Review Article - Journal of Cancer Immunology & Therapy (2024) Volume 7, Issue 6
Incidence and management of Cytokine release syndrome in CAR-T cell therapy for solid tumors: A systematic review of phase I trials.
Jasraj Singh Anand1*, Indermeet Anand2, Dr. Maheshvari Patel3
1Medical Biotechnology Section, Dr. D.Y. Patil Biotechnology & Bioinformatics Institute, Pune, India.
2Department of Pharmacology & Pharmacy Practice, Shri Sarvajanik Pharmacy College, Mehsana, Gujarat, India.
3NovoBliss Research Private Limited, 313, Silver Radiance 4, Gota, Ahmedabad-382481, Gujarat, India.
- *Corresponding Author:
- Jasraj Singh Anand
Medical Biotechnology Section
Dr. D.Y. Patil Biotechnology & Bioinformatics Institute
Pune, India
E-mail: Jasraj.anand1211@gmail.com
Received: 24-Oct-2024, Manuscript No. AAJCIT-24-150885; Editor assigned: 25-Oct-2024, PreQC No. AAJCIT-24-150885 (PQ); Reviewed: 11-Nov-2024, QC No AAJCIT-24-150885; Revised: 18-Nov-2024, Manuscript No. AAJCIT-24-150885 (R); Published: 28 -Nov-2024, DOI:10.35841/aajcit-7.5.235
Citation: Anand JS. Incidence and management of Cytokine release syndrome in CAR-T cell therapy for solid tumors: A systematic review of phase I trials. J Cancer Immunol Ther. 2024; 7(5):235
Abstract
Background: Chimeric Antigen Receptor T-cell (CAR-T) therapy has revolutionized treatment for hematologic cancers by genetically modifying T-cells to target cancer antigens, providing hope for patients with relapsed or refractory disease. The FDA approved second-generation CARs, like Kymriah and Yescarta, in 2017. However, applying CAR-T therapy to solid tumors presents challenges, including tumor heterogeneity and an immunosuppressive microenvironment. Toxicities such as Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) require careful management. Objectives: This systematic review aims to analyze CRS associated with CAR-T therapy in solid tumors. Key objectives include: • Evaluation of CRS: Assessthe incidence, severity, and clinical manifestations of CRS in solid tumors compared to hematologic malignancies. • Management Strategies: Evaluate the effectiveness of existing CRS management strategies and identify modifications needed for solid tumors. • Biomarker Exploration: Investigate the applicability of current biomarkers for predicting CRS in solid tumors and the potential need for novel biomarkers. • CAR-T Design Optimization: Compile findings to inform CAR-T cell design aimed at minimizing CRS risk and severity. Results: The review analysed data from 217 patients across Phase I clinical trials targeting solid tumors like colorectal cancer and glioblastoma. Of these, 75 patients (34.6%) experienced CRS, primarily mild to moderate (Grade 1-2), with severe cases (Grade 3-5) being rare. Management strategies included supportive care, corticosteroids, and tocilizumab for severe CRS. The incidence and severity of CRS in solid tumor trials were lower than in hematologic malignancies. Conclusion: While CRS is a concern in CAR-T therapy for solid tumors, its lower incidence and severity compared to hematologic cancers highlight the need for tailored management strategies. Future research should focus on optimizing patient outcomes and mitigating CRS risks to develop safer and more effective CAR-T therapies for solid tumors.
Keywords
Cytokine Release Syndrome, CAR-T Cell, Solid Tumours.
Introduction and Background
Chimeric Antigen Receptor T-cell (CAR-T) therapy represents a ground-breaking advancement in the treatment of various malignancies, particularly hematologic cancers. This innovative approach leverages the body's immune system by genetically modifying T-cells to express receptors specific to cancer antigens, thereby enabling targeted destruction of malignant cell [1,2]. Since its inception, CAR-T cell therapy has transformed the landscape of cancer treatment, offering hope to patients with refractory or relapsed cancers who have exhausted conventional therapeutic options [3].
