Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2017) Volume 7, Issue 62
Influence of the Zhichan capsule on the Expression of TNF-?, IL-1?, IL-6 and T, B Lymphocytes Function in PD Mice
Yongmao Liu1, Pengguo Zhang2, Yanling Wang1, Shuang Wu3, Lin Tian4 and Qingwei Zhou5*
1Department of Immunology, College of Basic Medical Sciences, Jilin University, China
2Imaging Unit, Second Hospital of Jilin University, China
3Department of Immunology, College of Basic Medical Sciences, Jilin University, China
4Department of Gastroenterology, First Hospital of Jilin University, China
5Department of Biomedical Engineering, School of Pharmaceutical Sciences, Jilin University, China
- *Corresponding Author:
- Qingwei Zhou
Department of Biomedical Engineering
School of Pharmaceutical Sciences
Jilin University, China
E-mail: Zhouqw@jlu.edu.cn
Accepted date: June 14, 2017
Abstract
Objective: To investigate the immunomodulatory effect of the Zhichan capsules on the Parkinson's disease (PD) induced mice. Methods: Intraperitoneal injection of MPTP induced in a PD model, reverse transcription polymerase chain reaction (RT-PCR)is performed to detect the gene expression of Interleukin 1 beta (IL-1β), IL-6 and Tumor Necrosis gene Factor (TNF-α); the lymphocyte transformation test and haemolytic plaque assay was done to analyse the effect of Zhichan capsules on T, B lymphocytes proliferation. Results: The expression of IL-1β and TNF-α mRNA were increased, and the IL-6 was reduced in PD model control group compared with normal control group. The expression of IL-1β and TNF-α mRNA were reduced, and the IL-6 was increased in the treatment group which were treated with the Zhichan capsules compared with the PD model control group, and the expression of them had significant differences (p<0.05); compared with the control group, the transformation rate of T cells and the number of plaques formed in treatment group were significantly increased, they both have statistical significance (p<0.05). Conclusion: Zhichan capsules have a down-regulation on the expression IL-1 β and TNF-α mRNA and an up-regulation on IL-6 and the T, B lymphocytes in PD mouse. It was indicated that Zhichan capsules have preventive and therapeutic effect on PD.
Keywords
Zhichan capsules; PD mice model; Immunity molecule; Immunity fuctions; Influence
Introduction
Parkinson's disease (PD) is one of the common chronic degenerative diseases of human nervous system. It was characterized by midbrain substantia nigra dopaminergic neuronal degeneration and loss, striatal dopamine levels drop, resting tremor, myotonia bradykinesia and postural abnormalities as the main clinical symptoms [1]. It was found that there are a large number of activated microglia cells, inflammatory cytokines and oxidative stress in nigrostriatal area of PD patients [2]. Chronic inflammatory cytokines are the pathological feature of majority of neurodegenerative diseases including AD, multiple sclerosis and especially PD [3]. More and more evidences indicate that inflammatory cytokines reaction participate in the metatropy of dopaminergic neurons and the disease progression of PD [4]. For example, a series of inflammatory cytokines including TNF-α, IL-6 and IL-1 all have been found in the cerebrospinal fluid and brain regions affected in PD patients [5-7]. For a long time, the traditional PD treatment strategies are adopted for the lack of dopamine replacement therapy, levodopa and compound preparations are primary drugs. However, with long-term use of these drugs, there will be drug-related motor fluctuations and dyskinesia and other adverse reactions, which in clinical applications has been limited [8]. In recent years, treatment of PD using traditional Chinese medicine has led to researcher’s attention. Many studies have confirmed that the traditional Chinese medicine treatment of PD has little side effects and the exact effect [9,10], which can be regarded as one of neuroprotective therapies. Zhichan Capsule as a compatibility of traditional Chinese medicine used in this study has been applied in clinical medicine for many years, it not only improves a variety of PD patient’s clinical symptoms and also their immune function [11]. The research group in preliminary studies confirmed that the effect of Zhichan capsule on Parkinson's disease rat shown that dopaminergic neurons signal transduction, apoptosis, MAO-B and TH plays a significant regulatory role [12,13]. To further investigate the immunomodulatory effects of Zhichan capsules on Parkinson's disease (PD) treatment, this study was used by preparing intraperitoneal injection of MPTP to induce mouse model of Parkinson's disease, and then intervene by oral administration of Zhichan capsules. RT-PCR is applied to detect the expression of IL-1β, IL-6 and TNF-α in mouse midbrain substantia nigra, and the lymphocyte transformation test and haemolytic plaque assay were used to analyze the effect of Zhichan capsules on T, B lymphocytes proliferation.
