Research Article - Biomedical Research (2017) Volume 28, Issue 16
Inhibition effect of ATRA nanostructured lipid carriers on IL-17, ICAM-1, MIP-2, MCP-1, and Ip-10 releasing and relative signal pathways induced by zymosan
Hongyan Zhou1, Xunyi Gao1, Wensong Zhang2*, Hongguang Zhang3 and Ning Kong4
1Department of Ophthalmology, China-Japan Union Hospital of Jilin University, Changchun, Jilin, PR China
2Department of Ophthalmology, the Second Hospital of Jilin University, Changchun, Jilin, PR China
3Department of Chemical Engineering and Application, College of Chemistry of Jilin University, Changchun, Jilin, PR China
4College of Pharmacy of Jilin University, Changchun, Jilin, PR China
- *Corresponding Author:
- Wensong Zhang
Department of Ophthalmology
The Second Hospital of Jilin University
Changchun, Jilin, PR China
Accepted on July 12, 2017
Abstract
Fungal Keratitis (FK) is the main corneal infection that is resistant to antifugi drugs. Exploring new agent will be important for the drug resistance and new infectious diseases. All-Trans-Retinoic Acid (ATRA), a bioactive derivative of vitamin A, is immunoregulatary and anti-inflammation agent by decreasing the expression of the pro-inflammatory cytokines and increasing the expression of antiinflammatory factors. The study was to investigate the potential role of ATRA to be a supplementary method for the therapy of FK. The aim of this study was to investigate the role of ATRA Nanostructured Lipid Carriers (NLC) in the zymosan induced cytokines (IL-17, ICAM-1, MIP-2, MCP-1 and Ip-10) by Corneal Fibroblasts (CFs) and to investigate the characters of ATRA-NCL. ATRA-NLC was prepared by the method of Emulsification process from scratch. ATRA-NCL concentration was calculated by High Performance Liquid Chromatography (HPLC) method. ATRA-NCL physical stability was observed. ATRA-NCL Cytotoxicity assay was performed by the detection of Lactate Dehydrogenase (LDH). The role of ATRA-NCL on the release of IL-17, ICAM-1, MIP-2, MCP-1, IP-10 induced by zymosan was examined by ELISA. The relative NF-KB-p65, P-ERK1, 2, P-IκBα cell signal pathway was assayed by immune blot analysis. The mean diameter of ATRA-NLC was 200 nm. ATRA-NCL physical stability study showed well at 4°C without precipitation and crystallization. The NLC particles were dispersed well under the scanning electron microscope (S-4800). Scanning electron microscope image of the ATRA particles were shown. ATRA-NCL showed no cytotoxic effect on KCs. ATRA-NCL inhibited zymosan-induced IL-17, ICAM-1, MIP-2, MCP-1, Ip-10 release by CKs. ATRA-NCL inhibited NF-KBp65, P-ERK, P-IκBα signalling pathways induced by zymosan in KCs. ATRA-NLC can inhibit the releasing of cytokines (IL-17, ICAM-1, MIP-2, MCP-1, Ip-10) induced by zymosan in CFs. It was supplementary selection for the therapy of fungal keratitis.
Keywords
Fungal keratitis (FK), All-trans-retinoic acid (ATRA), Nanostructured lipid carriers, Cytokines, Chemokines.
