Research Article - Journal of Agricultural Science and Botany (2019) Volume 3, Issue 2
Inheritance of albinism in grapefruit (Citrus paradisi Macf.; al2+al1-) and Hanayu (C. hanaju hort. ex Shirai; al1+al1-).
Ly Minh Le1, Akira Wakana1*, Kaori Sakai2*, Yuki Mizunoe1, Kohei Kajiwara2, Sayuri Kajihara2, Yukio Ozaki1
1Faculty of Agriculture, Kyushu University, Fukuoka, 819-0395, Japan
2Faculty of Agriculture, Kyushu University, Kasuya-gun, 811-2307, Japan
- *Corresponding Author:
- Wakana A
Laboratory of Horticultural Science
Faculty of Agriculture, Kyushu University
Fukuoka, 819-0395, Japan
E-mail: wakana@agr.kyushu-u.ac.jp
- *Corresponding Author:
- Sakai K
University Experimental Farm,
Faculty of Agriculture, Kyushu University
Kasuya-gun, 811-2307, Japan
E-mail: sakai.kaori.198@m.kyushu-u.ac.jp
Accepted Date: January 30, 2017
Citation: Yavisheva TM, Shcherbakov SD. Carcinogenesis from the viewpoint of the work of the morphofunctional zones and aging. J Aging Geriatr Psychiatry. 2017;1(2):1-10
Abstract
Albinism seen in very young seedlings from seeds infected with Alternaria fungi and that seen in variegated leaves is known in Citrus. However, genetic control of albinism has not been reported in Citrus accessions probably because of their apomixis, self-incompatibility, long generation time and cross breeding. In the present study, albino seedlings were observed for the first time in the zygotic seedlings obtained from the crosses or backcrosses with grapefruit and Hanayu with the segregation ratio of 1 albino:3 green and 1 albino:7 green. The 1:3 ratio indicated that Hanayu and grapefruit have independent single recessive gene for the albino seedlings. The albino genes detected in Hanayu and grapefruit designated al1 and al2 respectively. On the other hand, the 1:7 ratio indicated that Hanayu or Yuzu has independent single dominant gene designating R2 a green-restorer for restoration of genetic albinism in the crosses with grapefruit. Albinism observed in the seedlings showed the stability in terms of the two albino genes and all the albino seedlings showing slow growth died several months after seed germination. When grapefruit (pummelo×sweet orange?) was crossed to 46 monoembryonic Citrus accessions including 28 pummelo accessions, albino seedlings did not segregate in the progenies of all crosses. The albino seedlings found in this study may be useful as markers in various experiments and contribute to chloroplast research in Citrus.
Keywords
Albino gene alleles al1 + al2 +, Grapefruit, Green-restorer R 2, Hanayu.
Introduction
Citrus is one of the most important commercial and nutritional fruit crops in the world, and thus, molecular genetic analysis are in great progress [1]. However, genetic analysis of traits governed by recessive genes is especially difficult in Citrus accessions because of their apomixis, self-incompatibility, long generation time and cross breeding [2]. In the studies for self-incompatibility [3], male sterility [4] and parthenocarpy [5] in Citrus accessions, we carried out more than hundred self-pollinations, crosses and backcrosses to find out their inheritance. During this study, we happened to find out albino seedlings from self-pollination of Hanayu (Citrus hanaju hort. ex Shirai) and grapefruit (C. paradisi Macf.).
In Satsuma mandarin (C. unshiu Marcow.), variegated shoots with a white over green periclinal chimera structure occurred in the trees with a frequency of 10 shoots /1 ha /20 years. The variegated shoots with albinism in their germ layer 2 were also seen in many groups of Citrus plants such as citron, pummelo, calamondin, lemon, sweet orange and sour orange as well as trifoliate orange (Poncirus trifoliata (L.) Raf.) and kumquat (Fortunella spp.) [2]. Some of these variegated Citrus accessions are valuable as ornamental plants. Albino nucellar seedlings were steadily produced from polyembryonic seeds of these variegated accessions with white over green structure. However, one albino seedling steadily appeared with a certain rate in a polyembryonic seed of self-pollinated Hanayu, suggesting that albino seedling formation was not due to fungi infection that reported in Citrus seeds [6].