Materials and Methods
Animals
A total of 460 C57BL/6 male mice of clean grade, mice aged 10 weeks, weighing 22 to 25 g were selected in this study. Because of the research process involving the experimental animals, the Ethics Committee made a discussion and had made an approval of this project. The mice were provided by the Jilin University of Basic Medical Sciences, Experimental Animal Center, China.
Drugs
Zhichan powder was developed by the professor Guozhong Gai from university of Changchun Traditional Chinese Medicine. Prescriptions are comprised of Astragalus, Ginseng, Baishouwu, teasel, Magnolia, Jurchen child, Chuanxiong and other ingredients.
Reagents
MPTP, Con A (SIGMA, USA); Trizol reagent (U.S. Life Technologics); reverse transcription kit, Taq DNA polymerase, PCR primers (Takara Biotechnology Company, Japan).
Instruments
Biofuge primo R cold centrifuge (Heraeus, Germany); VDS gel imaging system (Pharmacia, Sweden); microplate reader (U.S. L & B company).
Experimental groups and preparation of the PD mouse model
The 90 of 460 C57BL/6 mice were randomly selected as normal control group, then the rest of 400 mice were used to prepare PD model by referencing method of Sheng-Di Chen et al. [14]. Each mouse was injected intraperitoneally at a dose of MPTP of 0.9 mg/0.5 ml, once a day for 7 days. The PD mouse models were screened using the pole test and electrical stimulation experiments, which lost the ability to climb the pole or unresponsive to stimulation were successful models. Then successful model mice were randomly divided into model control group and the Zhichan capsules treatment group to complete the experimental research. Grouping and administration of the specific process is as follows:
(1) 80 normal control mice, divided into 16 cages, each cage was with 5 mice. Each mouse of normal control group was treated with saline volume 0.4 mL/each time, twice a day. During the test, each time point, 10 mice (two cages) were randomly selected which were used for cytokines determination and the lymphocyte transformation test, and 5 of them (one cage) were used for the haemolytic plaque assay;
(2) Model control group has 80 of PD model mice they were feed and treated in the same way as the normal control group;
(3) Zhichan capsules treatment group has 80 PD model mice, divided into 16 cages also. The mice were feed and treated in the same way as the normal control group used Zhichan capsules instead of saline.
Sample collection
From the beginning of the administration of the first day onwards, every 10 days was an experimental point. Each time point, the mice in each group were randomly selected 5, and the hippocampus were taken in clean bench and put in liquid nitrogen for RT-PCR analysis, the spleen was prepared for lymphocyte transformation test. The remaining 5 mice injected with sheep red blood cells in the four days prior to each test point for haemolytic plaque assay.
Detection the expression of cytokine by RT-PCR
Preparation and characterization of total RNA: Total RNA of mouse hippocampus was extracted Using Trizol reagent according to the product manual procedure and the purity and quality of the RNA were analyzed using agarose gel electrophoresis and UV spectrophotometry.
Reverse transcription reaction RNA into cDNA: Reverse transcription reaction from mRNA into cDNA was completed according to the GenAmp RNA PCR kit operating instructions, specific operation as follows:
(1) 40 μl of dNTPs (10 mmol/l), 0.5 μl of RNase inhibitor, 0.5 μl of Random primer, and 2 μl of total RNA were taken and added into an EP tube, and incubated at 70 for 5 min after jog centrifugation, and then cooled on ice.
(2) 0.5 μl of AMV reverse transcriptase, 5.0 μl of 5 × RT buffer, 10.5 μl of DEPC-treated water was added into each EP tube.
(3)After slightly centrifugation, they were incubated at 37 for 60min, then 90 for 5min, and cooled rapidly in an ice bath. They are the cDNA solution used to PCR reaction.
