Research Paper - Archives of General Internal Medicine (2018) Volume 2, Issue 1
Increased All-cause Mortality, Total Cardiovascular Disease and Morbidity in Hospitalized Elderly Patients with Orthostatic Hypotension.
Isak Lindstedt1,2*, Lars Edvinsson2,3, Anne-Lie Lindberg3, Maria Olsson3, Claes Dahlgren3, Marie-Louise Edvinsson2,3
1Achima Care Ekeby vårdcentral, Storgatan 46, 26776, Ekeby, Sweden
2Department of Clinical Sciences, Division of Experimental Vascular Research, Lund University, Lund, Sweden
3Department of Emergency and Internal Medicine, Skåne University Hospital, Lund, Sweden
- *Corresponding Author:
- Isak Lindstedt
Achima Care Ekeby vårdcentral
Storgatan 46, 26776, Ekeby, Sweden
E-mail: isaklindstedt@hotmail.com
Accepted on January 12, 2018
Citation: Lindstedt I, Edvinsson L, Lindberg AL, et al. Increased all-cause mortality, total cardiovascular disease and morbidity in hospitalized elderly patients with orthostatic hypotension. Arch Gen Intern Med. 2018;2(1):8-15.
Abstract
Background: Orthostatic hypotension is a common finding in elderly patients and is associated with significant morbidity and mortality. Known risk factors for orthostatic hypotension are age, multiple medications, smoking, low BMI, hypertension and diabetes. Most of the knowledge on orthostatic hypotension and cardiovascular endpoints and mortality comes from prospective cohort data and there are few clinical studies performed in the very elderly.
Methods: From 2014 to May 2017 a team composed of a physiotherapist and an occupational therapist supervised by a medical doctor visited newly hospitalized patients at Lund’s University Hospital. The team measured BMI, blood pressure, pulse, saturation and registered the patients’ age, sex, number and types of medications and symptoms for admittance. They were also able to register blood tests taken, the patients’ final diagnosis, the number of days in hospital, the number of medications at discharge, the number of re-hospitalizations and the number of deaths at follow-up after 6 months. Patients with complete blood pressure measurements both in the lying position and standing position were included in our study (n=210). These patients were divided into two groups, the orthostatic hypotension group (OH-group, n=119) and the normotensive group (NT-group, n=91). The division was made based on the generally accepted definition of orthostatic hypotension. Calculations for comparison in-between the two groups were done using the Student’s T-test or Mann-Whitney U-test for continuous variables and the Pearsons Chi-square test for dichotomous variables.
Results: During follow-up 14 of 91 patients died in the NT-group compared to 32 of 119 in the OH-group (p<0,05). At discharge 41 of 91 patient had been diagnosed with cardiovascular disease in the NT-group compared to 70 of 119 patients in the OH-group (p<0,05). The patients in the NT-group stayed at the hospital for a mean of 8,38 days compared to the patients from the OH-group whom stayed for a mean of 9,86 days (p<0,05).
Conclusions: This study has shown that there is increased cardiovascular disease, morbidity and mortality in elderly patients with orthostatic hypotension compared to patients without orthostatic hypotension in a hospital setting. The study results indicate the importance of taking orthostatic blood pressure tests in elderly patients.
Keywords
Elderly patients, Orthostatic hypotension, Mortality, Morbidity.
Introduction
Orthostatic hypotension is a symptom due to multifactorial causes that occurs when the cardiovascular reflexes fail to maintain blood pressure when standing from a supine position. The impaired orthostatic response may lead to symptoms of cerebral hypoperfusion and syncopal attacks directly by abrupt or progressive falls in blood pressure, or indirectly by triggering the vasovagal reflex [1]. Of note, although some patients with orthostatic hypotension may present with symptoms of dizziness or syncope, most are asymptomatic, which may make orthostatic hypotension underdiagnosed in usual clinical settings [2]. The prevalence varies significantly depending on the population studied. Orthostatic hypotension is more prevalent in elderly people. Moreover, it is quite common in hospitalized patients [3]. Intensive studies during the past decades shed light on the clinical relevance and implications [4]. These studies not only link orthostatic hypotension to the incidence of many cardiovascular diseases, such as coronary artery disease [5], stroke [6-8], congestive heart failure [9-11], atrial fibrillation [12], and renal failure [13], but they also relate its presence to increased risk of non-cardiovascular-related conditions [14-16]. For example, Xin et al. published a meta-analysis of eight published articles from 7 cohorts, consisting of 64,782 participants. During a mean follow-up of 15.2 years, 5719 coronary heart disease events and 3657 stroke events occurs. The overall results of the meta-analysis indicate that orthostatic hypotension is associated with significant increased risk for incident coronary heart disease (HR: 1.32, 1.12-1.56) and stroke (HR: 1.19, 1.08-1.30), which are independent of conventional risk factors [8].
