- Biomedical Research (2016) Volume 27, Issue 3
Increased hepatic gluconeogenesis and decreased glucose uptake, and increased hepatic de novo lipogenesis in rat model of maternal diabetes.
Premila Abraham1*, Suganthy Rabi2, Deepak Vinod Francis2, Mohana Priya D1, Kasthuri Natarajan1, Anita Amaladass11Department of Biochemistry, Christian Medical College, Bagayam, Vellore 632002, Tamil Nadu, India
2Department of Anatomy, Christian Medical College, Bagayam, Vellore 632002, Tamil Nadu, India
Accepted date: February 17, 2016
Abstract
In pregnancies complicated by diabetes, hyperglycemia and lipid metabolism alterations are associated with both maternal and fetal complications. However the mechanism of metabolic alterations in mothers that contribute to hyperglycemia and hyperlipidemia is not fully understood. In the present study we investigated the mechanisms underlying hyperglycemia and hypertriglyceridemia in gestational diabetes using a rat model. Diabetes was induced in virgin rats by the administration of 50 mg Streptozotocin (STZ) i.p. After the STZ injection, the animals were mated. On the 19th day of pregnancy all the rats were laparotomised after withdrawal of blood by cardiac puncture. Blood was used for the determination of insulin, glucose, cholesterol. Liver and pancreas were removed and used for biochemical assays and light microscopy. Plasma glucose, cholesterol and insulin were significantly higher in the diabetic rats as compared with control. The mRNA expressions of the rate limiting gluconeogenic enzymes PEPCK and Glucose 6 phosphatase and de novo lipogenic enzymes acetyl CoA carboxylase and fatty acid synthase were higher in the livers of diabetic rats. The protein level and activity of GK was decreased in the livers of diabetic rats. The results of the present study suggest that hyperglycemia observed in diabetic pregnant rats may be due to increased gluconeogenesis, increased glucose output and decreased glucose uptake by the liver. Hyperlipidemia may be due to increased de novo lipid synthesis in the liver.
Keywords
Gestational diabetes, PEPCK, Glucose 6 phosphatase, Glucokinase, De novo lipogenesis, Liver, Rat.
Introduction
Maternal diabetes is one of the commonest complications during pregnancy affecting up to 7% of pregnancies. Its incidence is variable and occurs in 3 to 8% of pregnant women [1,2]. Several population studies show that the diabetes during pregnancy affects the health of both mothers and their infants [3-6]. Diabetes during pregnancy is associated with an increased risk of maternal and neonatal morbidity [3]. Maternal diabetes, which includes the occurrence of either Diabetes Mellitus 1 (DM1) or Diabetes Mellitus 2 (DM2) in pregnancy and Gestational Diabetes Mellitus (GDM), creates an unfavorable environment for embryonic and fetoplacental development. The altered metabolic environment in maternal diabetes during pregnancy interferes with normal placental and fetal development. In humans, as well as in rodents, there is an increased incidence of congenital malformations and fetal growth retardation. In pregnancies complicated by diabetes, hyperglycemia and lipid metabolism alterations are associated with both maternal and fetal complications [7,8]. However the mechanism of metabolic alterations in mothers that contribute to hyperglycemia and hyperlipidemia is not fully understood.
Liver is the central organ that controls intermediary metabolism. The liver is a major insulin-sensitive organ responsible for maintaining glucose and lipid homeostasis. In the liver, insulin acts through a complex signaling network and functions as an important regulator of carbohydrate and lipid homeostasis [9]. Liver is responsible for maintaining proper blood glucose levels by sensing hepatoportal glucose, via GLUT2 glucose transporter, and Glucokinase (GK), and regulating gluconeogenesis [10,11]. The key enzyme responsible for the regulation of glucose utilization is GK that catalyzes glucose phosphorylation as the first step of storage of glucose as glycogen and glucose disposal by glycolysis [12]. Conversely, Phosphoenol Pyruvate Carboxykinase (PEPCK) and glucose 6 phosphatase (G6Pase) are rate-controlling enzymes of gluconeogenesis in the liver [13]. GLUT-2, the liver-and pancreas-specific glucose transporter is involved in glucose uptake and release processes. The transcription of the GLUT2 gene is known to be upregulated in the liver during postprandial hyperglycemic states or in type 2 diabetes [14].
Hepatic De Novo Lipogenesis (DNL) is the biochemical process of synthesising fatty acids from acetyl-CoA subunits that are produced from a number of different pathways within the cell, most commonly carbohydrate catabolism. De novo synthesis of fatty acids is regulated by two enzymes-Acetyl CoA Carboxylase (ACACA) and Fatty Acid Synthase (FAS). Acetyl-CoA Carboxylase (ACC) is the rate-limiting enzyme in the synthesis of fatty acids, which converts acetyl CoA to malonyl CoA and fatty acid synthase, a large multifunctional enzyme that synthesizes palmitate from acetyl CoA and malonyl CoA [15].