PCR reactions: One PCR reaction tube was used for each sample, in which the following solutions were added: 2 μL of cDNA solution, 5 μL of 10 × Taq buffer, 2 μL of the dNTPs (10 mmol/L), 10 μL of the each specific gene primer, 5μL of the internal reference gene primers, 3 μL of Taq DNA polymerase, 3 μL of DEPC-treated water, total reaction volume was 50 μL. PCR reaction conditions (Tables 1 and 2).
| Gene | Primer | Length(bp) |
|---|---|---|
| β-actin | Sense 3’-GTGACCAACTGGGACGAC-5’ Antisense 3’-GAAGCGCTCGTTGCCGATG-5’ |
540 |
| IL-1β | Sense3’-CAACTGTCCCTGAACTCAA-5’ Antisense 3’-GCATTTTTGTTGTTC-5’ |
395 |
| IL-6 | Sense 3’-AGCTATGAAGTTTCTCTCCG-5’ Antisense3’-CACAAACTCCAGGTAGAA-5’ |
425 |
| TNF-α | Sense 3’-GTCTACTCCTCAGAGCCC-5’ Antisense 3’-AGGGGGAACCGTAAGGAAG-5’ |
380 |
Table 1: Primer sequence of the IL-1β, TNF-α and IL-6 Genes for RT-PCR
| Gene | Forecast denaturation | Denaturation | Annealing | Extend | Reverse extend | Cycles |
|---|---|---|---|---|---|---|
| β-actin | 94℃ 5 min | 94℃ 1 min | 56℃ 1 min | 72℃ 2 min | 72℃ 10 min | 35 |
| IL-1β | 94℃ 5 min | 94℃ 1 min | 56℃ 1 min | 72℃ 2 min | 72℃ 10 min | 35 |
| IL-6 | 94℃ 5 min | 94℃1 min | 56℃ 1 min | 72℃ 2 min | 72℃ 10 min | 35 |
| TNF-α | 94℃ 5 min | 94℃ 1 min | 56℃ 1 min | 72℃2 min | 72℃ 10 min | 35 |
Table 2: RT-PCR Amplification conditions of the IL-1β,TNF-αand IL-6 Genes in experimental mice brain
PCR product analysis: 0.5 g of Agarose powder was resolved in 50 ml of TAE buffer to make a 1% agarose gel containing Ethidium bromide (EB) with a final concentration of 0.5 μg/ml.
Sample preparation: 10 μl of PCR amplification product was mixed with 2 μl of 6× sample buffer, and load on agarose gel, the electrophoresis was completed at power supply of 10 V/cm for 30 min. After electrophoresis, separation result was observed under ultraviolet light, and pictures were taken using gel imaging system.
Lymphocyte transformation test
MTT assay was used to measure the transformation rate of T Lymphocyte, specific operation was as follows:
In the clean bench, the mouse spleen was taken and minced in Hanks solution to make the cell suspension;
After cell countthe number of cells were adjusted to a concentration of 5 × 106 cells/ml with RPMI1640, and then added to 96-well culture plates with a volume of 100 μL/holes. The experiment was operated with three holes for each mouse.
20 μl of ConA solution was added to each well with a final concentration of 5 μg/ml. and then the plate was incubated at 37, 5% CO2 for 44 h;
Discard supernatant of all well and added 100 μL of MTT solution with a final concentration of 5 μg/ml made with serum-free RPMI1640 medium, and continue to incubate at 37, 5% CO2 for 4 h;
After removing the supernatant of all wells, 100 μL of dimethylsulfoxide solution was added into each well; OD of 570 nm value was measured using microplate reader, and then the stimulation index, the group mean, standard deviation and P values were calculated.
Haemolytic plaque assay
Each group of mice for hemolytic plaque assay was immunized by intraperitoneal injection with sheep red erythrocyte before each test time point for 4 days; guinea pig serum was collected as complement solution. The spleen cell suspension was prepared in the clean bench, the concentration of cell was adjusted to 5 × 106/ml with RPMI1640; plaque assay: at first 5 ml of 1% agarose was melted and keep in 45 water bath, then 100 μl of the 10% SBRC and 40 μl of spleen cell suspension were added. After mixing, gel was pulled into the dish. After agar was concreted, then they were placed in 37incubator for 1 hour; follow 1 ml of complement with 10% concentration was added on gel surface and incubated at 37for another 1h; After observed and counted, the number of plaques hemolysis in per million splenocytes and the average value of each group were calculated.
Statistical analysis
Statistical software SPSS17.0 was used for data processing, measurement data to ± s, among groups using Analysis Variance by pairwise comparison with q test after a statistical significance with p<0.05 was considered statistically significant.