Even though orthostatic hypotension is linked to many conditions, the findings are not always consistent [17]. More importantly, there is accumulating evidence that orthostatic hypotension is significantly associated with a higher risk of future mortality events [18-20]. For example, Angelousi et al. in their meta-analysis of seven prospective studies found that orthostatic hypotension is associated with a significant increased risk for overall mortality (HR: 1.36 (1.13-1.63) [20]. Furthermore, studies on mortality and morbidity in orthostatic hypotension are largely longitudinal in character, based on population cohorts put together many years ago, and few study the very elderly (>80 years). Therefore, we constructed a study with a prospective design hypothesizing that a group of patients with a mean age in the eighties and recently admitted to the hospital with orthostatic hypotension would have greater mortality and morbidity than non-orthostatic elderly patients.
Methods
Over a four-year period (from 2014 to May 2017), a dedicated team composed of a physiotherapist and a medical doctor visited newly hospitalized patients at Lund’s University Hospital in Sweden. The team measured BMI, blood pressure, pulse, and oxygen saturation and also recorded the patients’ age, sex, types of medications, and symptoms prompting hospitalization.
The study included patients with complete blood pressure measurements in both the supine position and standing position (n=210). We divided the patients into two groups. The orthostatic hypotension group (OH-group, n=119) exhibited a fall in systolic blood pressure of ≥ 20 mm Hg upon standing up, and/or a fall in diastolic blood pressure ≥ 10 mm Hg upon standing up, or simply a systolic blood pressure <90 mm Hg. The study included only one patient based on the last criteria. The normotensive group (NT-group, n=91) included patients with systolic and/or diastolic blood pressure that did not fall more than 20 mm Hg or 10 mm Hg, respectively, upon standing. The orthostatic blood pressure was measured three minutes after the patient changed position.
Six months after each patient’s initial admission into the hospital and inclusion in the study, the team made a follow-up of each patient by recording blood tests taken during hospitalization, the number of days of hospital treatment, the patient’s final diagnosis, the number of medications the patient was discharged with, the number of re-hospitalizations, and the number of deaths.
For the patients in this study, there were a variety of presenting symptoms and diagnoses at both hospital admittance and discharge. Thus, we decided to only report data for those symptoms and diagnoses where the number of cases was sufficient to make meaningful comparisons. The team recorded and presented the main complaints at admission, i.e. the patients’ most frequently recurring symptoms. We did not attempt to describe the included patients’ previous diseases since the study was open to any elderly patient, making it rational to decide that the included study population would be a reasonable representation of the general Swedish population in their eighties coming to the hospital for medical attention.
Total cardiovascular disease was defined as a combined endpoint of the following diagnoses at discharge: heart failure, heart attack, arrhythmias, cerebral ischemia or bleeds, lung embolus, hypertension, hypotension, diabetes, and renal failure. Total lung disease was a combined endpoint of the following diagnoses: chronic obstructive pulmonary disease, asthma, pneumonia, lung cancer, and pneumothorax. Total infectious disease was a combined endpoint of the following diagnoses: sepsis, urinary tract infection, influenza, erysipelas, or infection unspecified. The final diagnosis was made based on good clinical practice and according to the ICD-10, International Statistical Classification of Diseases and Related Health Problems.
This study was approved by the Regional Ethical Review Board in Lund, Sweden, D.no 2016/819. The study conformed to the principles outlined in the Declaration of Helsinki.
Statistics
Calculations for comparison between the two groups were done using the Student’s T-test or Mann-Whitney U-test for continuous variables and the Pearson’s Chi-square test for dichotomous variables. Statistical significance was set at p <0.05. Statistics were performed using the IBM SPSS Statistics 22 program.
Results
Table 1 presents the demographic results, with a total of 210 patients – 119 with orthostatic blood pressure and 91 without orthostatic blood pressure. As can be seen in Table 1, the patients in the orthostatic group manifest a clear drop in both systolic and diastolic blood pressure from the supine to the standing position. The mean difference is 29.1 mm Hg for systolic blood pressure and 12.1 mm Hg for diastolic blood pressure. In comparison, the table shows an increase in blood pressure from the supine to the standing position in the non-orthostatic group, with a mean difference of 2.6 mm Hg for systolic blood pressure and 3.9 mm Hg for diastolic blood pressure. For the orthostatic patients, both systolic and diastolic resting blood pressure values are slightly higher than those for the patients in the non-orthostatic group; however, the difference is not statistically significant (p=0.17/ p=0.15). The data shows no major differences in either resting or standing heart rate or oxygen saturation between the groups, and the mean age is the same for both groups. There is a tendency towards a lower BMI in the orthostatic group (p=0.12). Gender has a statistically significant difference between the groups (p <0.01). In terms of relative risk, the results show women with a lower likelihood to exhibit orthostatic hypotension than men, RR 1.49 (1.19-1.86).