Introduction
Fungal Keratitis (FK) is a common inflammatory disease characterized by pro-inflammatory, chemotactic and regulatory cytokines releasing induced by fungi and its active factor. Currently, some antifungal agents are commonly used to treat FK. There are still some difficulties in the therapy of FK. Drug-resistance can lead to the invasive fungi infection. Antifungi agents may lead to secondary resistance to these agents were noticed [1]. It is important to develop novel antibiotics for the emergency of drug resistance and the spread of new infectious diseases [2]. The potential anti-fungal targets for patient-centered therapies and immune-enhancing strategies for the prevention and therapy of invasive fungal diseases should be explored [3]. A novel host-directed therapeutic strategy can complement the current approach for combatting infections [4]. Immune cells play a key role in the inflammatory process. Activated immune cells release pro-inflammatory cytokines and chemokine’s. These proinflammatory factors cause aggregation of monocytes and polymorphonuclear leukocytes and release more inflammatory cytokines. Effectively inhibiting the production of pro-inflammatory cytokines and reducing the production of inflammatory cytokines from excessive inflammation is significantly important [5]. Zymosan, a polysaccharide cell wall component derived from Saccharomyces cerevisiae, has been widely used as a model of acute inflammation. Zymosan-induced recruitment of monocytes and neutrophils can be used to study the effects of anti-inflammatory drugs [6]. Zymosan induced the production of Polymorphonuclear (PMN) immigration into the injury area. This response was associated with increase in the level of the chemokines Macrophage Inflammatory Protein (MIP)-1alpha, MIP-2, Monocyte Chemo-Attractant protein (MCP)-1 and cytokine-induced neutrophil chemo-attractant (KC), Interleukin-10 (IL-10) [7]. Zymosan was used in our experiment for the activation of fungi inflammation. All-Trans- Retinoic Acid (ATRA), a bioactive derivative of vitamin A, modulates immune responses by affecting T cells [8]. ATRA has shown immunomodulatory effects in many immune disorders. This effect was associated with a reduction in IL-17A production [9]. ATRA ameliorates the inflammation by decreasing the expression of the pro-inflammatory cytokines and increasing the expression of anti-inflammatory factors [10]. ATRA treatment ameliorates severity of uveitis and reduces the Th1/Th17 responses [11]. ATRA was found to have a promising regulatory effect on immune system and inflammatory responses in experimental research. The encapsulated ATRA has a higher potency over free ATRA in its immunomodulatory activity and also has a significant impact on reducing inflammation [12,13]. Our data suggest that waterinsoluble ATRA can be incorporated in Nanostructured Lipid Carriers (NCL) well enough. In this study, we investigated the impact of ATRA-NCL on the development of zymosan induced cytokines and chemokines expression in CFs and the pathophysiologic mechanisms by which ATRA might have anti-inflammation effects in fungal keratitis. ATRA may represent a new therapeutic agent for human fungal keratitis.
Materials and Methods
ATRA-NLC preparation
We prepared the ATRA-NLC by the method of Emulsification process from scratch. In brief, Oil phase: Whales wax palm, lecithin BHT 0.1 mg, oleic acid, soybean oil mixed and preheat to 70°C. Water phase: Poly yamanashi resin and distilled water preheat to 70°C. The water phase mixture and the oil phase mixture with magnetic stirrer under the speed of 1400 RPM, stir for 1 min. The pH value adjusted, then through 0.22 micron filter membrane filtration sterilization was done. The mixture was stored in dark sealed containers.
High performance liquid chromatography (HPLC) testing
All-trans-retinoic-acid was determined by High Performance Liquid Chromatography (HPLC) method. The size of C 18 reversed phase column is 5 μm, 150 × 5 mm. The composition of mobile phase was detected at 342 nm, the pH value was 4.2. The constitute ratio of mobile phase was as follows: Acetonitrile (84.5%), water (15% ice), acetic acid (0.5%). Sample amount was 20 mL, flow rate was 1.0 ml/min.
ATRA-NCL physical stability study
Precipitation and crystallization was observed at 4°C for one month, at high speed centrifugation status and at high hyperthermia conditions.
Scanning electron microscope observations
Appropriate ATRA-NLC dispersed liquid drop were put on the silicon slide and then dried in the oven at 60°C. The status and size of particles were observed under the scanning electron microscope (S-4800).
ATRA-NCL cytotoxicity assay
KCs (2 × 104 per well) were seeded in a 96-well plate with ATRA-NCL in MEM or without ATRA-NCL in MEM for 24 h. The amount of lactate dehydrogenase in the medium was measured with the use of an assay kit. A cell lysis solution was taken as positive control. Absorbance at 490 nm was measured.
Cell isolation
New England white rabbits (bodyweight 2.0 to 2.5 kg) were got from Animal Department of Jilin University. This study adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the Animal Experimental Committee of Jilin University. Rabbit Corneal Fibroblasts (CFs) were isolated as described previously [14]. Briefly, the enucleated eye was washed with DPBS containing antibiotic-anti-mycotic mixture, the endothelial layers were removed mechanically, and the remaining corneal tissue was incubated with dispase (2 mg/ml in MEM) for 2 h at 37°C. The remaining tissue was treated with collagenase (2 mg/ml in MEM) at 37°C until single-cell suspension of CFs was obtained. The isolated CFs were cultured under a humidified atmosphere at 37°C containing MEM with 10% FBS. Proliferating cells were harvested for experiments after four to seven passages in monolayer culturing.