Results
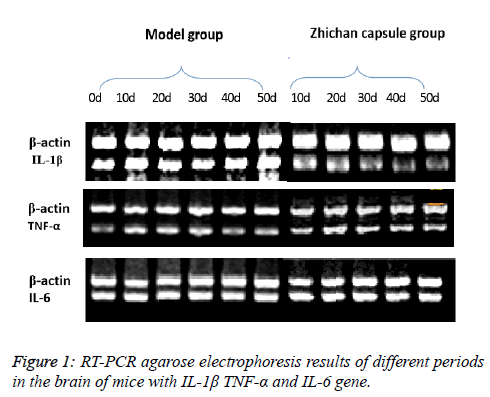
Analysis of electrophoresis density scan of RT-PCR products in the brain of experimental mice
The results show that IL-1β gene of C57BL/6 mice brain was amplified by PCR at different time points (0 d, 10 d, 20 d, 30 d, 40 d and 50 d), and results PCR products were analyzed using the gel imaging system to by UV scanning. The experimental results show that in the PD model group, IL-1 β gene expression level was significantly higher than that of Zhichan capsule group and normal control group. Compared with normal control group, p<0.01; compared with the Zhichan capsule group, p<0.05. The Zhichan capsule can lower the expression of IL-1β gene and alleviated expression of IL-1β which is induced by MPTP (Table 3 and Figure 1).
| Time(d) | Density ratio(IL-1β/β-actin) | |
|---|---|---|
| Model group | Zhichan capsule group | |
| 0 d | 0.4913 | 0.4913 |
| 10 d | 0.8931* | 0.6642* |
| 20 d | 0.6125* | 0.5315* |
| 30 d | 0.7123* | 0.4139* |
| 40 d | 0.8237* | 0.3338* |
| 50 d | 0.9255* | 0.3356* |
Table 3: Analysis of electrophoresis density scan of RT-PCR products in the brain of experimental mice (*p<0.01, Compare with model group; p<0.05,Compare with adjacent group).
Results of Density ratio of PCR products of TNF-α gene in the brain of experimental mice
The results show that TNF- α gene of C57BL/6 mice brain was amplified by PCR at different time points (0 d, 10 d, 20 d, 30 d, 40 d and 50 d), using the gel imaging system to analyze PCR products by UV scanning. The experimental results show that in the PD model group, TNF- α gene expression level was significantly higher than that of Zhichan capsule group and normal control group. Compared with normal control group, p<0.01; compared with the Zhichan capsule group, p<0.05. The Zhichan Capsule lowers TNF-α gene expression in mouse brain induced Parkinson disease (Table 4 and Figure 1).
| Time(d) | Density ratio(TNF-α/β-actin) | |
|---|---|---|
| Model group | Zhichan capsule group | |
| 0 d | 0.2351 | 0.2351 |
| 10 d | 0.6226* | 0.4356 |
| 20 d | 0.6132* | 0.4993 |
| 30 d | 0.6261* | 0.4135 |
| 40 d | 0.5133* | 0.4152 |
| 50 d | 0.5234* | 0.4149 |
Table 4: Results of Density ratio of PCR products of TNF-α gene in the brain of experimental mice (*p<0.01, Compare with model group; p<0.05, Compare with adjacent group)
Results of PCR products of IL-6 gene in the brain of experimental mice
The results show that IL-6 gene of C57BL/6 mice brain was amplified by PCR at different time points (0d, 10d, 20d, 30d, 40d and 50D) using the gel imaging system to analyze PCR products by UV scanning. The experimental results show that in the PD model group, IL-6 gene expression level was significantly lower than that of Zhichan capsule group and normal control group. Compared with normal control group, p<0.01; compared with the Zhichan capsule group, p<0.05. The Zhichan capsule can higher the expression of IL-6 gene and alleviate expression of IL-6 which was induced by MPTP (Table 5 and Figure 1).
| Time(d) | Density ratio(IL-6 /β-actin) | |
|---|---|---|
| Model group | Zhichan capsule group | |
| 0 d | 0.8325 | 0.8325 |
| 10 d | 1.3651* | 1.3581 |
| 20 d | 1.1532* | 1.1367 |
| 30 d | 1.1353* | 1.0152 |
| 40 d | 1.0164* | 0.9103 |
| 50 d | 0.9847 | 0.8334 |
Table 5: Results of PCR products of IL-6 gene in the brain of experimental mice (* p<0.01, Compared with model group; p<0.05, Compared with the Zhichan capsule group).