| Orthostatic | SEM | N | Non-orthostatic | SEM | N | p-value | |
|---|---|---|---|---|---|---|---|
| Male/ Female | 61/58 | 0,05* | 119 | 25/66 | 0,05* | 91 | < 0,01 |
| Age | 83,6 | 0,75 | 119 | 82,7 | 0,85 | 91 | 0,43 |
| BMI | 23,3 | 0,53 | 76 | 24,7 | 0,70 | 57 | 0,12 |
| Heart rate rest | 81,3 | 1,40 | 117 | 82,1 | 1,64 | 91 | 0,72 |
| Heart rate stand | 94,5 | 2,01 | 80 | 97,8 | 3,66 | 51 | 0,40 |
| DBP rest | 77,9 | 1,17 | 119 | 75,3 | 1,42 | 91 | 0,15 |
| DBP stand | 65,8 | 1,25 | 119 | 79,2 | 1,52 | 91 | < 0,01 |
| SBP rest | 142,2 | 2,02 | 119 | 137,7 | 2,56 | 91 | 0,17 |
| SBP stand | 113,1 | 2,07 | 119 | 140,3 | 2,70 | 91 | < 0,01 |
| SAT O2 rest | 95,5 | 0,26 | 109 | 95,3 | 0,31 | 89 | 0,58 |
Age (Years);
BMI (Weight(kg)/Length(m)2);
Heart rate (Heartbeats/minute);
DBP (Diastolic Blood Pressure (mm Hg));
SBP (Systolic Blood Pressure (mm Hg));
SAT (Oxygen Saturation (%));
rest = resting;
stand =standing;
SEM = Standard Error of the Mean;
* SEM calculated from mean of orthostatic = 1 and non-orthostatic = 0, Mean value not shown.
Table 1. Demographics.
Table 2 indicates the main symptoms patients presented when first entering the hospital, with significantly more patients from the non-orthostatic group presenting chest pain compared to the orthostatic group (p <0.05). The results show a tendency towards more syncope in the orthostatic group (p=0.09), but no difference between the groups in regard to vertigo or falls.
| Orthostatic | SEM* | N | Non-orthostatic | SEM* | N | p-value | |
|---|---|---|---|---|---|---|---|
| Breathlessness | 42 | 0,04 | 119 | 33 | 0,05 | 91 | 0,88 |
| Falls | 22 | 0,04 | 119 | 14 | 0,04 | 91 | 0,55 |
| Chest pain | 5 | 0,02 | 119 | 13 | 0,04 | 91 | < 0,05 |
| Syncope | 11 | 0,03 | 119 | 3 | 0,02 | 91 | 0,09 |
| Vertigo | 7 | 0,02 | 119 | 4 | 0,02 | 91 | 0,63 |
SEM = Standard Error of the Mean;
* SEM calculated from mean of orthostatic = 1 and non-orthostatic = 0, Mean value not shown.
Table 2. Symptoms at enrollment.
Table 3 provides the mean number of medicines taken and the number of medicines from the major pharmacological groups that patients were taking when discharged from the hospital.
| Orthostatic | SEM | N | Non-orthostatic | SEM | N | p-value | |
|---|---|---|---|---|---|---|---|
| Mean number of medicines at discharge | 10,2 | 0,46 | 119 | 10,7 | 0,58 | 91 | 0,47 |
| Diuretics | 66 | 0,05* | 119 | 44 | 0,05* | 91 | 0,31 |
| Beta-Blockers | 64 | 0,05* | 119 | 43 | 0,05* | 91 | 0,35 |
| Blood pressure medication | 31 | 0,04* | 119 | 28 | 0,05* | 91 | 0,45 |
| Diabetes medication | 12 | 0,03* | 119 | 6 | 0,03* | 91 | 0,37 |
| ARB | 16 | 0,03* | 119 | 11 | 0,03* | 91 | 0,77 |
| Spironolactone | 19 | 0,03* | 119 | 17 | 0,04* | 91 | 0,61 |
| Digitalis | 12 | 0,03* | 119 | 11 | 0,03* | 91 | 0,65 |
| Imdur | 12 | 0,03* | 119 | 11 | 0,03* | 91 | 0,65 |
| ACEi | 29 | 0,04* | 119 | 27 | 0,05* | 91 | 0,39 |
ARB (Angiotensin Receptor Blockers);
ACE (Angiotensin Converting Enzyme);
SEM = Standard Error of the Mean;
* SEM calculated from mean of orthostatic = 1 and non-orthostatic = 0, Mean value not shown.
Table 3. Medication at discharge.