Assay of cytokines and chemokines release
30000/1 ml CKs cultured in 24-well plates for 1 d with serumdeprived MEM alone. Serum-free CKs were incubated with or without ATRA-NLC in the additional presence of Zymosan for 6, 12, 24 h. IL-17, ICAM-1, MIP-2, MCP-1, IP-10 concentrations were examined with the use of a cytokine assay system. Standard proteins were dissolved in MEM for producing of standard curves.
Immunoblot analysis
Immunoblot analysis Immunoblot analysis of NF-KB-p65, P-ERK1, 2, P-IκBα was also performed as described previously. In brief, cells (5 × 105 per well of a 24-well plate) were cultured for 24 h in serumfree MEM and were then incubated first for 12 h with or without 0.01, 0.1 μmol/LATRA-NCL and then for 30 min in the additional absence or presence of zymosan (500 μg/ml). The cells were lysed, and the cell lysates (30 μg of protein) were then subjected to immunoblot analysis. Data are representative of three independent experiments.
Statistical analysis
Data are presented as mean values ± SD. All experiments were repeated at least three times. Statistical analysis was performed using SPSS19.0 statistical software for statistical analysis. Dunnett T analysis was used to investigate the differences in different groups. A P value of <0.05 was considered statistically significant.
Results
ATRA-NLC concentration detection
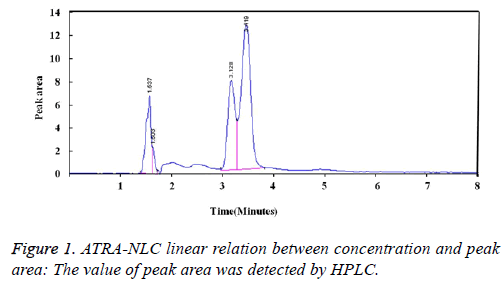
ATRA-NLC linear relation between concentration and peak area: The value of peak area was detected by HPLC. ATRA-NCL showed good light resistance. The calculated ATRA-NLC solution concentration was obtained by the calibration curve (Figure 1).
ATRA-NCL physical stability study
The physical stability was well enough that no precipitation and crystallization appeared after storage duration of one month at 4°C. There was no solid precipitation presenting even after 2 h centrifuge. The NCLs did not show resistant and thermo curing to high temperature on 120°C.
Scanning electron microscopy results
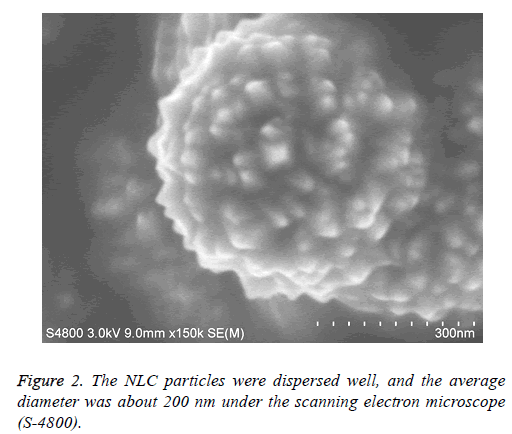
The NLC particles were dispersed well, and the average diameter was about 200 nm. ATRA-NLC particles dispersed in liquid drop were put on the silicon slide and were dried in the oven at 60°C. The status and size of particles were observed under the scanning electron microscope (S-4800). Scanning electron microscope image of the ATRA particles were shown. The scale bars represent 300 nm (Figure 2).
KCs cytotoxicity assay
KCs (2 × 104 per well) were seeded in a 96-well plate with ATRA-NCL in MEM or without ATRA-NCL in MEM for 24 h. The amount of lactate dehydrogenase released into the culture medium was then measured with the use of an assay kit (Cyto Tox96® Non-Radioactive Cytotoxicity Assay).