Lymphocyte transformation test results of Zhichan capsule in vivo
The results of OD 570 nm in the experimental group and control group are both shown in Table 3. At OD 570 nm determination of mean, lymphocyte transformation rate in Zhichan capsule group was significantly higher than that model control group, there were significant differences between tremor- stopping capsule group and the saline group, p<0.05 (Table 6).
| Time(d) | OD 570 nm | ||
|---|---|---|---|
| Normal control group | Model group | Zhichan capsule group | |
| 0 | 0.385 ± 1.553 | 0.283 ± 1.656* | 0.283 ± 1.656* |
| 10 | 0.374 ± 1.165 | 0.262 ± 1.912* | 0.459 ± 1.843*# |
| 20 | 0.382 ± 0.981 | 0.305 ± 1.036* | 0.436 ± 1.312*# |
| 30 | 0.369 ± 1.128 | 0.319 ± 0.980* | 0.413 ± 1.155*# |
| 40 | 0.376 ± 1.665 | 0.314 ± 0.875* | 0.413 ± 1.503*# |
| 50 | 0.366 ± 1.354 | 0.294 ± 1.369* | 0.408 ± 1.109*# |
Table 6: Lymphocyte transformation test results of Zhichan capsule in vivo (n=5,). (p<0.01, Compare with the contrast group; #p<0.05, Compare with the adjacent group).
Results of haemolytic plaque measurement number
The determination results of hemolytic plaque number per million splenocytes (Table 7). From the analysis of the formation of plaque number, compared with the normal control group, model control group decreased significantly (p<0.05), Zhichan capsule group increased significantly (p<0.05), and was significantly higher than the model group (p<0.01).
| Time(d) | Number of plaque forming | ||
|---|---|---|---|
| Normal control group | Model group | Normal control group | |
| 0 | 14.78 ± 2.000 | 11.45 ± 1.858* | 11.45 ± 1.858* |
| 10 | 13.69 ± 2.230 | 12.53 ± 1.367* | 13.63 ± 1.955# |
| 20 | 13.99 ± 1.569 | 12.36 ± 1.372* | 13.35 ± 1.955# |
| 30 | 13.56 ± 1.833 | 11.87 ± 1.663* | 13.87 ± 1.955# |
| 40 | 14.23 ± 1.964 | 11.59 ± 1.568* | 13.61 ± 1.955# |
| 50 | 14.37 ± 1.746 | 10.64 ± 1.931* | 14.58 ± 1.955# |
Table 7: Results of hemolytic plaque measurement number (n=5,). (*P<0.01, Compare with the normal control group; #P<0.05,Compare with the model group).
Discussion
Parkinson's disease (PD) is a common movement disorders disease among older peopleit is a kind of nervous system degeneratie disease characterized by static tremor, bradykinesia, myotonia and posture gait disorder. But its pathological mechanisms are undefinded at present. For further study of PD pathological mechanisms, we used MPTP intraperitoneal injection to set up the mice model. Researches show that MPTP is a kind of neurotoxin which can result in the dopaminergic neuron in substantia nigra degeneration and injury which is used for various kinds of animals with PD symptoms and pathological models [15]. The dopaminergic neuron in midbrain substantia nigra pars compacta is the most intensive and the content of the dopamine in corpus striatum is the highest, the reduction in the dopaminergic neuron can result in the change of PD pathology and clinical symptoms [16].
Currently, levodopa compound is used as the treatment of Parkinson's disease, but there are some side effects, which limits the clinical application of it [17-20]. Many studies have shown that traditional Chinese medicine in the treatment of Parkinson's disease is used for multi-factor, multi-target pathological complexity diseases which have proven a unique number of advantages [21,22], and it became a hot topic in recent years in prevention and treatment of Parkinson's disease. More and more information shows that the immune inflammatory response involved in this process [23-28]. Researches show immune inflammatory response participates in the pathogenic process of PD, the effect of inflammatory factors in the pathogenesis of PD has been accepted widely [29-31], including TNF-α, IL-1β, IL-6, IL-2, etc. [32,33]. Other information reported, TNF-α induce and promote Parkinson's disease degeneration of dopamine neurons [34] and IL-6 is in favor of regeneration and repair after neuronal degeneration [35]. Therefore, improving the ability of secretion IL-6 of the nigral DA neurons in PD and inhibiting the biosynthesis of TNF-α and IL-1β can have neuroprotective effect.