In the present study we investigated the mechanisms underlying hyperglycemia and hypertriglyceridemia in gestational diabetes using a rat model of GDM. Gestational DM conditions are reproduced in animals by administration of different doses of STZ before mating [16,17] or during pregnancy [18,19].The most often used experimental models are rodents (Wistar rat) because of their convenient maintenance, short length of pregnancy and multiparity. Therefore we used rat model to investigate alterations in glucose metabolism and fatty acid metabolism in the livers of pregnant rats with diabetes. We analysed the hepatic gene expression of key gluconeogenic enzymes-PEPCK, G6Pase, and key enzymes of de novo lipogenesis-ACAC, and FAS. We also assayed the activities of G6Pase and GK, protein expression of GK and GLUT2 in the livers of diabetic pregnant rats and compared with normal pregnant rats.
Materials and Methods
Induction of diabetes
Female virgin adult Wistar rats weighing 150 gm were used for the study. Diabetes was induced in the rats by the administration of i.p. injections of freshly prepared 50 mg streptozotocin in citrate buffer [17] after determining fasting blood glucose level. On day 3 of STZ injection glucose was estimated in the blood drawn from tail vein. Only rats with fasting blood glucose >300 mg/dl were used for the study, the others were rejected. The control rats were administered citrate buffer alone. Food intake, nonfasting blood glucose concentrations urine sugar and ketone bodies were recorded once weekly.
Induction of pregnancy
Two weeks after the STZ injection, the animals were mated by caging them with males in the ratio of 3:1 between 5 p.m. and 7 a.m. Control animals which had been treated with citrate buffer alone were mated simultaneously. Mating was confirmed by examination of vaginal smears for the presence of sperm immediately after separation from the male. The day when a positive vaginal smear was obtained was denoted as gestational day 0. On the 19th day of pregnancy all the rats were laparotomised under light ether anesthesia after withdrawal of blood by cardiac puncture. Blood was used for the determination of insulin, glucose, cholesterol. Liver and pancreas were removed. Sections of the pancreas and liver were fixed in buffered formalin for light microscopic studies. Liver was frozen at -70°C till analysis.
Real-time polymerase chain reaction analysis of gluconeogenic genes and lipogenesis genes
Total RNA was isolated from liver tissue using TRIzol reagent (Invitrogen, United States) according to the manufacturer’s instructions. Equal amounts of RNA were used to synthesize first strand cDNA (Promega, United States), and quantitative Real-Time Polymerase Chain Reaction (RT-PCR) was performed on an ABI PRISM 7300 PCR System (Applied Biosystems, United States) using Syber Green I GoTaq® qPCR Master Mix (Promega, United States). PCR was performed as: one cycle at 95 °C for 5 min, followed by 40 cycles of 95°C for 15 s, 58°C for 20 s and 72°C for 30 s. Then PCR products were analyzed by melting curve to confirm the specificity of amplification. The PCR primer sequences are shown in Table 1. Data for each gene of interest was normalized relative to the internal reference gene β actin. This was done by substracting the Ct values of beta-actin in each sample from that of the gene of interesat. The value obtained was referred to as the ΔCt value. The relative fold-change in the gene of interest was determined according to the comparative Ct method using the following formula: Relative fold change=2-(ΔCt) [20]
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| ACACA | TTGTGGAAGTGGAAGGCACAG | CTTATTATTGTCCCAGACGTAAGC |
| FAS | GGCTCACACACCTACGTATTGG | TGCTTAATGAAGAAGCATATGGCTT |
| G6Pase | GTGTTGACATCGGCCC | AACTGAAGCCGGTTAG |
| PEPCK | CGCAAGCTGAAGAAATATGACAA | TCGATCCTGGCCACATCTC |
| Beta actin | TTCTACAATGAGCTGCGTGTG | GGGGTGTTGAAGGTCTCAAA |
Table 1. PCR primer sequences.
Western blot analysis of GLUT2 and GK
Livers from diabetic and control rats were homogenized in lysis buffer (pH 7.4) containing 100 mM NaF, 50 mM HEPES, 150 mM NaCl, 10% Glycerol, 1.2% Triton X, 1 mM MgCl2, 1 mM EDTA, 1 mM Na3VO4, and protease inhibitor cocktail (Roche, 11836145001). Total protein concentration was determined by Bradford Protein Assay (Bio-Rad). Lysates (50 μg protein) were run in 8% SDS-PAGE and transferred to PVDF membrane (Millipore). Membranes were blocked for 10 minutes at room temperature with 5% milk and were incubated overnight at 4°C with antibodies against GLUT2, glucokinase or β-actin (1: 1,000, Santa Cruz Biotechnology).