In this study, the attempt was focused on the segregation of seedlings showing genetic albinism in various crosses with grapefruit and Hanayu to extend our knowledge as a basis for cross breeding. Coincidentally, albinism was widely surveyed with grapefruit as a pollen parent in various crosses with many Citrus accessions.
Materials and Methods
Plant materials
Plant materials used were about 30-year-old trees of Hanayu, ‘Foster Pink’ grapefruit, ‘Mash seedless’ grapefruit, ‘Variegated Daidai’ sour orange (C. aurantium L.), ‘Variegated Buddha’s Hand’ citron (C. medica L.). During our previous studies for inheritance of male sterility and parthenocarpy [4,5], we conducted various crosses and backcrosses from which the seedlings with various age were produced. From these hybrid seedlings, we selected monoembryonic materials useful for genetic analysis in the present study. The monoembryonic hybrids between Hanayu and Yuzu (C. junos Sieb. ex Tanaka) and those between Hanayu-Yuzu hybrids and grapefruit were about 10-year-old trees. Forty-six monoembryonic Citrus accessions containing 28 pummelos, 11 pummelo-relatives, four yuzu-relatives and three mandarin-relatives used for crosses with grapefruit were 20- to 30-year-old trees of the 28 pummelo accessions, 15 were collected from more than 100-year-old trees grown in southwest Japan while 13 were introduced from National Institute for Fruit Tree Science (NIFTS) and Kagoshima Prefectural Fruit Tree Experimental Station in Japan. These accessions were grown in the Experimental Farm of Kyushu University, Fukuoka, Japan.
Crosses with Hanayu
Thirteen accessions including polyembryonic Hanayu, Yuzu, ‘Variegated Daidai’, seedless ‘Variegated Buddha’s Hand’ and nine monoembryonic Hanayu hybrids were hand-pollinated with/to Hanayu. Pollination was made in May of 2008 to 2016 with at least three replicates in each cross. The flower buds one day before anthesis were emasculated, hand-pollinated with pollen of target accessions, bagged to prevent pollination with alien pollen, and harvested in November of the year.
Crosses with grapefruit
Ten accessions including Hanayu, ‘Foster Pink’ and ‘Marsh seedless’ grapefruit, (Hanayu×Yuzu)-No.16 and six monoembryonic hybrids between ‘Foster Pink’ and (Hanayu×Yuzu)-No.16 were pollinated with grapefruit. Since two grapefruit cultivars are highly polyembryonic, they were used as pollen parents. Three hybrids between ‘Foster Pink’ and (Hanayu×Yuzu)-No.16 were self-pollinated. Pollination was made in May of 2011 to 2018 with at least three replicates in each cross. All the pollinated flowers were bagged to prevent outcross, and harvested in November of the year.
Forty-six monoembryonic Citrus accessions including 28 pummelo accessions, 11 pummelo hybrid accessions, four Yuzu relative accessions and three mandarin accessions were pollinated with ‘Foster Pink’ grapefruit with the same procedure as mentioned above.
Seedling production and observation
The seeds were extracted from mature fruit in November of the year. The zygotic embryos extracted from the seeds were germinated under fluorescent light in a room maintaining at 25°C and allowed to grow for about six months in a greenhouse (13-30°C). The rate of zygotic or monoembryonic seeds was about 80% in crosses with polyembryonic ‘Hanayu’ as a seed parent and about 10% in crosses with polyembryonic ‘Yuzu’ as a seed parent. The polyembryonic seeds containing two or more embryos per seed were not used, and only monoembryonic seeds were selected for this study. In each of the 46 monoembryonic Citrus accessions pollinated with grapefruit, more than 100 seeds were used for the detection of albino seedlings.
As a control for genetic albinism in this research, open pollinated sour orange seeds were collected in November and sown in soil without disinfection and albinism in the infected seedlings were observed.
Assessment of albinism
Morphology of albino seedlings in various crosses with Hanayu and grapefruit was observed in comparison to the green seedlings. The rates for albino seedlings observed in various crosses was repeatedly examined for at least three years, and the result in each year was combined in each cross combination.
Statistical analysis
Segregation rates for albino seedlings were calculated and Chi- square test was performed to determine the probability.