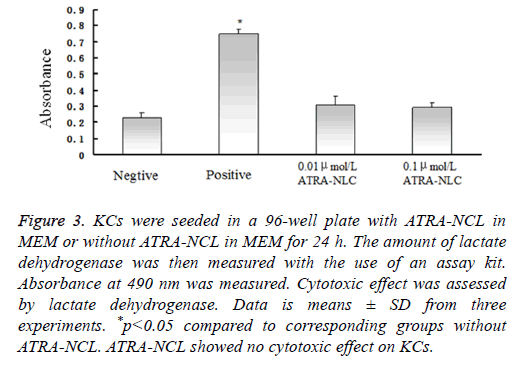
Absorbance at 490 nm was measured. Cytotoxic effect was assessed by LDH. The amount of LDH released from cells by a cell lysis solution was determined as positive control (Figure 3).
Figure 3: KCs were seeded in a 96-well plate with ATRA-NCL in MEM or without ATRA-NCL in MEM for 24 h. The amount of lactate dehydrogenase was then measured with the use of an assay kit. Absorbance at 490 nm was measured. Cytotoxic effect was assessed by lactate dehydrogenase. Data is means ± SD from three experiments. *p<0.05 compared to corresponding groups without ATRA-NCL. ATRA-NCL showed no cytotoxic effect on KCs.
Effects of ATRA-NCL on zymosan-induced IL-17, ICAM-1, MIP-2, MCP-1, Ip-10 release from CKs
The concentrations of IL-17, ICAM-1, MIP-2, MCP-1 and Ip-10 in culture supernatants were calculated (Figures 4-8).
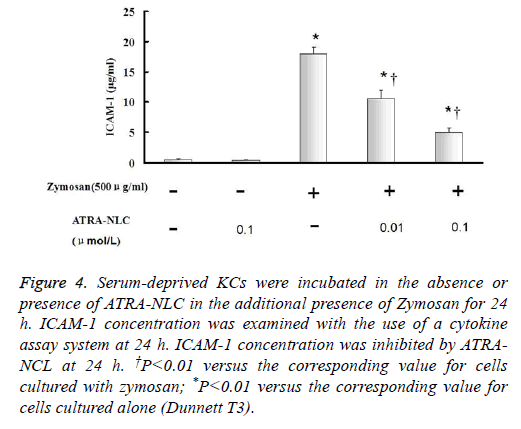
Figure 4: Serum-deprived KCs were incubated in the absence or presence of ATRA-NLC in the additional presence of Zymosan for 24 h. ICAM-1 concentration was examined with the use of a cytokine assay system at 24 h. ICAM-1 concentration was inhibited by ATRANCL at 24 h. †P<0.01 versus the corresponding value for cells cultured with zymosan; *P<0.01 versus the corresponding value for cells cultured alone (Dunnett T3).
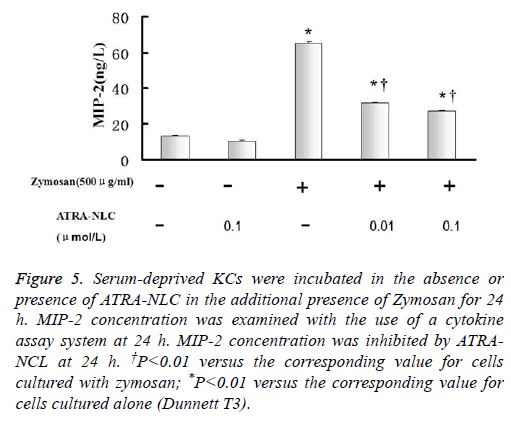
Figure 5: Serum-deprived KCs were incubated in the absence or presence of ATRA-NLC in the additional presence of Zymosan for 24 h. MIP-2 concentration was examined with the use of a cytokine assay system at 24 h. MIP-2 concentration was inhibited by ATRANCL at 24 h. †P<0.01 versus the corresponding value for cells cultured with zymosan; *P<0.01 versus the corresponding value for cells cultured alone (Dunnett T3).