The experimental results show that the expression of proinflammatory cytokines IL-1β and TNF-α gene mRNA is increasing in brain abdominal area of MPTP model group, IL-6 mRNA gene expression is reducing. At different time of Zhichan capsule treatment, the expression of IL-1β and TNF-α mRNA reduced, IL-6 mRNA increased, the most obvious at the time for 30 d. Compared with model group with a significant difference, p<0.05. Zhichan Capsule has a down-regulatory effect on the expression of IL-1β and TNF-α and up-regulatory effect on the expression of IL-6 in the midbrain substantia nigra of PD mouse, which can reduce and substantia nigra nerve inflammation induced by neurotoxin MPTP and DA neurons can survive.
Lymphocyte is the immunocompetent cell of body whose activation, proliferation and differentiation play an important part in the immune response process. Detecting the cellular immune function can by means of the lymphocyte transformation test and the hemolytic plaque test. Therefore, by obseving the influences of Zhichan capsule to the lymphopoiesis proliferation reaction, and researching the regulating effects of Zhichan capsule to immunologic function.
In this study, in vivo lymphocyte transformation test, the results showed that lymphocyte proliferation has a significant difference (p<0.05), Zhichan capsules treated group compared with the model control group. It shows that Zhichan capsules can promote the function of lymphocyte transformation, significantly enhanced T cell function in mice. In this study, further research found that the number of haemolytic plaque increased in Zhichan capsule group, compared with the model control group, with statistical significance, (p<0.05). It showed that Zhichan capsules can improve proliferation of B cells, suggesting that the Zhichan capsule can enhance B cell function.
Conclusion
In summary, the mechanism of Zhichan capsules treatment on PD shows that inhibiting the expression of IL-1β and TNF-α factor and increasing the expression of IL-6 in the midbrain substantia nigra can reduce the damage to the brain which was caused by excessive inflammatory cytokines. On the other hand, enhancing the humoral and cellular immune function of PD mouse model are relative to the activation of specific and non-specific killer cells natural killer cells (NK) and lymphokine-activated killer cells. In future, the Zhichan capsules may be used to treat Parkinson’s disease effectively.
References
- Oleg SG, Shoudong Li, Layla FS, Weijun C, Galina K, Fredric PM, Ronald JM, Nicholas M. The phosphorylation state of Ser -129 in human alpha -synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci USA. 2008; 105: 763-768.
- Tansey MG,Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010; 37: 510-518.
- Figueiredo-Pereira ME, Rockwell P, Schmidt-Glenewinkel T, Serrano P. Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front Mol Neurosci. 2015; 7: 104.
- Russo I, Bubacco L, Greggio E. LRRK2 and neuroinflammation:partners in crime in Parkinson’s disease. J Neuroinflammation. 2014; 11: 52.
- Hirsch EC, Hunot S. Neuroinflammation in parkinson's disease: a target for neuroprotection. Lancet Neurol. 2009; 8: 382-397.
- Yokoyama H, Kuroiwa H, Yano R, Araki T. Targeting reactive oxygen species, reactive nitrogen species and inflammation in MPTP neurotoxicity and Parkinsons disease. NeurolSci. 2008; 29: 293-301.
- Ferrari CC, Tarelli R. Parkinson s disease and systemic inflammation. Parkinsons Dis. 2011: 436813.
- FahnS. A new look at levodopa based on the Elldopa study. J Neural Transm Suppl. 2006: 419-426.
- Ma Yunji, Shi Juxin, Li Mei. Advances in Parkinson's disease Chinese medicine treatment for fear TCM Journal, 2011, 26: 104-106.
- Li Xuehua. Advances in medicine treatment of Parkinson's disease. Chinese J Clin Rat Drug Use. 2012; 5: 148.
- Yao Xin. The studies of Zhichan Capsules Clinical treatment Parkinson's disease. Jilin Medical. 2008; 29: 653-654.
- Jiajun Chen, Jinshu Ma, Yafei Qiu, Shihong Yi, Yongmao Liu, Qingwei Zhou. Effects of Zhichanpowder on signal transduction and apoptosis-associated gene expression in the substantia nigra of Parkinson’s disease rats. Neural Regen Res. 2012; 7: 2115-2122.