Results
Morphology of albino seedlings
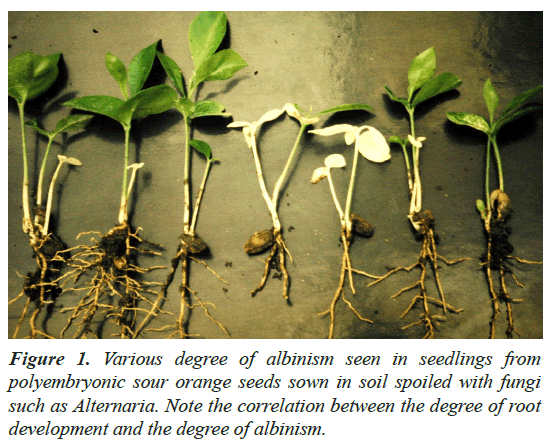
Sour orange seedlings grown in disinfected soil showed various degree of albinism and root development (Figure 1). In comparison to this non-genetic albinism, genetic albinism seen in zygotic seedlings was stable in morphology, i.e., white leaf color, white stem color, white cotyledon color, seedling high and root development (Figures 2 and 3). In comparison to the green zygotic seedlings in each cross, albino zygotic seedlings were small in height, leaf size, cotyledon size and root size (Figures 2 and 3). There was no year-to-year variation for the morphology of albino seedlings, and they did not turn green during their growth period and died several months after germination.
Figure 1: Various degree of albinism seen in seedlings from polyembryonic sour orange seeds sown in soil spoiled with fungi such as Alternaria. Note the correlation between the degree of root development and the degree of albinism.
Figure 2: Albino (upper) and green (lower) seedlings derived from the cross of [(Hanayu×Yuzu)-16×Foster Pink]-No.3×Foster Pink grapefruit three weeks after germination under the plant growth fluoescent light at 25?.
Figure 3: Segregation of albino seedlings (A) and morphology of albino and green seedlings (B) derived from the cross of Hanayu×(Hanayu×Yuzu)- No.14 under greenhouse conditions.
Segregation of albino seedlings in the crosses with Hanayu
In the self-pollination of Hanayu, albino seedlings and green seedlings segregated in a ratio of 123:380 (1:3), while in the reciprocal crosses between Hanayu and Yuzu only green seedlings appeared (Table 1). The 1:3 ratio for the segregation of albino seedlings were constant for four years observations (data is not presented but combined in Table 1). When Hanayu was backcrossed, albino seedlings and green seedlings segregated in five of nine backcross combinations with a ratio of 1:3, while only green seedlings appeared in four of the nine backcrosses (Table 1). Albino seedlings did not segregate in the cross of Hanayu×Variegate Daidai and that of Hanayu×Variegated Buddha’s Hand.
Table 1. Segregation of albino seedlings in crosses and backcrosses with/to Hanayu (al1+al1−).
Cross |
Genotypes | Observed ratio | Expected ratio | X??2 | p-value | |
|---|---|---|---|---|---|---|
| Albino seedling | Green seedling | |||||
| Hanayu×Hanayu | al1+al1-×al1+al1- | 123 | 380 | 01:03 | 0.183 | 0.669 |
| Hanayu×Yuzu | al1+al1-×al1-al1- | 0 | 117 | 00:01 | - | - |
| Yuzu×Hanayu | al1-al1-×al1+al1- | 0 | 101 | 00:01 | - | - |
| Hanayu×(Hanayu×Yuzu)-3 | al1+al1-×al1-al1- | 0 | 76 | 00:01 | - | - |
| Hanayu×(Hanayu×Yuzu)-10 | al1+al1-×al1+al1- | 16 | 35 | 01:03 | 1.105 | 0.293 |
| Hanayu×(Hanayu×Yuzu)-24 | al1+al1-×al1+al1- | 32 | 79 | 01:03 | 0.868 | 0.352 |
| Hanayu×(Hanayu×Kabosu)-22 | al1+al1-×al1-al1- | 0 | 129 | 00:01 | - | - |
| Hanayu×(Hanayu×Kabosu)-31 | al1+al1-×al1+al1- | 39 | 138 | 01:03 | 0.831 | 0.362 |
| (Hanayu×Yuzu)16×Hanayu | al1-al1-×al1+al1- | 0 | 200 | 00:01 | - | - |