After three washing series (10 minutes), the membranes were incubated for 1 hour at RT with antibodies against rabbit IgGHRP conjugate (1; 2000, #170-6515, Bio-Rad) or mouse IgGHRP conjugate (1; 2000, #170-6516, Bio-Rad).Proteins were detected with horseradish peroxidase-conjugated secondary antibody (Bio-Rad Laboratories, Hercules, CA, USA) and enhanced chemiluminescence reagent (Amersham Biosciences, Piscataway, NJ, USA)with the enhanced chemiluminescence detection system. The experiments were replicated three times. β-actin served as an internal loading control protein. The bands were documented using an AlphaEase FC gel documentation system (Alpha Innotech Corporation, CA.) and normalized to those for beta-actin.
Hepatic enzymes activities
Enzyme sources were prepared according to the method developed by Hulcher and Oleson with slight modification [21]. Liver samples were homogenized in 9 volumes of a buffer containing 50 mmol/L Tris-HCl, pH 7.4, 100 mmol/L KCl, 10 mmol/L mercaptoethanol and 1 mmol/L EDTA.
Homogenates were centrifuged at 100,000g for 1 h; the cytosol was used for the spectrophotometric assay of glucokinase as described by Davidson and Arion, in which the formation of glucose-6-phosphate from glucose at 37°C was coupled to its oxidation by G6PD and Nicotinamide Adenine Dinucleotide (NAD) [22] and microsomes for G6Pase.
Microsome was dissolved in ice cold homogenizing buffer 0.25 M sucrose, 5 mm HEPES buffer, pH 7.4. Glucose 6- phosphatase activity was assayed by measuring the amount of glucose and Pi released as byproducts of G6P hydrolysis. Inorganic phosphate was determined according to Fiske and Subbarow assay [23] at 700 nm. The protein concentrations were determined using the Lowry method [24] as modified by Peterson [25] using bovine serum albumin as standard.
Measurement of the plasma metabolic parameters
The concentrations of blood glucose, total cholesterol, were determined by using kits purchased from Wako Pure Chemical Industries. The plasma insulin concentration was measured by a radioimmunoassay, using a kit purchased from Shionogi &Co. (Osaka, Japan).
Statistical analysis
Statistical significance of the difference between two groups was analyzed by unpaired Student’s t-test for comparison of two groups. P<0.05 was accepted as significant.
Results
Weight gain and food intake
Pregnancy in diabetic pregnant rats was associated with a significantly lower weight gain as compared with normal rats. The weight gain in normal rats was 38.8 ± 12.2 gm and in diabetic rats it was 22.8 ± 10.3 gm, P<0.05).The food intake was higher in the diabetic rats as compared with control (50.11 ± 15.44 vs 86.24 ± 26.66 gm/kg/day, P<0.05).
Plasma glucose, cholesterol, and insulin
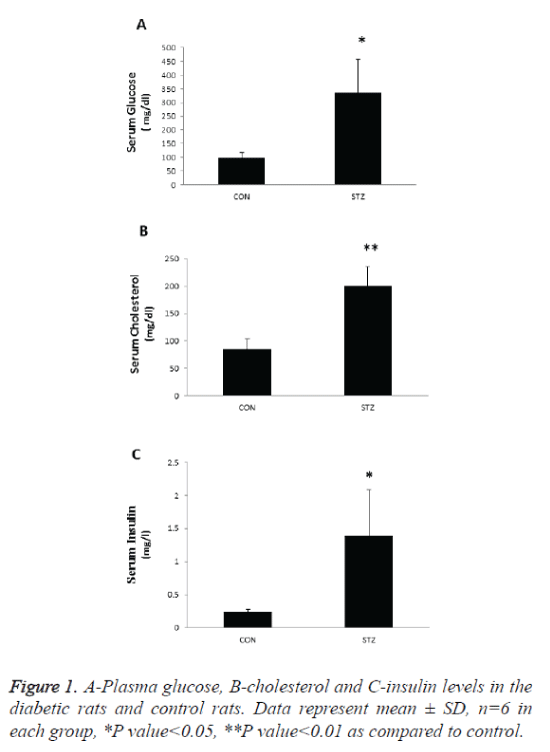
Plasma glucose, cholesterol and insulin was significantly increased the diabetic pregnant rats as compared with control (Figure 1). Thus, during pregnancy our experimental model of chemical diabetes shows that normal carbohydrate tolerance is not maintained. In that sense our experimental design mimics the development of gestational diabetes in women.