- Qingwei Zhou, Jiajun Chen, Shihong Yi, Yongmao Liu. Weimin Tang, Yongmao Liu, Pengguo Z. Zhichan powder regulates nigrostriatal dopamine synthesis and metabolism in Parkinson’s disease rats. Neural Regen Res. 2012; 7: 2017-2014.
- Chen Shengdi. Studies in Experimental Parkinson's disease animal models.Shanghai Pharmaceutical. 1987;18: 120-125.
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007; 2: 141-151.
- Nicotra A, Parvez S. Apoptotic molecules and MPTP-induced cell death. Neurotoxicol Teratol. 2002; 24: 599-605.
- Scapira AHV, Emreb M, Jenner P, Poewe W. Levodopa in the treatment of Parkinson’s disease. Eur J Neurol. 2009; 16: 982-989.
- Shen Hongyan, Henan XU, Chun HU. Survey on monoamine oxidases B inhibitors as therapy drug of Parkinson′s disease in clinical practice. Chinese J Med Chem. 2011; 21: 483-488.
- Tie WU, Feng Bing Hong. Pharmacology. Science Press, 2010: 195-200.
- Brunton L, Parkerk L. Manual of pharmacology and therapeutics. Science Press, Beijing, China, 2009: 324-332.
- Zhu Minglong. Method of active blood by Yu Yin treatment Parkinson’s disease Chinese Magazine of Clinical Medicinal Professional Research.
- Li Xuexin. Xifengdingchantang combined with Madopar treatment on Parkinson's disease in 27 cases. Chinese Med Res. 2008; 2: 232-233.
- Abramsky O, Litvin Y. Automimmune resonse to dopamine-receptor as a possible mechanism in the pathogensis of Parkinson’s disease and schizophrenia. Perspect Biol Med. 1978; 22:104-114.
- Sun Weimin. Cell Factor Methodology. People's Health Publishing House, 1999: 5-9.
- Chen Shengdi. Progress in the etiology and pathogenesis of Parkinson's disease. Front. Neurol. 1997; 24: 243-247.
- Thatte U, Dahanukar S. Apoptosis: clinical relevance and pharmacolcgical manipulation. Drugs. 1997; 54: 511-532.
- Mogi M, Nagatsu T. Neurotrophins and cytokine in Parkinsion’s disease. Adv Neural. 1999; 80: 135-139.
- Nagatsu T, Mogi M, Ichnose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Trasm. 2000; 60: S277-S299.
- Ya wu, Shaohua Liao,Yanling zhang. Preliminary screening of Parkinsons Disease substantia nigra autologous antigen in vivo.J Immunol. 2012; 28: 926-931.
- Zhongmei Chen, Yunpeng Yang, Xu Yang, Changqing Zhou, Fengqun Li, Peng Lei, Ling Zhong, Xin Jin, Guoguang Peng. Effects of immunization in optimization of nucleic acid vaccine to chronic MPTP mice of Parkinsons Disease. Neurol Sci. 2013; 28: 926-931.
- Shaodan Li, Yi Liu, Minghui Yang. Effects of reinforcing kidney activating blood pill to Parkinsons Disease model of tissue in mice brain nitric oxide, TNF-α and γ-interferon. Southern medical University. 2011; 31: 90-92.
- Mitchell GA, Gauthier N, Lesimple A, Wang SP, Mamer O, Qureshi I. Heredity and acquired diseases of acyl-coenzyme A metabolism. Molto Genet Metab. 2008; 94: 4-15.
- Sawada H, Hishida R, Hirata Y, Ono K, Suzuki H, Muramatsu S, Nakano I, Nagatsu T, Sawada M. Activated microglia affect the nigro-striatal dopamine Neurons differently in neonatal and aged mice treated with 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine. Neurosci Res. 2007; 85: 1752-1761.
- Nishimura M , MizutaI, Mizuta E , Yamasaki S, Ohta M, Kaji R, Kuno S. Tumor necrosis factor gene poly morphisms in patients with sporadic Parkinson's disease. Neurosci Lett. 2001; 311: 1-4.
- Muller T, Blum D, Przuntek H, Kuhn W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity on Parkinson's disease. Acta Neuro Scand. 1998; 98: 142 -144.