| (Hanayu×Yuzu)16×Yuzu | al1-al1-×al1-al1- | 0 | 100 | 00:01 | - | - |
| (Hanayu×Kabosu)14×Hanayu | al1+al1×al1+al1- | 19 | 52 | 01:03 | 0.117 | 0.732 |
| (Hanayu×Kabosu)34×Hanayu | al1-al1-×al1+al1- | 0 | 17 | 00:01 | - | - |
| (Mexican lime×Hanayu)-2×Hanayu | al1+al1-×al1+al1- | 29 | 90 | 01:03 | 0.025 | 0.874 |
| Hanayu×Variegated Daidai | al1+al1-×al1-al1- | 0 | 349 | 00:01 | - | - |
| Hanayu×Variegated Buddha’s Hand | al1+al1-×al1-al1- | 0 | 65 | 00:01 | - | - |
Segregation of albino seedlings in the crosses with grapefruit
In two crosses of Hanayu×Foster Pink grapefruit and (Hanayu×Yuzu)-No.16×Foster Pink grapefruit, albino seedlings did not segregate. In the three self-pollinations of ‘Foster Pink’ grapefruit hybrids, [(Hanayu×Yuzu)-No.16×Foster Pink]-No.3 generated albino and green seedlings with a ratio of 1:3, while in two of the three, albino seedlings did not segregate (Table 2).
Table 2. Segregation of albino seedlings in crosses and backcrosses with grapefruit, and genotypes for albino genes al1 and al2 and green restoration gene R2 for al2.
Cross |
Albino genotype for three genes al1, al2 and R2 | Observed ratio | Expected ratio | X? | p-value | |
|---|---|---|---|---|---|---|
| Albino seedling | Green seedling | - | - | |||
| Hanayu×Foster Pink | al1+al1-al2−al2−R2−or+R2−×al1-al1-al2+al2− R2−R2− | 0 | 800 | 00:01 | - | - |
| (Hanayu×Yuzu)-16×Foster Pink | al1-al1-al2−al2−R2−or+R2−×al1-al1-al2+al2− R2−R2− | 0 | 200 | 00:01 | - | - |
| [(Hanayu×Yuzu)-16×Foster Pink]-3×selfing | al1-al1-al2+al2−R2−R2−×al1-al1-al2+al2−R2−R2− | 38 | 117 | 01:03 | 0.019 | 0.889 |
| [(Hanayu×Yuzu)-16×Foster Pink]-3×selfing | al1-al1-al2−al2−R2−or+R2−×al1-al1-al2−al2−R2−R2− | 0 | 21 | 00:01 | - | - |
| [(Hanayu×Yuzu)-16×Foster Pink]-5×selfing | al1-al1-al2−al2−R2−or+R2−×al1-al1-al2−al2−R2−R2− | 0 | 24 | 00:01 | - | - |
| [(Hanayu×Yuzu)-16×Foster Pink]-1×Foster Pink | al1-al1-al2+al2−R2−R2−×al1-al1-al2+al2−R2−R2− | 27 | 84 | 01:03 | 0.027 | 0.869 |
| [(Hanayu×Yuzu)-16×Foster Pink]-2×Foster Pink | al1-al1-al2+al2−R2+R2−×al1-al1-al2+al2−R2−R2− | 67 | 474 | 01:07 | 0.004 | 0.948 |
| [(Hanayu×Yuzu)-16×Foster Pink]-2×Marsh | al1-al1-al2+al2−R2+R2−×al1-al1-al2+al2−R2−R2− | 35 | 250 | 01:07 | 0.008 | 0.929 |
| [(Hanayu×Yuzu)-16×Foster Pink]-3×Foster Pink | al1-al1-al2+al2−R2−R2−×al1-al1-al2+al2−R2−R2− | 66 | 200 | 01:03 | 0.005 | 0.944 |
| [(Hanayu×Yuzu)-16×Foster Pink]-6×Foster Pink | al1-al1-al2−al2−R2−or+R2−×al1-al1-al2+al2−R2−R2− | 0 | 34 | 00:01 | - | - |
When ‘Foster Pink’ grapefruit hybrids [(Hanayu×Yuzu)- No.16×Foster Pink] was backcrossed with ‘Foster Pink’ grapefruit, albino seedlings and green seedlings segregated in two of four backcross combinations with a ratio of 1:3 and in one of the four back cross combinations with a ratio of 1:7 (Table 2). On the other hand, only green seedlings appeared in one of the four backcrosses (Table 2). When ‘Foster Pink’ grapefruit hybrids [(Hanayu×Yuzu)-No.16 × Foster Pink]- No.2 was backcrossed with ‘Marsh seedless’ grapefruit, albino seedlings and green seedlings also segregated with a ratio of 1:7 (Table 2).