Hepatic gene expressions of rate limiting enzymes of gluconeogenesis and lipogenesis are upregulated in the diabetic pregnant rats
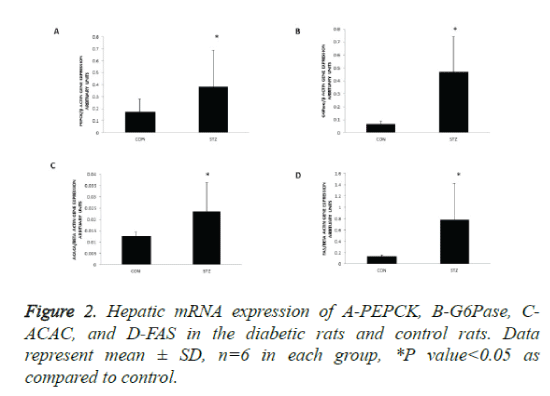
PEPCK and G6Pase are rate-controlling enzymes of gluconeogenesis in the liver. To understand the mechanism of hyperglycemia in diabetic pregnancy we quantified mRNA of key enzyme of glucose production and release in the liver, PEPCK, and G6Pase. To understand the mechanism of hypertriglyceridemia in maternal diabetes we analysed the mRNA of key enzymes of de novo fatty acid synthesis- ACACA and FAS by RT PCR. The mRNA expressions of PEPCK and G6Pase were increased 2 fold and 5 fold respectively in the livers of diabetic rats as compared with control (Figures 2A and 2B).
With respect to lipogenic enzyme genes, mRNA levels of acetyl CoA carboxylase and fatty acid synthase were increased 2 fold and 4 fold respectively in the livers of diabetic rats as compared with control (Figures 2C and 2D). Thus, in the livers of diabetic mothers the key gluconeogenic genes and lipogenic genes were upregulated, suggesting that increased glucose synthesis and de novo lipogenesis contribute to hyperglycaemia and hyperlipidemia observed in diabetic pregnant rats.
Hepatic activities of glucokinase and glucose 6 phosphatase are increased in the diabetic pregnant rats
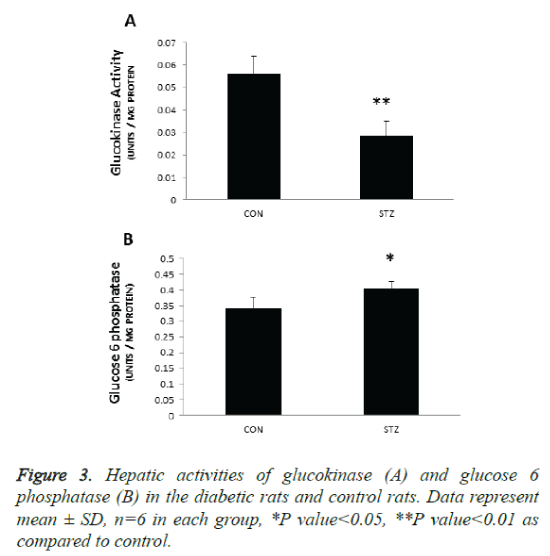
The key enzyme responsible for the regulation of glucose utilization is GK that catalyzes glucose phosphorylation as the first step of storage of glucose as glycogen and glucose disposal by glycolysis. The main function of this enzyme is to regulate hepatic glycolysis and glucose homeostasis. In the present study GK activity was reduced by 45% (p 0.001) in the liver microsomes of diabetic rats (Figure 3A). This was accompanied by significant increase in cytosolic G6 Pase activity (P<0.05) (Figure 3B) suggesting decreased glucose uptake and increased glucose release by the liver of diabetic rats. These results suggest that reduced glucose uptake and utilisation by the liver and increased glucose production (by glycogenolysis and/gluconeogenesis) from the liver contribute to hyperglycaemia observed in STZ treated pregnant rats.
Protein levels of GK and GLUT2 are decreased in the diabetic pregnant rats
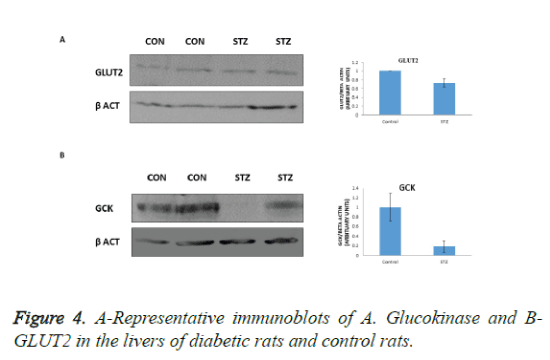
GLUT2 is the major glucose transporter of hepatocytes. In hepatocytes GLUT2 is involved in glucose uptake and release in the fed and fasted states, respectively. However, it is indispensable for glucose uptake. In the present study we observed decreased GLUT2 expression in the livers of diabetic rats (Figure 4A), suggesting decreased uptake of glucose by the liver. In addition, GK protein level was also lowered (Figure 4B) suggesting that decreased uptake, phosphorylation and utilization by the liver contributes to hyperglycemia observed in diabetic pregnant rats.