The journey of CAR-T cell therapy began in the late 1980s when researchers first conceptualized the idea of engineering T-cells to recognize and attack cancer cells. Initial experiments focused on developing chimeric receptors that combine antigen-binding domains with T-cell activating functions. The early 2000s marked significant milestones with the development of second-generation CARs, incorporating co-stimulatory domains such as CD28 and 4-1BB, which significantly enhanced T-cell proliferation, persistence, and anti-tumour activity. These advancements culminated in the approval of the first CAR-T cell therapies, such as Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel), by the U.S. Food and Drug Administration (FDA) in 2017 for the treatment of B-cell Acute Lymphoblastic Leukaemia (ALL) and Diffuse Large B-Cell Lymphoma (DLBCL), respectively [4,5].
The clinical success of CAR-T cell therapy has spurred extensive research into expanding its applicability beyond hematologic malignancies. Efforts were underway to develop CAR-T therapies targeting solid tumours, with promising preliminary results. However, the unique microenvironment and heterogeneity of solid tumours present significant challenges, necessitating further refinement of CAR designs and combinatorial approaches to enhance efficacy and overcome resistance mechanisms [6].
Despite the remarkable therapeutic potential of CAR-T cell therapy, its application is accompanied by notable adverse effects and toxicities. The most prevalent and severe toxicities include Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). CRS is characterized by a systemic inflammatory response resulting from massive cytokine release by activated CAR-T cells, manifesting as fever, hypotension, and multi-organ dysfunction. ICANS, on the other hand, involves neurotoxic symptoms ranging from mild confusion to severe encephalopathy and seizures. Understanding and managing these toxicities were critical to optimizing patient outcomes and expanding the safe use of CAR-T cell therapies [7,8].
The rationale for this systematic review arises from the pressing need to thoroughly investigate the adverse effects and toxicities associated with CAR-T cell therapy, particularly in the context of solid tumors. While the efficacy of CAR-T therapy in treating hematologic malignancies has been well-documented, its expanding application in solid tumors introduces new challenges, especially in terms of safety and adverse events such as Cytokine Release Syndrome (CRS). CRS is a critical factor influencing both therapeutic outcomes and patient safety, and its manifestation in solid tumors remains less understood compared to hematologic cancers [9].
As clinical trials increasingly focus on CAR-T therapy for solid tumors, understanding the frequency, severity, and management of CRS in this new context is essential. Current CRS management strategies were largely based on experiences from hematologic malignancies, but solid tumors present a distinct microenvironment and immune response, which may alter CRS incidence and progression. This review aims to evaluate the similarities and differences in CRS between hematologic and solid tumors, thereby informing clinical practices specific to solid tumor CAR-T therapy [10].
Furthermore, this review explores whether existing biomarkers for predicting CRS in hematologic malignancies were applicable to solid tumors or if novel biomarkers need to be identified. By synthesizing data across studies, this review will also provide insights into optimizing CAR-T cell design to minimize severe CRS and improve patient outcomes. Given the growing clinical interest in CAR-T therapies for solid tumors, it is critical to establish a comprehensive understanding of CRS in this setting to enhance patient safety, guide therapeutic decision-making, and support the development of more effective and safer CAR-T treatments [11].
Ultimately, this systematic review will contribute in refining CRS management strategies, tailoring them to the unique challenges posed by solid tumors, and has serve as a foundation for future research in optimizing CAR-T cell therapy across a broader spectrum of cancer types.
Methodology
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We aimed to synthesize research on Chimeric Antigen Receptor T-cell (CAR-T) therapy in cancer treatment, focusing on safety outcomes and associated toxicities.