In the pollination with ‘Foster Pink’ grapefruit as a pollen parent, no segregation of albino seedlings was observed in 45 monoembryonic Citrus accessions including 28 pummelos, 11 pummelo hybrids, four Yuzu relatives and three mandarins.
Discussion
Genetic studies in different crops show that albinism is a recessive trait governed by many loci [7]. The present study in Citrus crop also show that albinism is a recessive trait governed by at least two loci. In the self-pollinations and backcrosses with Hanayu, the albino seedlings and green seedlings segregated in a ratio of 1:3. This indicates that the genetic albinism is governed by single recessive gene designated al1. The nuclear gene al1 consists of functional allele al1 + and nonfunctional allele al1 −. In the cross of Hanayu×Foster Pink grapefruit, albino seedlings did not segregate, while in the self-pollinations of grapefruit hybrids and backcrosses with grapefruit, the albino seedlings and green seedlings segregated in a ratio of 1:3. This indicates that the genetic albinism is also governed by single recessive gene designated al2. The nuclear gene al2 consists of functional allele al2 + and nonfunctional allele al2 −.
The present study in Citrus crop also shows that albinism is restored by a green-restorer gene on at least one locus. In the backcrosses with grapefruit, the albino seedlings and green seedlings segregated in a ratio of 1:7. This indicates that the genetic albinism is restored by single dominant gene designated R2. The nuclear gene R2 consists of functional allele R2 + and nonfunctional allele R2 −. It is estimated that either Hanayu or Yuzu has allele R2 +. Since no clear difference in morphology and growth rate was found between albino seedlings as to al1 and al2 genes, their function to generate albino seedlings seems to be similar.
Conclusion
It seems that the genetic albinism of a given Citrus genotypes is a stable trait governed by one or more recessive nuclear genes. The albinism is not affected by environmental conditions and seedling age after seed germination, but recovered by greenrestorers. Some Citrus accessions with relation to grapefruit and Hanayu carry these genes. These accessions will be very useful materials for producing markers in experiments such as micrografting, breeding and chloroplast research in Citrus.
Acknowledgement
This study was partially supported by a Grant in Aid (KAKENHI No. 18380026) from the Japan Society for the Promotion of Science (JSPS).
References
- Gmitter Jr FG, Ollitrault P, Machado MA, et al. Genome sequence analysis and comparisons reveal ancestral hybridization and admixture events in the origins of some citrus cultivars. XII International Citrus Congress Abstract, Valencia (Spain). 2012:61.
- Cameron JW, Soost H. Genetics, breeding, and nucellar embryony. In: Reuther W, Batchelor LD, Webber HJ (Eds.), The Citrus Industry. California University Press. 1968:325-70.
- Ngo BX, Chu TD, Wakana A, et al. Patterns and strength of pollen tube arrest in self-incompatible Citrus accessions (Rutaceae). J Fac Agr Kyushu U. 2019;64(2):In press.
- Dewi PS, Wakana A, Tanimoto Y, et al. Precocious flowering of Citrus seedlings and its use for determination of cultivars generating male sterile progenies. Sci Hort. 2013;160(1):1-11.
- Zhou XH, Wakana A, Sakai K, et al. Early diagnosis of parthenocarpic seedlings within one year after pollination with grapefruit (Citrus paradisi Macf.). Sci Hort. 2018;239:282-8.
- Ryan GF. Albinism in citrus seedlings; nongenetic absence or deficiency of chlorophyll in seedlings prevented by treating freshly extracted seeds with fungicide. Calf Agric. 1958;12(3):7-12.
- Kumari M, Clarke HJ, Small I, et al. Albinism in plants: A major bottleneck in wide hybridization, androgenesis and doubled haploid culture. Crit Rev Plant Sci. 2009;28:393-409.