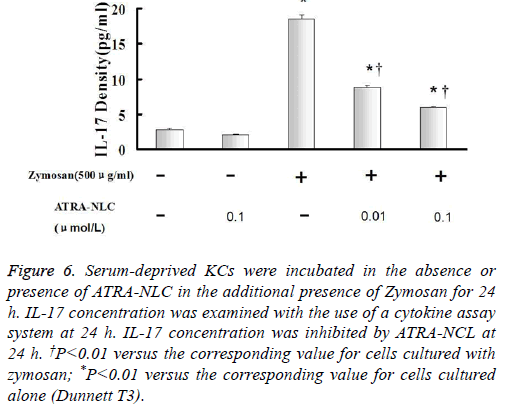
Figure 6: Serum-deprived KCs were incubated in the absence or presence of ATRA-NLC in the additional presence of Zymosan for 24 h. IL-17 concentration was examined with the use of a cytokine assay system at 24 h. IL-17 concentration was inhibited by ATRA-NCL at 24 h. †P<0.01 versus the corresponding value for cells cultured with zymosan; *P<0.01 versus the corresponding value for cells cultured alone (Dunnett T3).
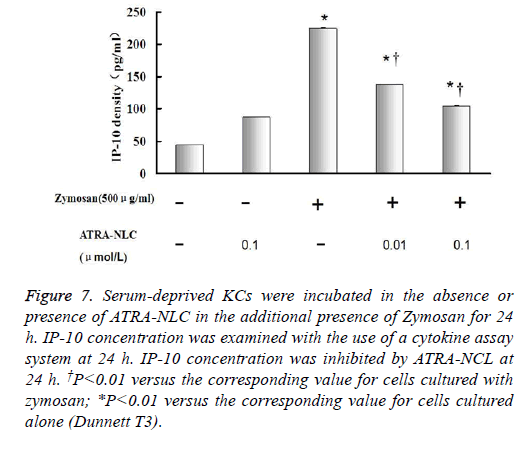
Figure 7: Serum-deprived KCs were incubated in the absence or presence of ATRA-NLC in the additional presence of Zymosan for 24 h. IP-10 concentration was examined with the use of a cytokine assay system at 24 h. IP-10 concentration was inhibited by ATRA-NCL at 24 h. †P<0.01 versus the corresponding value for cells cultured with zymosan; *P<0.01 versus the corresponding value for cells cultured alone (Dunnett T3).
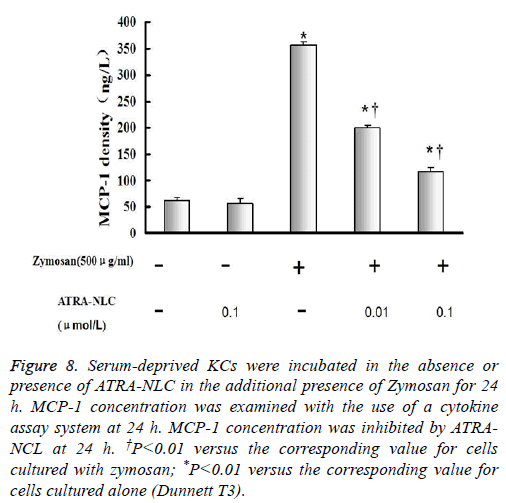
Figure 8: Serum-deprived KCs were incubated in the absence or presence of ATRA-NLC in the additional presence of Zymosan for 24 h. MCP-1 concentration was examined with the use of a cytokine assay system at 24 h. MCP-1 concentration was inhibited by ATRANCL at 24 h. †P<0.01 versus the corresponding value for cells cultured with zymosan; *P<0.01 versus the corresponding value for cells cultured alone (Dunnett T3).
Effects of ATRA-NCL on NF-KB-p65, P-ERK, PIκBα signalling pathways in KCs
The effects of ATRA-NCL on NF-ΚB-p65, p-ERK, P-IκBα signalling pathways in KCs induced by zymosan: KCs were incubated with or without ATRA-NCL (0.01, 0.1 μmol/L) for 24 h and then co-cultured with zymosan (500 μg/ml) for 30 min.
Immunoblot analysis showed that the levels of NF-KB-p65, PERK1, 2, P-IκBα were increased by stimulation with zymosan.
Immunoblot analysis manifested the amount of NF-ΚB-p65, p- ERK, P-IκBα could be inhibited by ATRA-NCL, as show in Figure 9.