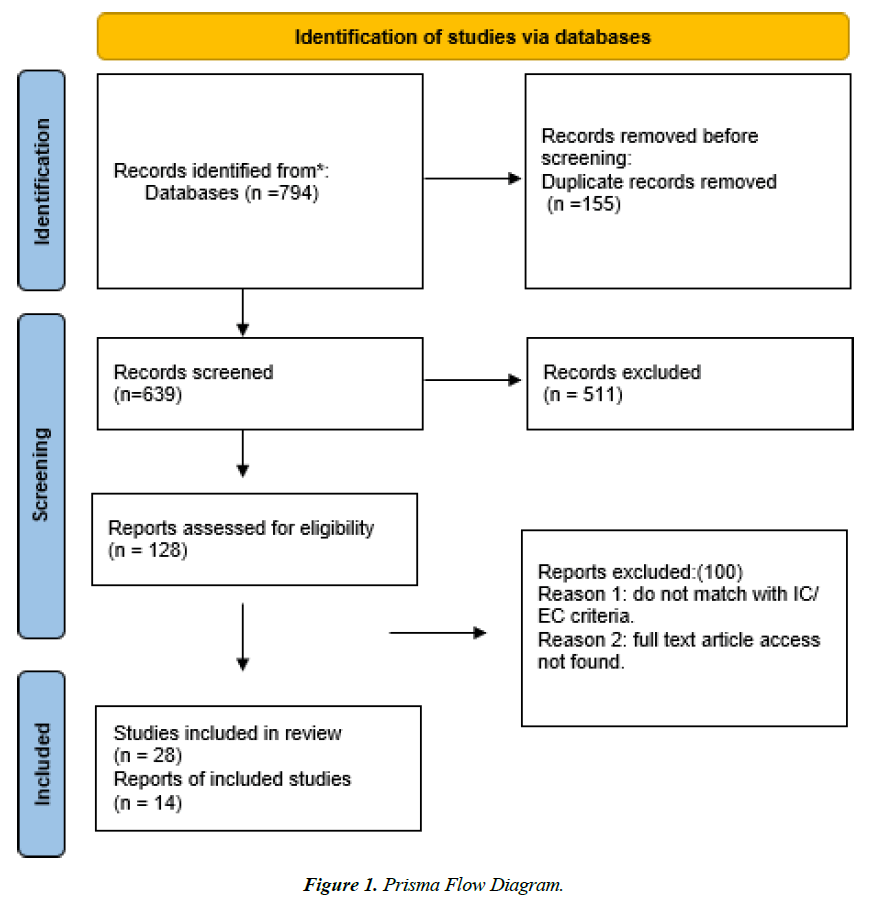
We conducted a comprehensive literature search using the PubMed database. Our search employed a combination of Medical Subject Headings (MeSH) terms and keywords related to CAR-T cell therapy, Cytokine Release Syndrome (CRS) and solid tumors. A search strategy was designed to identify relevant studies on Cytokine Release Syndrome (CRS) and Chimeric Antigen Receptor (CAR) T cell therapy. The search was conducted on PubMed, focusing on articles published within the last ten years (2014–2024) and including studies involving adults aged 18 to 90 years. Specific search terms were used, including "Cytokine release syndrome and chimeric antigen receptor or CAR T cell," which yielded 465 articles. Additionally, two more specific searches were conducted using the terms "Cytokine Release syndrome and solid tumors and chimeric antigen receptor T cell," yielding 178 articles, and "Cytokine release syndrome and CAR T cell and solid tumors," yielding 151 articles without any filter. In total, these searches produced 794 articles. The search encompassed all relevant studies published up to October 3rd, 2024, the date on which we concluded our search.
After removing 155 duplicate articles, 639 unique articles remained for further review. A title and abstract screening were performed on these articles, reducing the number to 370. Following this, 128 full-text articles were assessed, with 100 articles remaining after this stage. Finally, 28 studies were included in the review, with detailed data extraction performed on 14 of these studies. This methodology ensured a systematic and rigorous approach to selecting and evaluating the most relevant literature on CRS associated with CAR T cell therapy in the context of solid tumors.
Inclusion criteria encompassed all full-text articles of prospective clinical trials with outcome data available involving human subjects diagnosed with solid tumors and investigating CAR-T cell therapy, focusing on safety outcomes such as adverse events and toxicity. Exclusion criteria were non-human studies, paediatric population, non-English publications, studies focusing solely on CRS in hematologic malignancies (e.g., leukemia, lymphoma) without discussing solid tumors, Small case series or single case reports, CAR-T therapies unrelated to solid tumors and studies with incomplete or missing data, Unpublished or non-peer-reviewed data.
Information collected included study characteristics (title, authors, publication year, journal, study design, sample size), patient characteristics (age, sex, cancer type, disease stage, and prior treatments), intervention details (type of CAR-T cells used, administration protocol), and outcomes (incidence, efficacy measures, safety). We systematically recorded results, including main findings and reported adverse events.
The quality of the included studies was assessed using the Cochrane Risk of Bias Tool for randomized controlled trials and the Newcastle-Ottawa Scale for non-randomized studies. Results were reported following PRISMA guidelines, including a flow diagram of the study selection process and tables summarizing the characteristics and findings of the included studies (Figure 1).