Liver and pancreas histology
We examined whether there are any morphological changes in the liver and pancreas of diabetic rats. No morphological abnormalities were observed in the liver and pancreatic tissues of STZ treated rats and control rats. In normal and diabetic rats, a distinct and well-arranged hepatocytes, sinusoids, and central vein could be seen (Figure 5). The sections of pancreas showed that morphology and structure of pancreas were normal in control as well as pregnancy group (Figure 6).
Discussion
Diabetes in pregnant women is associated with an increased risk of maternal and neonatal morbidity and remains a significant medical challenge. Diabetes during pregnancy maybe divided into clinical diabetes (women previously diagnosed with type 1 or type 2 diabetes) and gestational diabetes, defined as any glucose intolerance detected during pregnancy that has evolved from a diagnosis associated with the metabolic risk of type 2 diabetes to a clinical condition associated with higher risks for maternal and perinatal morbidity [1,2].
Experimental models are developed with the purpose of enhancing understanding of the pathophysiological mechanisms of diseases that affect humans. With regard to diabetes in pregnancy, experimental findings from models will lead to the development of treatment strategies to maintain the closest to normal metabolic intrauterine milieu, improving perinatal development by preventing fetal growth restriction or macrosomia. Human type 2 DM and gestational DM conditions are reproduced in animals by administration of different doses of STZ before mating [16,17] or during pregnancy [18,19].
In pregnancies complicated by diabetes, hyperglycemia and lipid metabolism alterations are associated with both maternal and fetal complications [7,8]. However the mechanism of metabolic alterations in mothers that contribute to hyperglycemia and hyperlipidemia is not fully understood. Therefore we used rat model of GDM to investigate the role of hepatic gluconeogenic enzymes and enzymes of de novo fatty acid synthesis in hyperglycemia and hyperlipidaemia observed in diabetic pregnancy. GDM was induced in the rats using STZ. Severe diabetic rats had glycemia levels above 300 mg/dl throughout pregnancy. This result was expected and is in agreement with other studies previously performed in other laboratory [26,27], reproducing the hyperglycemia that some women with uncontrolled clinical diabetes present during pregnancy. In addition, the diabetic pregnant rats had significantly higher serum cholesterol and insulin level as compared with control. The liver is a major insulin-sensitive organ responsible for maintaining glucose and lipid homeostasis. A failure of insulin to increase hepatic glucose utilization and to suppress hepatic endogenous glucose production is a major factor contributing to hyperglycemia in diabetes [28].
GDM is characterised by insulin resistance [29], suggesting that hyperglycemia observed in diabetic pregnancy may be due to increased gluconeogenesis and increased release from the liver. Gluconeogenesis is the reverse process of glycolysis except that the four energy barrier steps are bypassed by four key enzymes: PEPCK, Pyruvate Carboxylase (PC), G6Pase, and Fructose-1, 6-bisphophatase (F1, 6BPase). PEPCK and G6Pase are rate-controlling enzymes of gluconeogenesis in the liver [13]. Glucose-6-phosphatase (G6Pase) catalyzes the terminal step in the glycogenolytic and gluconeogenic pathways, and Phosphoenolpyruvate Carboxykinase (PEPCK) is a key regulatory enzyme driving gluconeogenesis [13]. The last step in hepatic gluconeogenesis is the hydrolysis of glucose-6-phosphate to glucose by glucose-6-phosphatase in the lumen of the endoplasmic reticulum. Newly produced glucose is presumably transported back into the cytoplasm and then exits the cell via the facilitative glucose transporter GLUT2 in the plasma membrane. During the diabetic process, the expression of the liver PEPCK gene is markedly increased by the lack of insulin and the rise of glucagon levels [30].
Glucose 6-phosphatase enzymatic complex operates within the Endoplasmic Reticulum (ER) of liver [31], kidney [32], pancreatic β-cells [33]. The main function of this enzyme is to regulate hepatic glycolysis and glucose homeostasis [34]. Insulin suppresses gluconeogenesis by inhibiting the transcription of PEPCK and G6Pase [35]. Thus in GDM in which insulin resistance is observed, these enzymes are upregulated contributing to hyperglycemia. Therefore we analysed the mRNA expressions of PEPCK and G6Pase by RT PCR and the activity of G6Pase in the livers of diabetic rats and control rats. We found increase in mRNA expression of these genes, suggesting that hyperglycaemia observed in may be due to increased gluconeogenesis and glucose output from the liver. In addition to a selective dysregulation of individual glucose production pathways, increased production of glucose during hyperinsulinemia in GDM may result from inadequate suppression of all the supporting fluxes of glucose production [36].
