis B, et al. Analysis of expression of MHC class I molecules and TAP genes in malignant human cell lines. Folia Histochemica et Cytobiologica. 2007;45(3):205-214.
- Wells AD, Rai SK, Salvato MS, et al. Restoration of MHC class I surface expression and endogenous antigen presentation by a molecular chaperone. Scandinavian journal of immunology. 1997;45(6):605-612.
- Zhang Y, Wang Y, Wang Z, et al. In Vitro Evaluation of Anticancer Drugs with Kinetic and Static Alternating Cell Culture System. Journal of Cancer Therapy. 2017;8(09):845.