Results
Incidence of CRS in Solid Tumors
In this review, a total of 217 patients were included from various Phase I clinical trials evaluating CAR-T cell therapies in solid tumors. The patient ages ranged between 18 and 90 years across these studies. The trials targeted a variety of solid tumors, including colorectal cancer, glioblastoma multiforme, hepatocellular carcinoma, pancreatic ductal adenocarcinoma, and pleural tumors, among others (Table 1).
| Type of Tumor | Target Antigen | Research Type | Total No. Of Patients | No. Of Patients with CRS (Any Grade) | Type of CRS Grade |
|---|---|---|---|---|---|
| Colorectal Cancer [12] | Cea, Tag72 | Phase I Study | 14 | 6 | CRS Grade 1-2 |
| Crc (Colorectal Carcinoma) [13] | Cea (Carcinoembryonic Antigen) | Phase I Study | 10 | 3 | CRS Grade 2-3 |
| Cea-Positive Tumors [14] | Cea (Carcinoembryonic Antigen) | Phase I Study | 14 | 4 | CRS Grade 1-2 |
| Biliary and Pancreatic Cancers [15] | Her2 | Phase I Study | 11 | 0 | - |
| Pdac (Pancreatic Ductal Carcinoma) [16] | Mesothelin | Phase I Study | 6 | 0 | - |
| Hcc (Hepatocellular Carcinoma), Pancreatic Carcinomas, Colorectal Carcinomas [17] | Glypican-3, Mesothelin, Cea | Phase I Study | 23 | 1 | CRS Grade 1-2 |
| Gbm (Glioblastoma Multiforme) [18] | Egfrv3 | Phase I Study | 18 | 2 | CRS Grade 1-2 |
| Pleural Tumors [19] | Mesothelin | Phase I Study | 20 | 4 | CRS Grade 1-2 |
| Hepatocellular Cancer [20] | Glypican-3 | Phase I Study | 13 | 8 | CRS Grade 1-2 |
| Gastrointestinal Cancers [21] | Cldn18.2 | Phase I Study | 37 | 35 | CRS Grade 1-2 |
| Prostate Cancer [22] | Tgfβ | Phase I Study | 13 | 5 | 4 With CRS Grade 1-2, 1 With CRS Grade 5 |
| Solid Tumors (Synovial Sarcoma, Melanoma, Breast Cancer, Ovarian Cancer, Myxoid Liposarcoma) [23] | Ny-Eso-1 | Phase I Study | 9 | 3 | CRS Grade 1-2 |
| 3 With Metastatic Melanoma (Mm), 4 With Metastatic Triple- Negative Breast Cancer (Mtnbc) [24] | Cmet | Phase I Study | 7 | 1 | CRS Grade 1-2 |
| Solid Tumors (Ovarian Cancer, Germ Cell Tumors, Endometrial Cancer, Sarcoma) [25] | Cldn6 | Phase I Study | 22 | 7 | CRS Grade 1-2 |
Table 1. Shows the incidence of CRS associated in CAR t cell in various solid tumors.
Colorectal Cancer (Target Antigen: CEA, TAG72) in this early Phase I trial targeting colorectal cancer, CAR-T cells were designed to target CEA and TAG72. Out of the 14 patients, 6 experienced mild CRS (Grade 1-2). The CRS incidence rate was relatively high at approximately 42.9%, reflecting the early nature of the trial. Symptoms included fever and flu-like conditions, with no severe CRS cases observed, indicating mild toxicity of the therapy [12].
Colorectal Carcinoma (Target Antigen: CEA) in this Phase I study targeting CEA in colorectal carcinoma (CRC), 10 patients were treated. 3 patients (30%) developed CRS, with symptoms graded between Grade 2-3, indicating some cases of moderate severity. This trial highlighted the potential for slightly more severe CRS in certain solid tumors compared to milder cases in others. The relatively low patient count in early-phase studies emphasizes the need for larger cohorts to validate findings [13].
CEA-positive Tumors (Target Antigen: CEA) this Phase I study involved 14 patients with CEA-positive tumors, and 4 patients (28.6%) experienced Grade 1-2 CRS. This trial further supports the trend that CEA-targeted therapies may lead to a moderate incidence of CRS with mild to moderate symptoms. Fever and hypotension were the primary manifestations, with no patients experiencing severe toxicity [14].