The key enzyme responsible for the regulation of glucose utilization is GK that catalyzes glucose phosphorylation as the first step of storage of glucose as glycogen and glucose disposal by glycolysis. The regulation of GK activity is primarily due to changes in the transcription of its gene. In the liver, the primary regulated step that determines glucose uptake is glucose phosphorylation and not glucose transport. Hepatocytes are able to respond to fluctuations in blood glucose levels by parallel changes in the rate of glucose phosphorylation. The key enzyme in the regulation of this process is glucokinase. This enzyme is induced by insulin. Therefore we next assayed the activity and protein expression of GK in the liver. In the diabetic pregnant rat livers the protein levels and the activity of GK were significantly decreased when compared with control suggesting that decreased uptake and utilisation of glucose by the liver may contribute to hyperglyemia observed in diabetic mother rats.
GK activity is controlled by the partnership between insulin signaling and substrate-dependent regulation. The liver expresses hexokinase IV (i.e. glucokinase), which is inactive when associated with Glucokinase Regulatory Protein (GKRP) in the nucleus. The interaction of GK with GKRP is regulated by metabolic intermediates (glucose-6-phosphate and fructose-1-phosphate) that accumulate in response to hyperglycemia and dietary carbohydrates (e.g. fructose). These glycolytic intermediates compete with the binding of GK to GKRP; thus, this step is not directly controlled by glucose [37]. In the present study, G6Pase is high suggesting a decrease in the hepatic levels of glucose 6 PO4. This may facilitate the binding of GK to GKRP and its accumulation in inactive form. This may account for decreased GK activity observed in the livers of diabetic pregnant rats.
Blood glucose enters hepatocytes via GLUT2, a plasma membrane glucose transporter. GLUT2 is mainly expressed in the liver, beta-cells of the pancreas, and the basolateral membrane of kidney proximal tubules and plays an important role in glucose homeostasis in living organisms. GLUT-2 is a bifunctional glucose transporter which is involved in glucose uptake and release processes in the liver [38]. However, it is indispensable for glucose uptake [39]. Hepatocyte-specific deletion of GLUT2 blocks hepatocyte glucose uptake; however, deletion of GLUT2 does not affect hepatic glucose production in the fasted state [39].The transcription of the GLUT2 gene is known to be upregulated in the liver during postprandial hyperglycemic states or in type 2 diabetes to facilitate uptake [14]. Therefore we analysed the protein levels of GLUT2 in the liver by western blot. In the present study we observed decreased expression of GLUT2 protein in the livers of diabetic rats as compared with control. This suggests that decreased uptake of glucose by the livers of diabetic pregnant contribute to hyperglycemia observed in these rats.
All these findings suggest that hyperglycaemia observed in maternal rats with diabetes may be due to increased gluconeogenesis (increased PEPCK and G6Pase expression) and glucose output from the liver (G6Pase expression) and decreased uptake of glucose (decreased GK and GLUT2 expression) by the liver.
To date, the pathophysiology of hypertriglyceridemia in GDM is incompletely understood. We hypothesised that hyperlipidaemia observed in GDM may be due to increased hepatic de novo lipogenesis. Hepatic De novo lipogenesis (DNL) is the biochemical process of synthesising fatty acids from acetyl-CoA subunits that are produced from a number of different pathways within the cell, most commonly carbohydrate catabolism. De novo synthesis of fatty acids is regulated by two enzymes-acetyl CoA carboxylase and FAS. Acetyl-CoA carboxylase (ACAC) is the rate-limiting enzyme in the synthesis of fatty acids, which converts acetyl CoA to malonyl CoA. Fatty acid synthase is a large multifunctional enzyme that synthesizes palmitate from acetyl CoA and malonyl CoA [15]. In the present study we observed increase in the expression of these genes in livers of diabetic rats, suggesting increased de novo lipogenesis.
It is surprising to find increased de novo synthesis of fatty acids in the liver in the presence of insulin resistance because insulin is required for hepatic lipid synthesis. This may be explained by the ‘selective insulin resistance’ hypothesis. Recent studies show that the role of insulin resistance with regard to hepatic lipid metabolism is complex. Insulin is required for hepatic lipid synthesis. Thus, one might hypothesize that hepatic insulin resistance would decrease hepatic fatty acid and triglyceride synthesis. In contrast, liver and plasma triglycerides are increased in metabolic disease. To resolve this paradox, investigators have proposed that there may be pathway-selective insulin resistance, which has been observed in vascular tissues [40,41]. This would suggest that there are distinct insulin-sensitive signaling pathways that independently modulate glucose and lipid metabolic pathways. The model proposes that the pathways are differentially altered in metabolic disease. With selective insulin resistance, insulin fails to adequately suppress hepatic glucose production or augment hepatic glucose uptake, and yet still augments or at least sustains hepatic lipogenesis and TG accumulation, contributing to hypertriglyceridemia.