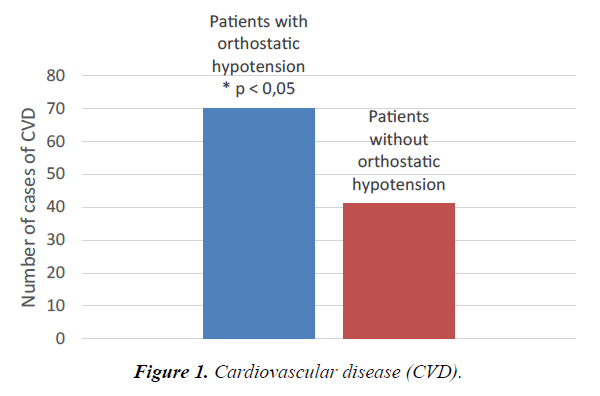
Table 4 shows the patients’ diagnoses at the time of hospital discharge. In order to increase statistical power, some of the diagnoses are shown together in a combined endpoint of total cardiovascular disease, total lung disease, or total infectious disease (See the Methods section for explanation). There are fewer total cardiovascular disease diagnoses in the nonorthostatic group (p <0.05). Put in terms of relative risk, the results show the chance of not having cardiovascular disease to be 33% higher in the non-orthostatic group compared to the orthostatic group, RR 1.33 (1.00-1.77). See also Figure 1. There are no differences between groups when it comes to total lung and infectious diseases, heart failure, chronic obstructive pulmonary disease, or cancer. There is a statistically significant higher occurrence of renal failures (p <0.05) and a tendency towards more atrial fibrillations in the orthostatic group (p=0.08).
| Orthostatic | SEM* | N | Non-orthostatic | SEM* | N | p-value | |
|---|---|---|---|---|---|---|---|
| Total Cardiovascular Disease | 70 | 0,05 | 119 | 41 | 0,05 | 91 | < 0,05 |
| Total Lung Disease | 29 | 0,04 | 119 | 25 | 0,05 | 91 | 0,61 |
| Total Infectious Disease | 9 | 0,02 | 119 | 8 | 0,03 | 91 | 0,75 |
| Heart failure | 33 | 0,04 | 119 | 29 | 0,05 | 91 | 0,52 |
| Cancer | 14 | 0,03 | 119 | 10 | 0,03 | 91 | 0,86 |
| COPD | 15 | 0,03 | 119 | 12 | 0,04 | 91 | 0,90 |
| Atrial fibrillation | 15 | 0,03 | 119 | 5 | 0,02 | 91 | 0,08 |
| Kidney failure | 7 | 0,02 | 119 | 0 | 0,00 | 91 | < 0,05 |
Total Cardiovascular Disease (heart failures, heart attacks, arrhythmias, cerebral ischemias or bleeds, lung embolus, hypertension, hypotension, diabetes, kidney failures);
Total Lung Disease (chronic obstructive pulmonary disease, asthma, pneumonia, lung cancer, and pneumothorax);
Total Infectious Disease (sepsis, urinary tract infections, influenzas, erysipelas, or infections unspecified);
COPD (Chronic obstructive pulmonary disease);
SEM = Standard Error of the Mean;
* SEM calculated from mean of orthostatic = 1 and non-orthostatic = 0, Mean value not shown.
Table 4. Diagnosis at discharge.
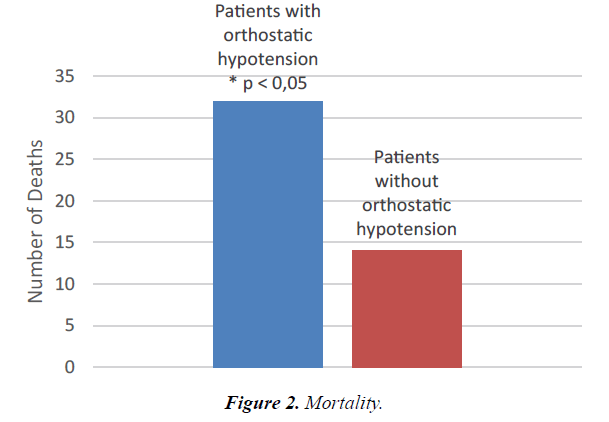
Table 5 gives the results for mortality and different measures of morbidity. The number of deaths is significantly lower in the non-orthostatic group compared to the orthostatic group (p <0.05). In terms of relative risk, the results show the chance of not dying to be 16% higher in the non-orthostatic group compared with the orthostatic group, RR 1.16 (1.01-1.33). See also Figure 2. The non-orthostatic group also records significantly fewer days in the hospital as compared to the orthostatic group (p<0.05). There is a tendency towards more hospital readmissions in the non-orthostatic group for the six-month period following initial hospitalization (p=0.11).
| Orthostatic | SEM | N | Non-orthostatic | SEM | N | p-value | |
|---|---|---|---|---|---|---|---|
| Deaths | 32 | 0,04* | 119 | 14 | 0,04* | 91 | < 0,05 |
| Mean number of days enrolled | 9,9 | 0,61 | 119 | 8,4 | 0,63 | 91 | < 0,05 |
| Re-enrollments within 30 days | 21^ | 0,04* | 91 | 11^ | 0,05* | 62 | 0,43 |
| Number of re-enrollments within 6 months | 76^ | 0,11* | 90 | 64^ | 0,22* | 62 | 0,11 |
SEM = Standard Error of the Mean;
* SEM calculated from mean of orthostatic = 1 and non-orthostatic = 0, Mean value not shown.