Biliary and Pancreatic Cancers (Target Antigen: HER2) in this Phase I study involving 11 patients with biliary and pancreatic cancers, none of the patients experienced CRS. This study stands out because of the absence of CRS, suggesting that HER2-targeted CAR-T therapy might elicit a lower inflammatory response in this cancer type. However, the sample size is small, and further research is necessary to confirm these findings [15].
Pancreatic Ductal Carcinoma (Target Antigen: Mesothelin) in this trial targeting mesothelin in PDAC patients, 6 individuals were treated, but none experienced CRS. Like the study by Feng et al., this lack of CRS could suggest that certain solid tumors, like pancreatic cancers, have a lower predisposition for triggering immune-mediated toxicities, or that mesothelin may not strongly stimulate CRS pathways in this context [16].
Hepatocellular Carcinoma (HCC), Pancreatic and Colorectal Carcinomas (Target Antigens: Glypican-3, Mesothelin, CEA) this trial involved 23 patients with a mix of HCC, pancreatic carcinomas, and colorectal carcinomas, with only 1 patient (4.3%) developing Grade 1-2 CRS. The low incidence and mild severity may be attributed to the combination of target antigens and the specific characteristics of the solid tumor types involved, highlighting the varied response of solid tumors to CAR-T therapy [17].
Glioblastoma Multiforme (GBM) (Target Antigen: EGFRv3) in this Phase I trial with 18 patients targeting EGFRv3 in GBM, 2 patients (11%) experienced mild CRS (Grade 1-2). As GBM is a highly aggressive and immunologically distinct tumor, the low incidence and mild severity of CRS were expected, as solid tumors generally provoke a lower immune response than hematologic malignancies [18].
Pleural Tumors (Target Antigen: Mesothelin) this Phase I study targeting mesothelin in pleural tumors included 20 patients, with 4 patients (20%) developing Grade 1-2 CRS. The moderate incidence and mild to moderate severity were consistent with other solid tumor trials, particularly those targeting mesothelin, where mild CRS is more common [19].
Hepatocellular Cancer (Target Antigen: Glypican-3) in this trial targeting Glypican-3 in HCC, 13 patients were treated, and 8 patients (61.5%) developed Grade 1-2 CRS. This high incidence rate suggests that Glypican-3 might be a potent target for eliciting immune responses, although the severity was limited to mild and moderate cases, with no severe CRS observed [20].
Gastrointestinal Cancers (Target Antigen: CLDN18.2) in this Phase I study involving 37 patients with gastrointestinal cancers, a remarkable 35 patients (94.6%) experienced Grade 1-2 CRS. This extremely high incidence, paired with the mild to moderate severity, indicates that CLDN18.2 is a highly immunogenic target in gastrointestinal cancers. The study emphasizes the potential for frequent but manageable CRS in this patient population [21].
Prostate Cancer (Target Antigen: TGFβ) this trial targeting TGFβ in prostate cancer involved 13 patients, of which 5 patients (38.5%) experienced CRS. Interestingly, 4 patients had Grade 1-2 CRS, while 1 patient experienced a severe Grade 5 CRS. This trial is unique in reporting a case of fatal CRS, underscoring the need for close monitoring in future studies targeting this pathway in prostate cancer [22].
Various Solid Tumors (Target Antigen: NY-ESO-1) in this Phase I trial involving 9 patients with a mix of synovial sarcoma, melanoma, breast cancer, ovarian cancer, and myxoid liposarcoma, 3 patients (33.3%) developed Grade 1-2 CRS. The relatively moderate incidence and mild severity suggest that NY-ESO-1-targeted therapies in these solid tumors were generally safe with manageable toxicities [23].
Metastatic Melanoma and Triple-Negative Breast Cancer (Target Antigen: cMET) this Phase I trial involved 7 patients with metastatic melanoma and triple-negative breast cancer, of whom 1 patient (14.3%) experienced Grade 1-2 CRS. The low incidence of CRS in this trial aligns with the overall trend in solid tumor trials, where CRS occurs less frequently and with lower severity [24].