The phenomenon of selective hepatic insulin resistance, in which hepatic glucose metabolism becomes unresponsive to insulin but hepatic lipogenesis continues unabated, is a long standing paradox in type 2 diabetes (T2D) [42,43].Evidence for the importance of DNL in insulin-resistant hepatic triglyceride synthesis includes reports of increased hepatic DNL in both humans with insulin resistance and humans with NAFLD [44], as well as in leptin-deficient insulin-resistant ob/ob mice [45].
Attempts to resolve the mechanism by which triglyceride synthesis paradoxically increases in the insulin-resistant liver have focused on the regulation of de novo lipogenesis (DNL) by the insulin-dependent sterol regulatory element binding protein 1c(SREBP-1c) [46]. Carbohydrate responsive element binding protein (ChREBP) is a master transcriptional activator of hepatic lipogenesis [47]. ChREBP is activated by increased glucose influx in liver, and together with SREBP-1, drives expression of genes involved in fatty acid synthesis and esterification. Hepatic DNL is regulated independently by insulin and glucose, through the activation of SREBP-1c [48] and ChREBP [49] which transcriptionally activate nearly all genes involved in de novo lipogenesis. Data from studies conducted in mouse models demonstrate that hepatic overexpression of SREBP-1c or hyperinsulinemia stimulate lipogenesis and cause hepatic steatosis, [50,51] whereas the levels of all the enzymes involved in DNL are reduced in ChREBP knockout mice [52]. Data indicate that glucose and fructose ingestion can very efficiently lead to the induction of lipogenic genes via these SREBP-1c pathways without insulin [53].
Conclusion
In summary, the results of the present study suggest that increased gluconeogenesis, increased glucose output and decreased glucose uptake by the liver contribute to hyperglycemia observed in diabetic pregnant rats. Hyperlipidemia observed in these rats may be due to increased de novo lipid synthesis in the liver. It is suggested that these effects may be mediated via the induction of SREBP-1c pathways by glucose.
References
- Maganha CA, Vanni DGBS, Bernardini MA, Zugaib M. Tratamento do Diabetes Melito Gestacional. Revista da Associação Médica Brasileira 2003; 49: 330-334.
- Schmidt MI, Matos MC, Reichelt AJ, Forti AC, de Lima L, Duncan BB. Prevalence of gestational diabetes mellitus - do the new WHO criteria make a difference? Brazilian Gestational Diabetes Study Group. Diabetic Medicine 2000; 17: 376-380.
- Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med 2010; 23: 199-203.
- Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 2006; 91: 3718-3724.
- Aerts L, Holemans K, Van Assche FA. Maternal diabetes during pregnancy: consequences for the offspring. Diabetes Metab Rev 1990; 6: 147-167.
- Persaud OD. Maternal diabetes and the consequences for her offspring. J Develop Disab 2007; 1: 101-134.
- Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005; 115: 485-491.
- Mestman J. Interaction between pregnancy, gestational diabetes, and long-term maternal outcome. In Diabetes in women: Adolescence, pregnancy and menopause Edited by: Reece EA, Coustan DR, Gabbi SG. Philadelphia: Lippincott Willians & Wilkins; 2004: 233-241.
- Seitz HJ, Müller MJ, Krone W, Tarnowski W. Coordinate control of intermediary metabolism in rat liver by the insulin/glucagon ratio during starvation and after glucose refeeding. Regulatory significance of long-chain acyl-CoA and cyclic AMP. Arch Biochem Biophys 1977; 183: 647-663.
- Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes 2000; 49: 1643-1648.
- Postic C, Shiota M, Magnuson MA. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog Horm Res 2001; 56: 195-217.
- Winzell MS, Coghlan M, Leighton B, Frangioudakis G, Smith DM, Storlien LH, Ahren B. Chronic glucokinase activation reduces glycaemia and improves glucose tolerance in high-fat diet fed mice. Eur J Pharmacol 2011; 663: 80-86.
- Yabaluri N, Bashyam MD. Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosciences 2010; 35: 473-484.
- Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015; 58: 221-232.
- Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity.Crit Rev Biochem Mol Biol. 2010; 45:199-214.
- Caluwaerts S, Holemans K, van Bree R, Verhaeghe J, Van Assche FA. Is low-dose streptozotocin in rats an adequate model for gestational diabetes mellitus? J Soc Gynecol Investig 2003; 10:216-221.
- Eriksson U, Dahlstrom E, Larsson KS, Hellerstrom C. Increased incidence of congenital malformations in the offspring of diabetic rats and their prevention by maternal insulin therapy. Diabetes 1982; 31:1-6.
- Lopez-Soldado I, Herrera E. Different diabetogenic response to moderate doses of streptozotocin in pregnant rats, and its long-term consequences in the offspring. Exp Diabesity Res 2003; 4:107-118.