^ For reenrollments, the same patient can have had several visits to the hospital within 1 or 6 months.
Table 5. Mortality and morbidity.
Table 6 shows the mean results from blood tests taken at the time of hospitalization. Mean blood glucose levels are significantly lower in the orthostatic group as compared to those of the non-orthostatic group (p<0.01). The “orthostatics” also have a significantly lower mean NT-proBNP (p=0.02). The data shows a tendency towards lower mean CRP levels in the orthostatic group (p=0.08).
| Orthostatic | SEM | N | Non-orthostatic | SEM | N | p-value | |
|---|---|---|---|---|---|---|---|
| CRP | 46,4 | 5,73 | 119 | 64,5 | 8,17 | 84 | 0,08 |
| Glucose | 7,08 | 0,18 | 111 | 8,45 | 0,33 | 78 | < 0,01 |
| NT-proBNP | 4259 | 954 | 50 | 6784 | 1356 | 44 | 0,02 |
| TNT | 36,1 | 3,72 | 71 | 79,5 | 39,1 | 66 | 0,71 |
| Creatinine | 104,7 | 4,90 | 119 | 106,1 | 8,71 | 85 | 0,68 |
| Urate | 600,3 | 59,3 | 6 | 631,3 | 168 | 3 | 0,55 |
| Sodium | 138,4 | 0,37 | 119 | 138,8 | 0,44 | 85 | 0,40 |
| Potassium | 3,99 | 0,05 | 119 | 3,95 | 0,05 | 85 | 0,78 |
| Hemoglobin | 123,8 | 1,61 | 119 | 122,2 | 1,82 | 85 | 0,53 |
| e-GFR | 50,0 | 2,56 | 96 | 52,9 | 4,13 | 65 | 0,99 |
CRP (C-reactive protein (mg/L));
Glucose (mmol/L);
NT-proBNP (ng/L);
TNT (Troponin T (ng/L));
Creatinine (µmol/L);
Urate (µmol/L); Sodium (mmol/L);
Potassium (mmol/L); Hemoglobin (g/L);
e-GFR (mL/min/1,73 m2);
SEM (Standard Error of the Mean)
Table 6. Blood tests (Means).
Discussion
The results of this study indicate that the presence of orthostatic hypotension in elderly patients (mean >80 y) may represent a risk factor for total mortality, cardiovascular disease, and morbidity. Our results, based on following patients for six months after their initial admission to the hospital, show a greater occurrence of deaths, total cardiovascular disease, and lengths of hospital stay in elderly patients with orthostatic hypotension as compared to elderly patients without orthostatic hypotension. Viewed in another way, the absence of orthostatic hypotension can be seen as a protective factor, with the chance of not dying increasing by 16% and the chance of not being discharged from the hospital with cardiovascular disease increasing by 33%, relative to elderly patients with orthostatic hypotension. Our results are in line with the results of prospective cohort studies [21]; yet reveal important insights. To the best of our knowledge, our results differ from previous studies, because our study encompass patients studied from inclusion to follow-up, patients with a very high mean age (about 83 years), and recent data, being taken from patients receiving current treatment regimens.
The mechanisms contributing to the association between orthostatic hypotension and increased mortality risk are not fully understood by medical professionals and researchers today. Orthostatic hypotension may confer an increased risk by interacting with classic risk factors. Aging, diabetes, hypertension, Parkinson’s disease, and carotid arterial disease are all associated with both orthostatic hypotension and increased mortality [22]; and all share the potential to have an impact on autonomic mechanisms that regulate blood pressure regulation. Baroreflex dysfunction, a marker of autonomic nervous system imbalance implicated in the pathogenesis of orthostatic hypotension, is characterized by enhanced sympathetic activity and withdrawal of parasympathetic control [23]; and it has long been recognized as an important mediator of increased cardiovascular morbidity and mortality [24]. Individuals with orthostatic hypotension are likely to have increased blood pressure variability related to body posture, and a large proportion of blood volume from the upper body may be transferred to lower limbs due to gravity during changes in posture. This in turn may lead to reduced filling of the heart and decreased cardiac output. Thus, intermittent ischaemia of important organs, such as heart, brain, and kidney, may often occur; and perfusion status may lead to permanent damage of these organs during orthostatic hypotension [21]. Moreover, blood pressure variability and supine hypertension associated with orthostatic hypotension may cause increased afterload, leading to permanent end-organ damage such as left ventricular hypertrophy and decreased renal function [21,22]. The orthostatic patients in our study show slightly higher systolic and diastolic supine blood pressure values than the patients in the non-orthostatic group, but the difference is not statistically significant (for example, resting systolic blood pressure of 142.2 mm Hg vs. 137.7 mm Hg, p=0.17).