Various Solid Tumors (Target Antigen: CLDN6) in this Phase I trial involving 22 patients with ovarian cancer, germ cell tumors, endometrial cancer, and sarcoma, 7 patients (31.8%) developed Grade 1-2 CRS. The trial demonstrated a moderate incidence rate with predominantly mild to moderate symptoms, consistent with other studies targeting solid tumors [25].
This highlights that while CRS is a known complication of CAR-T therapies in solid tumors, its incidence appears lower than in hematologic malignancies.
CRS Severity and Grading
The severity of cytokine release syndrome (CRS) in CAR-T cell therapy trials targeting solid tumors is generally mild to moderate. Among the 217 patients included in the reviewed studies, 75 experienced CRS, with 71 presenting Grade 1-2 CRS, which manifests with symptoms like fever, fatigue, hypotension, and elevated cytokine levels. Severe CRS (Grade 3-4) was rare, with only 1 patient experiencing Grade 5 CRS in a prostate cancer trial, and 3 patients in a colorectal cancer study showing Grade 3 CRS. These findings highlight that CRS in solid tumors is less frequent and less severe than in hematologic malignancies, likely due to differences in tumor biology and CAR-T cell activity in solid tumors.
CRS Management Strategies
In the studies reviewed, management strategies for Cytokine Release Syndrome (CRS) typically include several key approaches tailored to the severity of the condition. Close monitoring of patients is essential for the early identification of CRS symptoms, allowing for timely intervention. Supportive care is often administered, which includes intravenous fluids and the management of electrolyte imbalances to stabilize patients. For those experiencing more severe CRS (grades 3-5), corticosteroids were frequently employed to mitigate the inflammatory response. Additionally, symptomatic treatments, such as medications to control fever and pain, were provided to enhance patient comfort. In cases of severe CRS, the IL-6 receptor antagonist tocilizumab may be administered to help manage the clinical symptoms. Overall, the specific management strategies can vary across studies and were influenced by the severity of CRS experienced by patients, highlighting the need for a personalized approach in the clinical setting.
Discussion
The exploration of Cytokine Release Syndrome (CRS) associated with Chimeric Antigen Receptor T-cell (CAR-T) therapy for solid tumors has gained prominence as researchers strive to understand its implications for patient safety and treatment efficacy. This review synthesizes findings from multiple Phase I clinical trials that investigate various target antigens across distinct cancer types, with a focus on CRS incidence, severity, and management strategies.
The data compiled from the reviewed studies indicate a notable trend in the incidence and severity of CRS among patients receiving CAR-T cell therapies for solid tumors. In total, out of 217 patients evaluated, 75 experienced CRS, with 71 classified as Grade 1-2, presenting mild to moderate symptoms like fever, fatigue, and hypotension. These findings were consistent with earlier reports, which documented CRS incidences of 42.9% and 30%, respectively, in early trials targeting colorectal cancer [12, 13]. The mild nature of CRS observed in these solid tumor studies contrasts sharply with the experience in hematologic malignancies, where CRS incidence and severity were notably higher.
In hematologic settings, studies had reported severe CRS in up to 70% of patients undergoing CAR-T therapy for Acute Lymphoblastic Leukemia (ALL). This highlights a critical distinction between the responses elicited by solid tumors versus hematological malignancies, raising questions about the underlying biological mechanisms driving these differences [26].
Conversely, some studies had reported higher CRS incidences of 61.5% and 94.6%, respectively, particularly in trials targeting Glypican-3 and CLDN18.2 [20, 22]. These findings may imply that certain target antigens can provoke a more robust immune response, necessitating further investigation into the mechanisms that contribute to this variability. The pronounced CRS incidence associated with CLDN18.2 suggests that targeting this antigen could be both beneficial and risky, warranting caution in clinical application.
The discrepancy in CRS incidence and severity between solid and hematologic tumors may stem from differences in tumor biology and the associated immune microenvironment. Solid tumors frequently exhibit a more immunosuppressive microenvironment, which can limit T-cell activation and cytokine release. For instance, studies targeting mesothelin in pancreatic ductal carcinoma [16] and pleural tumors [19] reported no cases of CRS, suggesting that these specific tumors may elicit a diminished inflammatory response.