Figure 9: The effects of ATRA-NCL on NF-ΚB-p65; p-ERK; P-IκBα signalling pathways in KCs induced by zymosan. KCs were incubated with or without ATRA-NCL for 24 h and then co-cultured with zymosan. Immunoblot analysis manifested NF-ΚB-p65; p-ERK; PIκBα induced by zymosan were inhibited by ATRA-NCL in dose manner.
Discussion
The proinflammatory cytokine Interleukin-17 (IL-17) is produced by activated memory T lymphocytes but stimulates innate immunity and host defence. IL-17 family members play a role in inflammatory diseases, autoimmune diseases, and cancer [15]. IL-17, mainly produced by neutrophils and CD4+ T cells in the corneas, is essential in the pathogenesis of Candida keratitis [16,17]. The IL-17R is expressed by Human Corneal Fibroblasts (HCF). IL-17A plays a protective role in response to corneal infection, but is different from the pathogenic role in autoimmune or chronic inflammatory diseases contributing to both microbial clearance as well as inflammation-associated tissue damage. Topical treatment with polyclonal antibodies to IL-17 resulted in significant reductions in corneal pathology and also lowered bacterial counts after infection providing a promising new way for therapy of corneal infections [18-20]. The adhesion molecule ICAM-1 appears to play a significant role in development of the severe pathology characteristic of P. Aeruginosa eye infections, and is essential to margination of PMNs in Staphylococcus aureus infection model [21,22]. The expression of ICAM-1 was focally increased on keratocytes, corneal endothelial cells, and vascular endothelial cells (particularly at the site of lymphoid infiltration) in areas of inflammation of diseased corneas [23,24]. Chemokine levels and inflammatory cells (neutrophils, macrophages, dendritic cells) recruitment will be considered aggravated organ injury in inflammation process [25]. In our experiment, ATRA-NCL can inhibit zymosan induced IL-17, ICAM-1, MIP-2, Mcp-1, Ip-10 releasing at 24 h. ATRA-NCL cannot inhibit cytokines and chemokine’s releasing at the early stage. The data showed that ATRA can decrease zymosan associated corneal tissue damage in our experiment rather than inducing the cytokines responded by immune cells in the early stage of inflammations. That means ATRA can be an anti-inflammation agent in the therapy of fungal keratitis. We demonstrate a simple and robust route to fabricate nanoparticles and then show their efficacy through ocular drug delivery in zymosan induced inflammatory chemokines and cytokines releasing. NCL resulted in 200 nm particles with Emulsification process. Collectively, our results suggest that ATRA-NCL can target inflammatory cytokines and chemokines levels such as IL-17, ICAM-1, MIP-2, Mcp-1, Ip-10 induced by zymosan in CFs. Furthermore, ATRA-NCL inhibited the phosphorylation of IκB and blocked the activation of Nuclear Factor (NF)-κB and p-ERK induced by Zymosan. ATRA-NCL showed no cytotoxic effect on KCs. NCL can increase the bioavailability of ATRA thus opening the possibility of future applications in the therapy of FK patients.
Acknowledgement
This work was supported by the grants from Natural Science Foundation of China (No. 81300727) and this study was supported by Science and Technology Department of Jilin Province Research Fund (20160101011JC).
Conflicts of Interest
None.
References
- Kushima H, Tokimatsu I, Ishii H, Kadota J. Antifungal susceptibility and drug-resistant mechanism of Trichosporon. Med Mycol J 2015; 56: 123-128.
- Zhao HY, Anbuchezhian R, Sun W, Shao CL, Zhang FL, Yin Y, Yu ZS, Li ZY, Wang CY. Cytotoxic nitrobenzoyloxy-substituted sesquiterpenes from sponge derived endozoic fungus Aspergillus insulicola MD10-2. Curr Pharm Biotechnol 2016; 17: 271-274.
- Ravikumar S, Win MS, Chai LY. Optimizing outcomes in immune compromised hosts: Understanding the role of immunotherapy in invasive fungal diseases. Front Microbiol 2015; 6: 1322.
- Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, Ai X, Serhan CN, Levy BD. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol 2016; 9: 1278-1287.