In addition, orthostatic hypotension is also a condition of impaired haemodynamic homeostasis, where compensatory neuroendocrine mechanisms are intermittently activated. These mechanisms may activate platelets or the coagulation cascade, potentially facilitating the occurrence of cardio- or cerebrovascular events [21]. Also, activation of the endothelin system has been observed in patients diagnosed with syncope due to orthostatic hypotension [25]. Thus, vasoconstrictors, such as endothelin, may play favorable roles as adaptive mechanisms countering hypotension during changes in posture. On the other hand, endothelin plays a negative role by promoting atherosclerosis and thrombosis in susceptible individuals in the long term [21]. Studies have also found associations between orthostatic hypotension and circulating endogenous diuretic/ vasodilator neuropeptides such as ANP and BNP, which may contribute to orthostatic hypotension [26]. Some hypotheses assert that BNP levels increase in orthostatic hypotension [27], and that BNP levels elevate in cardiovascular diseases like hypertension [28] and heart failure. However, our results show a significant decrease in BNP levels in the orthostatic hypotension group compared to the non-orthostatic group (p=0.02). One possible explanation may be that levels of BNP are reduced in a compensatory manner in order to alleviate the hypotensive effects in these patients.
Our records indicate that patients with orthostatic hypotension stayed in the hospital significantly longer than patients without orthostatic hypotension. There are several possible explanations for this. One obvious possibility is that they were simply sicker. The fact that more of them had died prior to the six-month follow-up supports this hypothesis. It is also possible that the orthostatic patients were frailer, as they were a little older (mean age 83.6 vs. 82.7 years). There are significantly more males in the orthostatic group (61 vs. 25, p <0.01). Given that males have shorter life expectancies than women, it is not impossible that a gender factor may contribute to the results. The orthostatic patients also had a lower BMI (23.3 vs. 24.7; p=0.12), implying that the patients with orthostatic hypotension were less well-nourished and less resistant to stressors than their counterparts. The “orthostatics” also had slightly more medications at the time of hospital admittance (data not shown), which could imply that they suffered from conditions that needed more aggressive treatment and/or were more susceptible to side effects. The fact that they had a greater tendency towards seeking medical attention based on syncope than the non-orthostatics (11 vs. 3, p=0.09) could suggest both overmedication and/or greater blood pressure falls, but also concomitant cardiovascular diseases like arrhythmias.
Another interesting finding from our study is the significantly lower mean glucose level in the orthostatic hypotension group compared to the non-orthostatic group (p<0.01) at admission.
This finding also supports the hypothesis that the orthostatic patients were frailer, possibly caused by malnourishment resulting in lower BMI. Even though this argument seems to fit our data, it still seems contrary to previous research that has found a strong association between diabetes and orthostatic hypotension [29]. We see no such association in our study, in which we have the same number of diabetics in both groups (data not shown), no differences in diabetes medications (p=0.37), and no differences in medicines that can increase glucose levels (beta-blockers, p=0.35; diuretics, p=0.31). Since the relationship between lower glucose levels and orthostatic hypotension is so statistically strong in our results (p<0.01), we hypothesize that there might be other novel pathophysiological mechanisms at work here where hypoglycemia and orthostatic hypotension could interact. As an example, Radikova et al. found that orthostatic stress may affect the neuroendocrine response to subsequent hypoglycemia [30].
Our results also indicate that more patients in the orthostatic group were discharged from the hospital with the diagnosis of renal failure as compared to patients in the non-orthostatic group. Specifically, the former group documents seven cases of renal failure compared to none in the latter. We acknowledge that, although this is a statistically significantly difference between groups, it is difficult to draw any conclusions based on so few cases. Nevertheless, the result is in line with previous research. For example, a cross-sectional study by Canney et al., in a cohort from The Irish Longitudinal Study on Ageing, record participants with eGFR <60 mL/min per 1.73 m2 as approximately twice as likely to have sustained orthostatic hypotension and, moreover, the results show a graded association between eGFR categories and impaired orthostatic BP stabilization, particularly within the first minute of standing [31].
We see in our data a tendency towards more cases of atrial fibrillations at discharge in the orthostatic group (15/119) compared to the non-orthostatic group (5/91), p=0.08. This is also in line with previous observational research. One example is the study by Agarwal et al. examining associations between supine-to-standing changes in systolic blood pressure and incident atrial fibrillations in 12,071 African American and white men and women aged 45-64 years enrolled in the Atherosclerosis Risks in Communities (ARIC) study. During an average follow-up of 18.1 years, 11.9% of study participants developed atrial fibrillations. At a rate of 9.3 vs. 6.3 per 1,000 person years, p<0.001 [32], the study records a higher rate of incident atrial fibrillations among those with orthostatic hypotension than those without.
We do not find any difference in our results for the incidence of heart failure between the orthostatic and non-orthostatic groups. Records show heart failure diagnoses at discharge from the hospital for 33 of 119 orthostatic patients and 29 of 91 non-orthostatic patients in our study (p=0.52). This result is in line with previously published data that shows increased incidence of heart failure in younger individuals with orthostatic hypotension, but no such association in older individuals. The meta-analysis of four prospective cohort studies by Xin et al. shows a significant association between incident heart failure and orthostatic hypotension in age-groups <45 years, HR 2.05 (1.31-3.22), and 45 to 65 years, HR 1.38 (1.08-1.75), but not >65 years, HR 1.19 (0.89-1.59) [10].
Our current study, as documented here, has many strengths. One major strength is that, to the best of our knowledge, ours is the first clinical study in many years to follow patients in a structured way from inclusion to follow-up with the same professional hospital team to assess the prognostic meaning of blood pressure changes in patients from supine to standing positions. Another strength is the high mean patient age of about 83 years-an age in which outcomes like cardiovascular endpoints and mortality are common, reducing the need for a large number of study participants. Since there still are so few studies performed on the very elderly, our study can be used by researchers in the immediate future as a foundation for power calculations in similar upcoming studies. Furthermore, our study provides results from the modern clinical praxis in the southern parts of Sweden. The study population is quite representative for its region and age group since we did not attempt to deny any patient participation into the study based on any exclusion criteria. Taking an orthostatic blood pressure test is inexpensive, non-invasive, does not cause pain, is highly repeatable, and can be used as an educational tool for patient care. Furthermore, the orthostatic blood pressure test is an easy test to perform and, as this study shows, can be performed by a dedicated team to obtain valuable information; for example, signs of orthostatic hypotension pertaining to the risk of falls in the elderly population [33].
Our study does have several weaknesses. First, the number of patients studied is relatively low and may not be indicative of a larger group of patients. Second, the study is not randomized, controlled, matched, or blinded. Third, we have not attempted to adjust for any confounders. There are significantly more males in the orthostatic group than in the non-orthostatic group (p<0.01). This is also a very important finding, because it underlines the importance of measuring orthostatic blood pressure in elderly males. In the future, it will be important to determine whether the results in this study are caused by orthostatic hypotension alone or are influenced by other factors such as gender. Also, most of the patients had severe illnesses such as acute coronary syndromes, acute heart failures, infectious diseases and so forth, and it would be relevant to take into account the underlying cause of hospitalization in further studies in order to evaluate the impact of orthostatic hypotension per se. Fourth, our study does not allow us to draw conclusions as to whether orthostatic hypotension is a marker of generally increased risk of death, an intermediate variable in the causal pathway of cardiovascular risk factors, a simple measure of disease severity, or an independent causal mechanism. These will be important questions to address in upcoming studies.
Summary
Orthostatic hypotension is a symptom due to multifactorial causes that occurs when the cardiovascular reflexes fail to maintain blood pressure when standing from a supine position. This study shows increased cardiovascular disease, morbidity, and mortality in elderly patients with orthostatic hypotension compared to patients without orthostatic hypotension in a hospital setting. Our results differ somewhat from previous longitudinal studies, because our study encompass patients studied from inclusion to follow-up, patients with a very high mean age (about 83 years), and the data underlying the results has been recorded recently. The study results convey the importance of taking orthostatic blood pressure measurements in elderly patients. The orthostatic blood pressure test is a very inexpensive and easy test to perform and, considering the prognostic implications of this study, should be largely recommended in clinical practice.
References
- Joseph A, Wanono R, Flamant M, et al. Orthostatic hypotension: A review. Nephrol Ther. 2017; 13(Suppl 1):S55-S67.
- Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19:508-19.
- Shibao C, Grijalva CG, Raj SR, et al. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120:975-80.
- Fedorowski A, Melander O. Syndromes of orthostatic intolerance: a hidden danger. J Intern Med. 2013;273:322-35.
- Luukinen H, Koski K, Laippala P, et al. Orthostatic hypotension and the risk of myocardial infarction in the home-dwelling elderly. J Intern Med. 2004;255:486-93.
- Hossain M, Ooi WL, Lipsitz LA. Intra-individual postural blood pressure variability and stroke in elderly nursing home residents. J Clin Epidemiol. 2001;54:488-94.
- Eigenbrodt ML, Rose KM, Couper DJ, et al. Orthostatic hypotension as a risk factor for stroke: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1996. Stroke. 2000;31:2307-13.
- Xin W, Mi S, Lin Z, et al. Orthostatic hypotension and the risk of incidental cardiovascular diseases: A meta-analysis of prospective cohort studies. Preventive Medicine. 2016;85:90-7.
- Fedorowski A, Engstrom G, Hedblad B, et al. Orthostatic hypotension predicts incidence of heart failure: the Malmo preventive project. Am J Hypertens. 2010;23:1209-15.
- Xin W, Lin Z, Li X. Orthostatic Hypotension and the Risk of Congestive Heart Failure: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE. 2013;8(5):e63169.
- Jones CD, Loehr L, Franceschini N, et al. Orthostatic hypotension as a risk factor for incident heart failure: the atherosclerosis risk in communities study. Hypertension. 2012;59:913-8.
- Fedorowski A, Hedblad B, Engstrom G, et al. Orthostatic hypotension and long-term incidence of atrial fibrillation: the Malmo Preventive Project. J Intern Med. 2010;268:383-9.
- Franceschini N, Rose KM, Astor BC, et al. Orthostatic hypotension and incident chronic kidney disease: the atherosclerosis risk in communities study. Hypertension. 2010;56:1054-9.
- Veronese N, De Rui M, Bolzetta F, et al. Orthostatic changes in blood pressure and mortality in the elderly: The Pro. V.A Study. Am J Hypertens. 2015;28:1248-56.
- Frith J. The association of orthostatic hypotension with falls-an end to the debate? Age and Ageing. 2017;46:540-1.
- Yap PLK, Niti M, Yap KB, et al. Orthostatic Hypotension, Hypotension and Cognitive Status: Early Comorbid Markers of Primary Dementia? Dement Geriatr Cogn Disord. 2008;26:239-46.
- Casiglia E, Tikhonoff V, Caffi S, et al. Orthostatic hypotension does not increase cardiovascular risk in the elderly at a population level. Am J Hypertens. 2014;27:81-8.
- Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290-2295.
- Fedorowski A, Stavenow L, Hedblad B, et al. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85-91.
- Angelousi A, Girerd N, Benetos A, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens. 2014;32:1562-71.
- Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. 2015;36:1609-17.
- Shibao C, Biaggioni I. Orthostatic hypotension and cardiovascular risk. Hypertension. 2010;56:1042-4.
- Autonomic failure. A textbook of clinical disorders of the autonomic nervous system, 5th Edition. Edited by Christopher J Mathias and Sir Roger Bannister. OUP, Oxford, 2013. ISBN 0198566344, 9780198566342.
- Robertson D. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res. 2008;18(Suppl. 1):2-7.
- Berger R, Pacher R. The role of the endothelin system in myocardial infarction-new therapeutic targets? Eur Heart J. 2003; 24:294-6.
- Krishnan B, Benditt DG. Neuropeptides and peptide hormones in syncope and orthostatic intolerance. Cardiol J. 2014;21:591-600.
- Krishnan B, Patarroyo-Aponte M, Duprez D, et al. Orthostatic hypotension of unknown cause: Unanticipated association with elevated circulating N-terminal brain natriuretic peptide (NT-proBNP). Heart Rhythm. 2015;12:1287-94.
- Lindstedt IH, Edvinsson ML, Edvinsson L. Reduced responsiveness of cutaneous microcirculation in essential hypertension-a pilot study. Blood Press. 2006;15(5):275-80.
- Zhou Y, Ke S-J, Qiu X-P, et al. Prevalence, risk factors and prognosis of orthostatic hypotension in diabetic patients. A systematic review and meta-analysis. Medicine. 2017;96(36):e8004.
- Radikova Z, Penesova A, Koska J, et al. Does orthostatic stress influence the neuroendocrine response to subsequent hypoglycemia in humans? Ann N Y Acad Sci. 2004;1018:576-81.
- Canney M, O’Connell MDL, Sexton DJ, et al. Graded Association Between Kidney Function and Impaired Orthostatic Blood Pressure Stabilization in Older Adults. J Am Heart Assoc. 2017;6:e005661.
- Agarwal SK, Alonso A, Whelton SP, et al. Orthostatic change in blood pressure and incidence of atrial fibrillation: results from a bi-ethnic population based study. PLoS One. 2013;8(11):e79030.
- Stenhagen M, Ekström H, Nordell E, et al. Falls in the general elderly population: a 3- and 6- year prospective study of risk factors using data from the longitudinal population study “Good ageing in Skane”. BMC Geriatrics. 2013;13(81):1-11.