In contrast, hematologic malignancies often trigger a robust immune response, which leads to a higher incidence of severe CRS. This has been observed in multiple studies, which demonstrated severe CRS rates of 13% to 47% in CAR-T-treated patients [27]. The variability in CRS outcomes reinforces the need for tailored CAR-T approaches in treating solid tumors, accounting for the unique immunological challenges posed by these cancers.
The management strategies employed for CRS in the reviewed trials highlight the importance of early identification and supportive care. Close monitoring allows for timely interventions, ultimately improving patient outcomes. Standard management strategies typically include intravenous fluids and electrolyte management to stabilize patients experiencing mild symptoms. For moderate to severe CRS, corticosteroids were frequently utilized to mitigate the inflammatory response, consistent with clinical guidelines.
The use of tocilizumab, an IL-6 receptor antagonist, has emerged as a critical therapeutic option for managing severe CRS. Its efficacy in hematologic settings is well-established; however, its application in the context of solid tumors remains less defined. A systematic review discusses the necessity for further research to optimize CRS management in solid tumors, noting that while tocilizumab is effective in reducing IL-6 levels, its impact on long-term outcomes in solid tumor patients requires investigation [28].
Additionally, personalized treatment approaches may enhance the management of CRS. For instance, researchers were exploring the role of predictive biomarkers to identify patients at risk for severe CRS. The incorporation of such biomarkers could facilitate a more tailored approach to CAR-T therapy, potentially improving patient safety.
Future Directions
The findings from this review underscore the need for larger, multicentre studies to validate observed trends in CRS incidence and severity. Integrating biomarker studies could elucidate predictors of CRS and inform strategies to mitigate risks. Furthermore, exploring novel CAR-T cell designs and combinatory approaches with immune checkpoint inhibitors may enhance the efficacy and safety profiles of CAR-T therapies in solid tumors.
Standardizing criteria for CRS grading and management is crucial for improving the overall understanding of this complex syndrome and facilitating comparisons across studies. Emphasizing patient safety and effective CRS management will be paramount in advancing CAR-T therapies for solid tumors.
Conclusion
In conclusion, while CRS remains a notable concern in CAR-T therapy for solid tumors, the data suggest that its incidence and severity were generally lower than those observed in hematologic malignancies. Understanding the intricate dynamics of CRS across different cancer types, along with the establishment of effective management strategies, is essential for advancing CAR-T therapies. Future research should focus on optimizing patient outcomes and mitigating CRS risks, paving the way for more effective and safer treatments for patients with solid tumors.
Acknowledgements
I would like to express my sincere gratitude to Dr Maheshvari Patel, PhD Scholar (Pharmacology) and Dr Indermit singh Anand, PhD as Medical Reviewers, for their invaluable guidance, thoughtful suggestions, and continuous assistance throughout the preparation of this review article. Their expertise in medical review and critical insights were pivotal in shaping the structure, content, and overall quality of the manuscript. I deeply appreciate Dr. Hirva Desai, Doctor of Pharmacy, & Ms. Shambhavi Srivastava, Master of Pharmacy in Pharmacology as Medical Writers, for their time and effort in refining this work to meet high academic standards.
References
- Buechner J, Kersten MJ, Fuchs M, Salmon F, Jäger U. Chimeric antigen receptor-T cell therapy: practical considerations for implementation in Europe. HemaSphere. 2018;2(1):e18.
- Tchernonog E, Moignet A, Anota A, Bernard S, Bouguet G, Colin F, Rioufol C, Ysebaert L, Gyan E. Health-related quality of life in patients with hematologic malignancies treated with chimeric antigen receptor T-cell therapy: review and current progress. Haematologica. 2024;109(8):2401.
- Zhang C, Durer S, Thandra KC, et al. Chimeric Antigen Receptor T-Cell Therapy. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2024.
- Miliotou AN, Papadopoulou LC. CAR T-cell therapy: a new era in cancer immunotherapy. Current pharmaceutical biotechnology. 2018;19(1):5-18.
- Chen YJ, Abila B, Mostafa Kamel Y. CAR-T: what is next?. Cancers. 2023;15(3):663.
- Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, Shomali N, Chartrand MS, Pathak Y, Jarahian M, Izadi S. CAR T cells in solid tumors: challenges and opportunities. Stem cell res ther. 2021;12:1-6.
- Xiao X, Huang S, Chen S, Wang Y, Sun Q, Xu X, Li Y. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res. 2021;40:1-23.