- Oh W, Gelardi NL, Cha CJ. Maternal hyperglycemia in pregnant rats: its effect on growth and carbohydrate metabolism in the offspring. Metabolism 1988; 37:1146-1151.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101-1108.
- Hulcher FH, Oleson WH. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J Lipid Res 1973; 14:625-631.
- Davidson AL, Arion WJ. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: Physiological implications of higher cellular activity. Arch. Biochem. Biophys 1987; 253:156-167.
- Seddon B, Fynn GH. Ortophosphate analysis by the Fiske-SubbaRow method and interference by adenosine phosphates and pyrophosphate at variable acid pH. Anal Biochem 1973; 56:566-570.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin Phenol reagent .J Biol Chem 1951;193:265-275.
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 1973;83:346-356.
- Correia-Santos AM, Suzuki A, Vicente GC, Dos Anjos JS, Pereira AD. Effect of maternal use of flaxseed oil during pregnancy and lactation on glucose metabolism and pancreas histomorphometry of male offspring from diabetic rats. Diabetes Res Clin Pract. 2014; 106:634-42.
- Kiss AC, Lima PH, Sinzato YK, Takaku M, Takeno MA, Rudge MV, Damasceno DC. Animal models for clinical and gestational diabetes: maternal and fetal outcomes. Diabetol Metab Syndr 2009; 1: 21.
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000; 6: 87-97.
- Kelstrup L, Damm P, Mathiesen ER, Hansen T, Vaag AA, Pedersen O, Clausen TD. Insulin resistance and impaired pancreatic β-cell function in adult offspring of women with diabetes in pregnancy.J Clin Endocrinol Metab 2013;98:3793-3780.
- Tilghman SM, RW Hanson, L Reshef, MF Hopgood, FJ Ballard. Rapid loss of translatable messenger RNA of phosphoenolpyruvate carboxykinase during glucose repression in liver. Proc Nat Acad Sci USA 1974; 71:1304-1308.
- Benedetti A, Fulceri R, Romani A, Comporti M. MgATP-dependent glucose 6-phosphate-stimulated Ca2+ accumulation in liver microsomal fractions. Effects of inositol 1, 4, 5-trisphosphate and GTP.J Biol Chem 1988;263:3466-3473.
- Fulceri R, Romani A, Pompella A, Benedetti A. Glucose 6-phosphate stimulation of MgATP-dependent Ca2+ uptake by rat kidneymicrosomes.Biochim Biophys Acta 1990;1022:129-133.
- Senesi S, Marcolongo P, Kardon T, Bucci G, Sukhodub A. Immunodetection of the expression of microsomal proteins encoded by the glucose 6-phosphate transporter gene. Biochem J 2005; 389:57-62.
- Bánhegyi G, Marcolongo P, Fulceri R, Hinds C, Burchell A, Benedetti A. Demonstration of a metabolically active glucose-6-phosphate pool in the lumen of liver microsomal vesicles.J Biol Chem 1997;272:13584-13590.
- Yabaluri N, Bashyam MD. Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosci 2010; 35:473-484.
- Jin ES, Szuszkiewicz-Garcia M, Browning JD, Baxter JD, Abate N, Malloy CR. Influence of liver triglycerides on suppression of glucose production by insulin in men. J Clin Endocrinol Metab 2015; 100:235-243.
- Choi JM, Seo MH, Kyeong HH, Kim E, Kim HS. Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase. Proc Natl Acad Sci USA 2013; 110:10171-10176.
- M Lachaal, Rampal AL, Ryu J, Lee W, Hah JS, Jung CY. Characterization and partial purification of liver glucose transporter GLUT2. Biochimica et Biophysica Acta Biomembranes 2000; 1466: 379-389.
- Seyer P, Vallois D, Poitry-Yamate C, Schutz F, Metref S. Hepatic glucose sensing is required to preserve beta cell glucose competence. J Clin Invest 2013; 123:1662-1676.
- Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am 2008;37:685-711.
- Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium.Curr Opin Clin Nutr Metab Care. 2007; 10:523-530.
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 2010; 107:3441-3446.
- Brown MS, Goldstein JL. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab 2008; 7:95-96.
- Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes 2012; 61:2711-2717.
- Diraison F, Moulin P, Beylot .Contribution Diabetes Metab 2003; 29:478-485.
- Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Okazaki H. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes 2004;53:560-569.
- Poupeau A, Postic C. Cross-regulation of hepatic glucose metabolism via ChREBP and nuclear receptors. Biochim Biophys Acta 2011; 1812:995-1006.
- Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA 1999; 96:13656-13661.
- Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA 2001; 98:9116-9121.
- Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 1997; 99:846-854.
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem 1999; 274:30028-30032.
- Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA 2004; 101:7281-7286.
- Koo HY, Miyashita M, Cho BH, Nakamura MT.Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus..Biochem Biophys Res Commun 2009;390:285-289.