- Li YM, Wang HB, Zheng JG, Bai XD, Zhao ZK, Li JY, Hu S. Dimethyl sulfoxide inhibits zymosan-induced intestinal inflammation and barrier dysfunction. World J Gastroenterol 2015; 21: 10853-10865.
- Cash JL, White GE, Greaves DR. Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods Enzymol 2009; 461: 379-396.
- Ajuebor MN, Virág L, Flower RJ, Perretti M, Szabó C. Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunol 1998; 95: 625-630.
- Son HL, Park HR, Park YJ, Kim SW. Effect of retinoic acid in a mouse model of allergic rhinitis. Allergy Asthma Immunol Res 2015; 7: 590-598.
- Behairi N, Belkhelfa M, Mesbah-Amroun H, Rafa H, Belarbi S, Tazir M, Touil-Boukoffa C. All-trans-retinoic acid modulates nitric oxide and interleukin-17A production by peripheral blood mononuclear cells from patients with Alzheimer's disease. Neuroimmunomodulation 2015; 22: 385-393.
- Cao W, Chen W, Liang X, Zhou J, Wei C, Cui S, Liu J. All-trans-retinoic acid ameliorates the inflammation by inducing transforming growth factor beta 1 and interleukin 10 in mouse epididymitis. Am J Reprod Immunol 2014; 71: 312-321.
- Keino H, Watanabe T, Sato Y, Okada AA. Anti-inflammatory effect of retinoic acid on experimental autoimmune uveoretinitis. Br J Ophthalmol 2010; 94: 802-807.
- Grace VM, Siddikuzzaman, Rimashree B. Liposome encapsulated all trans retinoic acid (ATRA) has enhanced immunomodulatory and inflammation reducing activities in mice model. Anticancer Agents Med Chem 2015; 15: 196-205.
- Siddikuzzaman, Grace VM. Antioxidant potential of all-trans retinoic acid (ATRA) and enhanced activity of liposome encapsulated ATRA against inflammation and tumor-directed angiogenesis. Immunopharmacol Immunotoxicol 2013; 35: 164-173.
- Hongyan Zhou, Kazuhiro Kimura, Tomoko Orita, Teruo Nishida, Koh-Hei Sonoda. Inhibition by female sex hormones of collagen degradation by corneal fibroblasts. Mol Vis 2011; 17: 3415-3422.
- Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity 2004; 21: 467-476.
- Zhang H, Li H, Li Y, Zou Y, Dong X, Song W, Jia C, Li S, Xi H, Liu D, Wang Y. IL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneas. Eur J Immunol 2013; 43: 2671-2682.
- Karthikeyan RS, Vareechon C, Prajna NV, Dharmalingam K, Pearlman E, Lalitha P. Interleukin 17 expression in peripheral blood neutrophils from fungal keratitis patients and healthy cohorts in Southern India. J Infect Dis 2015; 211: 130-134.
- Zaidi TS, Zaidi T, Pier GB, Priebe GP. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect Immun 2012; 80: 3706-3712.
- Taylor PR, Leal SM Jr, Sun Y, Pearlman E. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol 2014; 192: 3319-3327.
- Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol 2002; 169: 5897-5903.
- Cole N, Hume EB, Khan S, Garthwaite L, Conibear TC, Willcox MD. The role of CXC chemokine receptor 2 in Staphylococcus aureus keratitis. Exp Eye Res 2014; 127: 184-189.
- He P, Srikrishna G, Freeze HH. N-glycosylation deficiency reduces ICAM-1 induction and impairs inflammatory response. Glycobiol 2014; 24: 392-398.
- Philipp W, Göttinger W. Leukocyte adhesion molecules in diseased corneas. Invest Ophthalmol Vis Sci 1993; 34: 2449-2459.
- Cheng T, Bai J, Chung CS, Chen Y, Biron BM, Ayala A. Enhanced innate inflammation induced by Anti-BTLA antibody in dual insult model of hemorrhagic shock/sepsis. Shock 2016; 45: 40-49.
- Guo S, Wu LX, Jones CX, Chen L, Hao CL, He L, Zhang JH. Allergic airway inflammation disrupts interleukin-17 mediated host defense against streptococcus pneumoniae infection. Int Immunopharmacol 2015; 31: 32-38.