- Yang C, Nguyen J, Yen Y. Complete spectrum of adverse events associated with chimeric antigen receptor (CAR)-T cell therapies. J Biomed Sci. 2023;30(1):89.
- Chaudhary N, Roy S, Lin CF, Tandon M, Kwan A, Kuebler P, Shewade A. Real-World Incidence, Characteristics and Management of Cytokine Release Syndrome Induced By Chimeric Antigen Receptor T-Cell Therapy across Hematologic Malignancies. Blood. 2023;142:5150.
- Leclercq G, Steinhoff N, Haegel H, De Marco D, Bacac M, Klein C. Novel strategies for the mitigation of cytokine release syndrome induced by T cell engaging therapies with a focus on the use of kinase inhibitors. Oncoimmunol. 2022;11(1):2083479.
- Roselli E, Faramand R, Davila ML. Insight into next-generation CAR therapeutics: designing CAR T cells to improve clinical outcomes. J Clin Investig. 2021;131(2).
- Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J immunotherap cancer. 2017;5:1-4.
- Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J, Luo Y. Phase I escalating-dose trial of CAR-T therapy targeting CEA+ metastatic colorectal cancers. Mol Ther. 2017;25(5):1248-58.
- Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW, Sharma SK, Chester KA. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol, Immunother. 2017;66:1425-36.
- Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H, Yang Q, Wang Y, Han W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein & cell. 2018;9(10):838-47.
- Beatty GL, O’Hara MH, Lacey SF, Torigian DA, Nazimuddin F, Chen F, Kulikovskaya IM, Soulen MC, McGarvey M, Nelson AM, Gladney WL. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterol. 2018;155(1):29-32.
- Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, Liu Y, Huang J, Lv H, Luo C, Feng KC. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunol. 2018;7(7):e1440169.
- Goff SL, Morgan RA, Yang JC, Sherry RM, Robbins PF, Restifo NP, Feldman SA, Lu YC, Lu L, Zheng Z, Xi L. Pilot trial of adoptive transfer of chimeric antigen receptor–transduced T cells targeting EGFRvIII in patients with glioblastoma. Int J Immunother. 2019;42(4):126-35.
- Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O'Cearbhaill RE, Zhu A, Cheema W, Chintala NK, Halton E, Pineda J. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti–PD-1 agent pembrolizumab. Cancer Discov. 2021;11(11):2748-63.
- Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, Lu Q, Gao H, Jiang H, Wang H, Yuan D. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clinl Cancer Res. 2020;26(15):3979-89.
- Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, Zhang M, Peng Z, Zhou J, Cao Y, Zhang X. Claudin18. 2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nature medicine. 2022;28(6):1189-98.
- Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, Hwang WT, Lal P, Carpenter EL, Maude SL, Plesa G. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28(4):724-34.
- Ishihara M, Kitano S, Kageyama S, Miyahara Y, Yamamoto N, Kato H, Mishima H, Hattori H, Funakoshi T, Kojima T, Sasada T. NY-ESO-1-specific redirected T cells with endogenous TCR knockdown mediate tumor response and cytokine release syndrome. J immunotherap cancer. 2022;10(6).
- Shah PD, Huang AC, Xu X, Orlowski R, Amaravadi RK, Schuchter LM, Zhang P, Tchou J, Matlawski T, Cervini A, Shea J. Phase I trial of autologous RNA-electroporated cMET-directed CAR T cells administered intravenously in patients with melanoma and breast carcinoma. Cancer res commun. 2023;3(5):821-9.
- Mackensen A, Haanen JB, Koenecke C, Alsdorf W, Wagner-Drouet E, Borchmann P, Heudobler D, Ferstl B, Klobuch S, Bokemeyer C, Desuki A. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat Med. 2023;29(11):2844-53.
- Wei J, Liu Y, Wang C, Zhang Y, Tong C, Dai G, Wang W, Rasko JE, Melenhorst JJ, Qian W, Liang A. The model of cytokine release syndrome in CAR T-cell treatment for B-cell non-Hodgkin lymphoma. Signal Transduct Target Ther. 2020;5(1):134.
- Zhang X, Zhu L, Zhang H, Chen S, Xiao Y. CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front immunol. 2022;13:927153.
- Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813-22.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref