Research Article - Journal of Pharmaceutical Chemistry & Chemical Science (2023) Volume 7, Issue 1
In vivo evaluation of wound healing activity of cinnamon oil loaded hydrogel
Amira Nour1*, Aalaa Youssef2, Aya Beltagy2, Sara Elbadry2, Nada Khamis2, Sara M Osman3, Basma B Elwakil4, Mohamed Abd Al Moaty1, El- Sayed El Ashry1, Salma S Elshewemi5, Zakia A Olama3
1Department of Chemistry, Faculty of Science, Alexandria University, Egypt
2Industrial microbiology and applied chemistry Program, Department of Botany and Microbiology, Faculty of Science, Alexandria University, Egypt
3Departmentof Botany and Microbiology, Faculty of Science, Alexandria University, Egypt
4Department of Medical Laboratory Technology, Faculty of Applied Health Sciences Technology, Pharos University in Alexandria, Alexandria, Egypt
5Department of Zoology, Faculty of Science, Alexandria University, Egypt
- *Corresponding Author:
- Amira Nour
Department of Chemistry, Faculty of Science
Alexandria University, Egypt
E-mail: nrommh96p41z336n@studenti.unical.it
Received: 23-Jan-2023, Manuscript No. AAPCCS-22-76161; Editor assigned: 25-Jan-2023, PreQC No. AAPCCS-22-76161(PQ); Reviewed: 09-Feb-2023, QC No. AAPCCS-22-76161; Revised: 13-Feb-2023, Manuscript No. AAPCCS-22-76161(R); Published: 20-Feb-2023, DOI:10.35841/aapccs-7.1.132
Citation: Nour A. In vivo evaluation of wound healing activity of cinnamon oil loaded hydrogel. J Pharm Chem Chem Sci. 2023;7(1):132
Abstract
Extraction of Cinnamomum verum Essential Oil (CEO) was implemented using hydro distillation method. The antimicrobial activity of CEO was evaluated against Candida albicans, gram negative bacteria namely: Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis and gram positive bacteria namely: Methicillin Resistant Staphylococcus aureus (MRSA) and Staphylococcus aureus. The maximum Inhibition Zone Diameters (IZD) of CEO were against S. aureus and MRSA (24,83 ± 1.76 and 27.97 ± 1.18 mm), respectively. Further evaluation was done using Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) which reported the highest effect against MRSA (29.20 ± 2.82, 112.03 ± 2.05 ?g/mL), respectively. GC-MS and FTIR analyses proved that the major compound of CEO was cinnamaldehyde. Experimentally induced wounds were implicated in rats. Assessment of wound healing using wound contraction percentage and histopathological evaluation showed significantly high wound contraction percentage, reepithelialization and collagen deposition in the CEO hydrogel treated group in relation to the other tested groups.
Keywords
Cinnamomum verum, Antibiotic resistance, Gram-positive bacteria, Gram-negative, Hydrogel, Powder edible gelatin.
Introduction
Antibiotic resistance has recently become a worldwide problem. A wide range of Gram-positive and Gram-negative bacteria showed natural resistance towards many antibiotics and antiseptics in which they retain their activity for long periods of time. These bacteria can survive under drastic conditions of limited nutrients and can colonize traumatized skin [1]. MRSA is highly resistant to many currently used drugs and often causing postsurgical wound infections almost exclusively of hospital origin, associated with high morbidity and mortality [2,3]. It also rates among S. aureus clinical isolates scoring the highest in Egypt compared to other African countries and to southern and eastern Mediterranean countries [4,5]. Therefore, the development of novel and effective antibacterial strategies for treating bacterial infection is urgently needed.
Natural products exhibited effective antibacterial and antifungal actions. Medicinal plants possess numerous bioactive secondary metabolites including tannins, alkaloids, saponins and flavonoids with many physiological actions [6]. Cinnamomum verum, a common spice from the Laurel family, is obtained from the inner bark of trees from the genus Cinnamomum. CEO has been used to treat many diseases because of potential antimicrobial activity of its major component, cinnamaldehyde [7]. It also has many therapeutic applications. Isolated cinnamaldehyde has been shown to effectively inhibit the growth of an array of microorganisms such as bacteria, molds, and yeasts, as well as having been reported to inhibit toxin production by micro-organisms [8]. In previous studies, it was shown that cinnamaldehyde is responsible for the induction of collagen synthesis and the stimulation of human endothelial cell proliferation in vitro [8].
Hydrogels are polymeric materials with unique threedimensional structures. They are insoluble in water; however, they absorb thousand-fold of their weight in water. The presence of some hydrophilic domains such as OH, -CONH-, CONH2- and -SO3H in the hydrogel structure give them such characteristic property of absorbing water. Another unique property is their controllable porous structure by altering the density of the cross-links in their gel matrix [9]. This property can be used to introduce therapies or active agents into the gel matrix that can diffuse at a rate-dependent manner to target sites. They can be engineered to imitate the extracellular environment of the body’s tissue, hence, make themselves worthy to be used in soft tissue engineering and drug delivery [10]. Hydrogels help fibroblast proliferation and migration of keratinocytes by their ability to retain wound exudate. The occurrence of complete epithelialization and wounds healing depends on the last two processes. Moreover, the cross-links in the hydrogel mesh add to its tightness which gives protection against wound infection [11]. The objective of the present study was to evaluate the antimicrobial activity of CEO, investigate the active fraction of CEO and assess its healing effect on MRSA infected rat wounds by incorporating it into a synthesized hydrogel.
Materials and Methods
Microorganisms
The bacterial strains used throughout the present study namely: E. coli (ATCC 25922), K. pneumoniae (ATCC 13883) and P. mirabilis (ATCC 35659) were obtained from the NAMRU-3 while MRSA, S. aureus, S. epidermidis, K. pneumoniae, and C. albicans were kindly provided by Alexandria University Main Hospital, Egypt.
Essential oil extraction
C. verum bark was purchased from a local company (Al Garas for Spices and Herbs Co.). Cinnamon bark was ground, and a total of 50 g of cinnamon powder in 300 ml of distilled water were subjected to hydro-distillation for 5h at 100°C. The final extract was subjected to rotary evaporator for solvent removal and was stored in sealed vials at 4°C [12].
Antimicrobial assay
Disc diffusion method: Antimicrobial assay of CEO was performed by disc diffusion method as described by Kirby- Bauer [13]. 0.1ml (107 CFU/ml) of each microorganism was inoculated onto the surface of Mueller Hinton (MH) agar plates. Sterile filter paper discs (5 mm diameter) were loaded with 25μl CEO extract. The plates were left at ambient temperature for 15 minutes and then incubated at 37°C for 16h. After incubation, the zone of inhibition diameters (mm) was recorded. This assay was done in triplicates and the results were expressed as mean ± SD [14,15]. An analysis of variation (ANOVA) and plotting was performed using Post Hoc Test (Tukey) and results were considered statistically significant at P< 0.05
Determination of MIC and MBC: MIC and MBC of CEO extract were evaluated by micro-dilution technique using microtiter plates. Each well was filled with 80μl of sterile broth and 100μl of CEO, followed by serial dilution technique. Then, the wells were inoculated with 100μl of 0.5 McFarland standard bacterial suspensions and incubated at 37°C for 24h. The MBC is the lowest concentration that kills the organisms completely. 20μl aliquots from each well that exhibited no microbial growth were plated onto MH agar, incubated at 37°C for 18h and observed for bacterial growth [16].
Fractionation of the cinnamon oil extract
CEO was fractionated by column chromatography using normal silica gel (mesh particle size: 230-400, particle size 40 63μm and of pore size 60 ?) and eluted with a mixture of ethyl-acetate/hexane (1:3 v/v) to afford different major subfractions. The sub-fractions obtained were monitored by Thin Layer Chromatography (TLC) which was conducted on silica gel and spots were detected under UV light at 254nm and each fraction was tested for antibacterial activity one at a time [17].
Characterization of the active fraction: A Bruker Tensor 37 FTIR spectrophotometer analysis was done in a KBr matrix. IR spectra were recorded in the 400-4000cm-1 range with a resolution of 4cm-1. The room was kept at a controlled ambient temperature (25?) and relative humidity (30%). CEO was coated on the KBr tablets to form thin liquid films for infrared spectrometry analysis. Analysis of the active fraction was done using Gas Chromatography-Mass Spectrometry (GCMS). 1.0μl of the sample was injected into a Varian 4000 GC/ MS/MS fused with a 30mx 0.25mm x 0.39mm DB-5 capillary column. The carrier gas was Helium and was set to maintain a capillary flow of 1 ml/min. The initial temperature of 40 °C was held for 4min followed by an increase of 5°C/min to a final temperature of 230°C [18].
Preparation of CEO loaded hydrogel
The powder edible gelatin (10g) was added slowly, under agitation, in the smallest volume of sterile distilled water (14ml). The mixture was placed in a microwave reactor until it was completely dissolved (1-2min) [19]. Four ml of CEO was added into a 2ml of Tween-20 solution (25% v/v in distilled sterile water) and homogenized by vigorous agitation. Finally, all the components mentioned above were mixed with 180ml of sterile distilled water. The control hydrogel was prepared by the same method but without the addition of CEO. All prepared hydrogels were poured in a petri-dish and homogenized at room temperature. They were left in the refrigerator at 4°C and the thickness of the wound dressings were recorded (0.3cm).
Characterization of the prepared hydrogel
Swelling Measurements: Swelling properties of the CEO loaded hydrogel were evaluated in Phosphate Buffered Saline (PBS) solution pH ∼ 7.4. Hydrogel cubes were pre-weighed, placed in test tubes filled with 5ml distilled water at 37 °C and taken out from the test tubes every 24 h for 2 weeks, blotted its surface and weighed. The Equilibrium Swelling Degree (ESD) at different time intervals was calculated as the ratio of the swollen weight to the dried weight from the following equation:
ESD (%) = (w_s-w_d)/w_d ×100
Where Ws is the weight of swollen hydrogel sample (in grams) at time intervals and Wd is the weight of the dry sample (in grams) through an interval period [20].
Hydrolytic degradation: Hydrolytic degradation or weight loss (%) of CEO loaded hydrogel as a function of gelatin contents in the formed hydrogels was implemented in PBS solution (pH 7.4, at 37? for 2 weeks). The degradation of hydrogel was investigated after reaching the equilibrium swelling weight of swollen hydrogels. Then, the sample was removed from PBS solution at time intervals and was wiped gently using soft filter papers. The weight loss (%) of hydrogel was determined as a function of incubation time using the following formula:
Weight loss (%) = (w_o-w_t)/w_o ×100
Where Wo is the initial weight of hydrogel, Wt. is the removed hydrogel from PBS and weighed at function of specific incubation time intervals. All experiments have been performed in duplicate [21].
Morphology investigation by SEM: Surface morphology of the CEO loaded hydrogel was determined by Field Emission Scanning Electron Microscope (JSM-6390LV-JEOL). Dehydration was done using freeze drying technique. The dried hydrogel was coated with gold coater before scanning [22].
In vivo evaluation
Animals, Experimental Protocol and Wound induction: This experiment was conducted in the animal house of Faculty of Pharmacy and Drug Manufacturing, Pharos University. All studies were approved by the Ethics Committee at Faculty of Science, Alexandria University. Twenty female albino rats, four months of age with a body weight ranging between 210 and 250 g were used in this study. They were kept under standard laboratory conditions and veterinary supervision with no restriction on water and food, maintained in a 12 h light/12 h dark cycle in a temperature-controlled room at 20-25°C. The rats underwent anesthesia by ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally. A circular area on the dorsal aspect of skin 100mm2 was excised using sterilized forceps, a surgical blade, and pointed scissors [23]. Rats were randomly divided into four groups (n=5): Group I (MRSA infected wounds), Group II (non-infected wounds), Group III (MRSA infected wounds treated with hydrogel) and Group IV (MRSA infected wounds treated with CEO loaded hydrogel).
In group I and II, wounds were cleaned with cotton wetted with normal saline daily. In the other groups, hydrogel and CEO loaded hydrogel were applied once daily from the day of the operation until complete healing. Skin lesions were measured for the assessment of wound healing on days 3, 5, 7, 9 and 11. Skin biopsies were excised on days 7 and 11 under anesthesia with ketamine (85 mg/kg, intraperitoneally) and xylazine (10 mg/kg, intraperitoneally) for histopathological studies [24].
Wound Contraction: The wound was monitored, and the healing area was calculated. The area was measured daily, and the percentage wound closure was calculated by the following formula:
% Wound Contraction = (wound area on day 0-wound area on day n)/ (wound area on day 0) ×100
Histopathological study: Specimen tissues were stored in 10% formalin solution, embedded in paraffin, cut into 5 mm pieces, and stained with Hematoxylin and Eosin (H and E). Sections were visualized under a light microscope at 100X magnifications. Fields were examined regarding the degree of epithelization, presence or absence of inflammatory cells, and the nature of collagen and fibrous tissue [25].
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The Kolmogorov-Smirnov test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, Standard Deviation (SD) and standard error of mean. Significance of the obtained results was judged at the 5% level. F-test (ANOVA) was used for normally distributed quantitative variables, to compare between more than two groups, and Post Hoc test (Tukey) for pairwise comparisons.
Results and Discussion
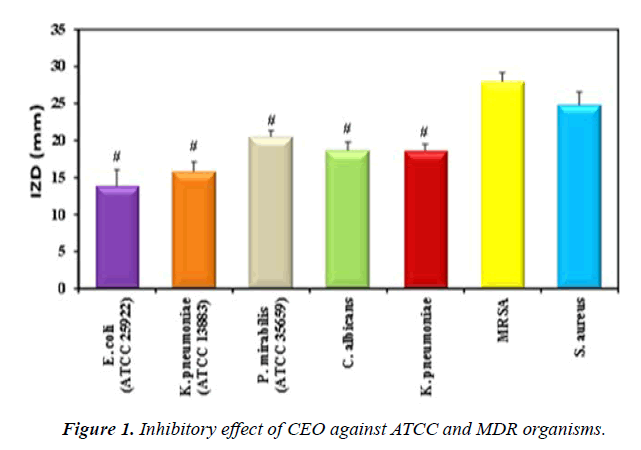
Antimicrobial activity of CEO extract against ATCC and MDR organisms: Disc diffusion method results showed that CEO (1.94 mg/ml, dry basis) had the most significant IZD against MRSA (27.97 ± 1.18mm) and S. aureus (24.83 ± 1.76 mm) compared to the other studied organisms. Grampositive bacteria were found to be more susceptible to CEO than Gram-negative (Figure 1, Table 1). Evidently, the highest antibacterial activity against the Gram-positives resides in the polar and semi-polar fractions of cinnamon. Solubility in water of cinnamon fractions could raise the idea of their easy formulation into drugs [26]. This phenomenon was probably due to structural differences in the outer membrane of bacteria. Data of the present study revealed that E. coli showed the least zone of inhibition. Gram-negative bacteria (E. coli) have a thick layer of lipopolysaccharide outer membrane covering the cell wall. This structure has shown to be more resistant to hydrophobic substance compared with the grampositive bacteria, which possess single peptidoglycan layer structure [27]. In contrast to the present findings, another study was conducted on the in vitro antibacterial activity of the aqueous extract of Cinnamon; the maximum zone of inhibition was recorded against E. coli and P. mirabilis. The antibacterial activity of C. verum aqueous extract had significant positive correlation with the total phenol content in their dose-dependent study [28]. In addition, proved that Cinnamaldehyde can inhibit the yeast growth (C. albicans) with fungicidal effect through cellular membrane damage [29].
| Tested pathogen | IZD (mm) Mean ± SD |
|---|---|
| E. coli (ATCC 25922) | 13.87 ± 2.19 |
| K. pneumoniae (ATCC 13883) | 15.83 ± 1.27 |
| P. mirabilis (ATCC 35659) | 20.53 ± 0.83 |
| C. albicans | 18.67 ± 1.15 |
| K. pneumoniae | 18.63 ± 0.87 |
| MRSA | 27.97* ± 1.18 |
| S. aureus | 24.83* ± 1.76 |
Table 1. Antimicrobial activity of CEO against ATCC and MDR organisms.
MIC and MBC of CEO: According to the present finding, the lowest MIC (29.20 ± 2.82, 64.73 ± 2.84 μg/mL) and MBC (112.03 ± 2.05, 241.90 ± 2.95 μg/ml) values were obtained with CEO extract on MRSA and S. aureus, respectively (Table 2). On the contrary the obtained data were slightly different from that [30]. who reported CEO MIC against MRSA (19.5312 ± 0.7071 μg/ml) and the corresponding MBC (39.0625 ± 0.7071 μg/ml). This can be attributed to the extraction method adopted in their research by using sequential extraction with hexane, ethyl acetate, methanol, and water, in addition to the different Cinnamomum species used.
| Test pathogen | CEO | |
|---|---|---|
| MIC (μg/mL) (mean ± SD) | MBC (μg/mL) (mean ± SD) | |
| E. coli (ATCC 25922) | 98.16 ± 0.65 | 456.33 ± 4.04 |
| K. pneumoniae (ATCC 13883) | 92.80 ± 0.4 | 395.66 ± 8.14 |
| P. mirabilis (ATCC 35659) | 74.56 ± 6.47 | 274.33 ± 7.50 |
| C. albicans | 87.33 ± 2.45 | 392.46 ± 4.08 |
| K. pneumoniae | 84.46 ± 0.94 | 392.16 ± 8.60 |
| S. aureus | 64.73 ± 2.84 | 241.90 ± 2.95 |
| MRSA | 29.20 ± 2.82 | 112.03 ± 2.05 |
Table 2. MIC and MBC values of CEO against the tested pathogens.
Disc diffusion method of the active fractions: The present results revealed that the first fraction which was obtained by column chromatography gave the highest zone of inhibition (mm) against MRSA (13.86 ± 0.31) (Table 3).
| Fractions | Zone of inhibition (mm) (mean ± SD) | ||
|---|---|---|---|
| Pathogen | 1 | 2 | 3 |
| E. coli (ATCC 25922) | 9.63 ± 0.58 | - | - |
| K. pneumoniae (ATCC 13883) | 8.76 ± 0.56 | 6.87 ± 0.21 | - |
| P. mirabilis (ATCC 35659) | - | - | - |
| C. albicans | - | - | - |
| K. pneumoniae | 7.76 ± 0.47 | - | - |
| MRSA | 13.86 ± 0.31 | 7.67 ± 0.40 | 5.87 ± 0.31 |
| S. aureus | 6.13 ± 0.21 | - | - |
Table 3. Antimicrobial activity of CEO fractions against tested organisms.
FTIR of CEO
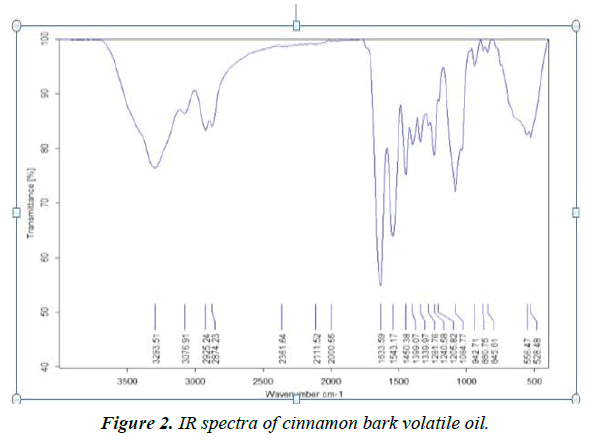
The volatile oils are complex mixture systems. The IR spectra show a total overlap of each absorption spectrum of various components. The IR characteristic fingerprint peaks for the cinnamon samples are mostly in the range of 4000-400 cm-1. The typical spectra (Figure 2) of cinnamons were analyzed and several characters can be extracted, such as the peak at 1543.17cm-1, corresponding to the aromatic ring C=C skeleton vibration of an aromatic substance. IR (Nujol) νmax 1633.59, 2874.23 and 2925.24 cm-1 correspond to the stretching vibration of an aldehyde carbonyl C=O, C-H and=C-H, respectively. These peaks reflect the large amount of cinnamaldehyde and aldehydes in the volatile oil of cinnamon bark. The data obtained from these characteristic peaks of the analyzed essential oil attributed to the high content of phenolic and aromatic compounds, especially cinnamaldehyde.
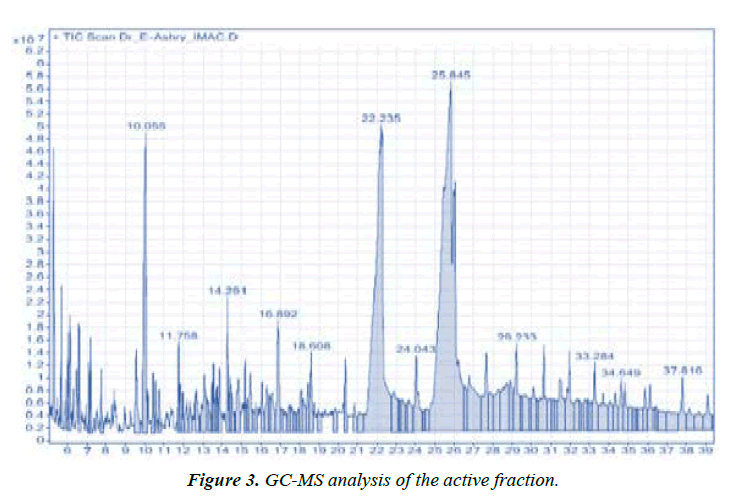
Identification of volatile compounds in cinnamon bark oils: GC-MS data detected three major compounds. Cinnamaldehyde was the most predominant compound (55%). The other two components were n-Hexadecenoic acid (27%) and trans-13-Octadecenoic acid (18%) (Figure 3). Cinnamaldehyde is an effective inhibitor for the bacterial growth through biofilm formation inhibition of MRSA isolates [31-33]. Revealed that Cinnamomum zeylanicum essential oil showed antibacterial activity with varying values. They recorded significant inhibition zones against E. coli, K. pneumoniae and P. mirabilis with inhibition zone diameter 30, 30 and 28mm, respectively. It was correlated with the major compound of the oil namely (E)-cinnamaldehyde (68.95%) which is also known to inhibit bacterial acetyl-CoA carboxylase and responsible for major antibacterial activity.
Characterization of the prepared hydrogel
Phosphate buffer solution was used to evaluate the swelling and degradation of the prepared hydrogel because it mimics the physiological conditions of the human body. Hydrogels are considered as one molecule regardless of its size. As all the polymer chains are cross linked to each other either physically or chemically and thus called infinitely large molecules or super macromolecules. One of the variables that affect capacity of water absorption is the degree of cross linking and the type of cross-linking agent used [34]. The prepared CEO loaded hydrogel showed Equilibrium Swelling Degree (ESD) of 89.3% and hydrolytic degradation of 35.6% after two weeks. Such degree of swelling reflects the high degree of crosslinking density and how it will retain the hydrogel shape regardless of the solvent amount. Moreover, the low percentage of degradation emphasizes the rigidity of the hydrogel and the low rate of releasing CEO extract. This showed the prepared CEO hydrogel high durability and boosts its application in skin tissue engineering.
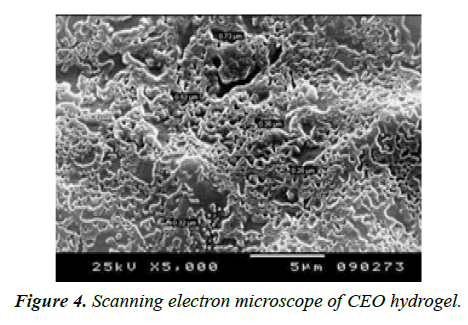
Scanning electron microscope: SEM analysis of the hydrogel sample was evaluated for the examination of the morphological changes and hydrogel pore size. SEM images of CEO Hydrogels revealed that the pore size range was 5-50 μm (Figure 4). This small pore size of the hydrogel improves the entrapment efficiency of the cinnamon oil and increases the durability of hydrogel. The obtained pore size also increases the mechanical strength of the hydrogel due to the bonding strength [35]. The pore size distribution and its interconnections are essential factors of a hydrogel matrix that are often challenging to compute and are generally included together in the parameter called tortuosity.
In vivo evaluation of CEO Hydrogel CEO
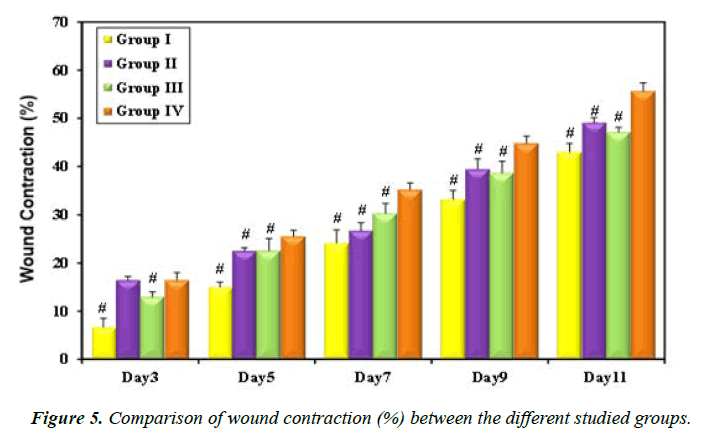
Loaded hydrogel treated rat group showed the highest percentage of wound contraction (44.84 ± 1.49 and 55.68 ± 1.66%) on days 9 and 11, respectively (Figure 5, Table 4). Recent research reported similar results and explained the antibacterial effect of cinnamon oil by inhibition of nitric oxide production which is responsible for inflammatory conditions in the human body. Moreover, cinnamon has also been shown to prevent the production of cyclooxygenase-2 enzyme, a proinflammatory agent and this may explain the healing effect of cinnamon oil extract on rat ulcers [36].
| Animal group | Wound contraction (%) ± SD | ||||
|---|---|---|---|---|---|
| Day3 | Day5 | Day7 | Day9 | Day11 | |
| Group I | 6.68 ± 1.85 | 15.01 ± 1.04 | 24.15 ± 2.70 | 33.25 ± 1.80 | 43.03 ± 1.79 |
| Group II | 16.47 ± 0.76 | 22.56 ± 0.62 | 26.72 ± 1.65 | 39.46 ± 2.18 | 49.16 ± 0.96 |
| Group III | 12.99 ± 1.04 | 22.58 ± 2.48 | 30.31 ± 2.10 | 38.70 ± 2.38 | 47.16 ± 0.97 |
| Group IV | 16.47 ± 1.51 | 25.54 ± 1.29 | 35.21 ± 1.39 | 44.84 ± 1.49 | 55.68 ± 1.66 |
| P | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
Table 4. Wound contraction (%) of the treated rats during different time intervals.
Morphological findings: It was noticed that bleeding following wound induction was minimal in all groups. No clear-cut clotting was observed by naked eye on wounds in all groups post-injury. Significant reduction of wound area was observed in group IV when compared to the other studied groups (Table 4).
Histopathological findings
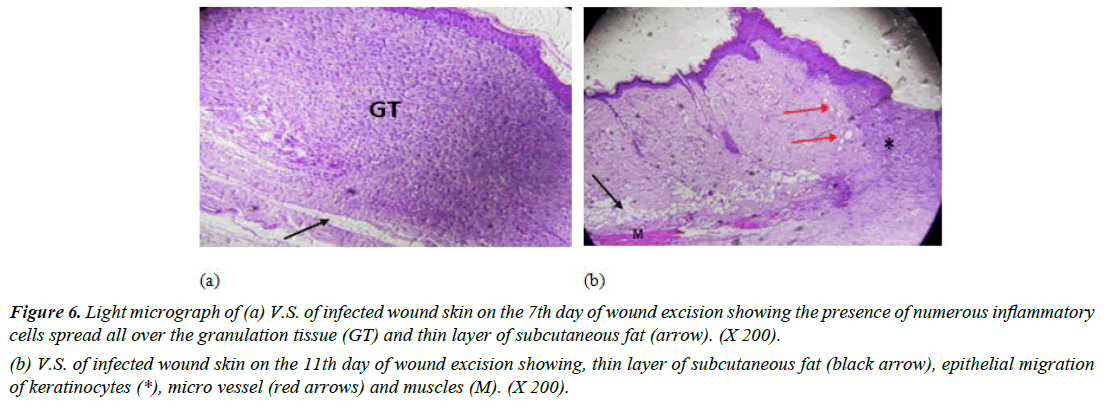
Group I (Positive control): Skin sections from rats in day 7 showed many inflammatory cells in the dermis layer with absence of demarcation line (which separates the necrotic tissue from vital tissue). A thin layer of subcutaneous fat was recognized (Figure 6a). Moreover, light micrograph-stained vertical skin sections of infected wounded rat after 11 days revealed minimal epidermal regeneration, micro vessels and some muscle tissue (Figure 6b). Another study showed similar results with delayed wound healing and re-epithelialization of MRSA-infected wounds in diabetic mice. Granulation tissue formation was enhanced in MRSA-infected wounds of diabetic mice compared to normal mice [37].
Figure 6: Light micrograph of (a) V.S. of infected wound skin on the 7th day of wound excision showing the presence of numerous inflammatory cells spread all over the granulation tissue (GT) and thin layer of subcutaneous fat (arrow). (X 200).
(b) V.S. of infected wound skin on the 11th day of wound excision showing, thin layer of subcutaneous fat (black arrow), epithelial migration of keratinocytes (*), micro vessel (red arrows) and muscles (M). (X 200).
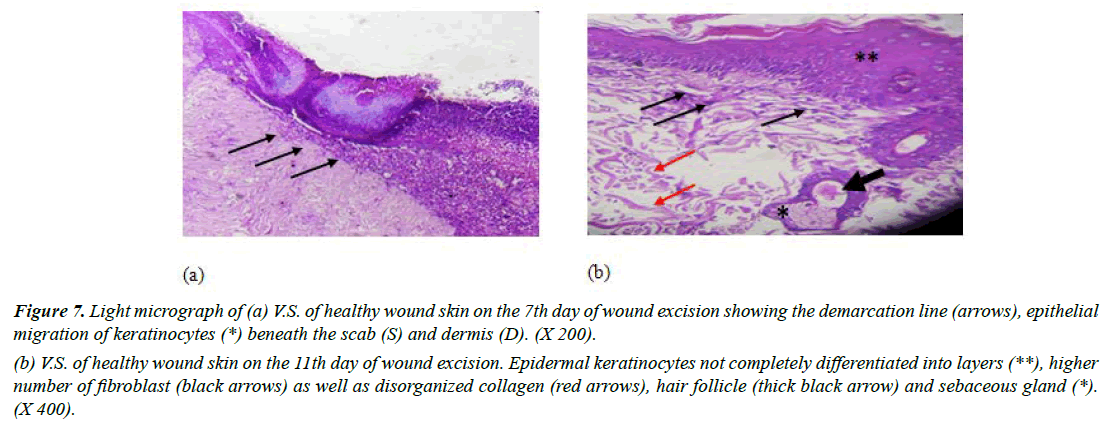
Group II (negative control): Examination of light micrograph-stained vertical skin sections in day 7 revealed some features of wound repair. It was observed that the most superficial layer of the wound, a scab, which consists mainly of dead tissue, many inflammatory cells and damaged collagen fibers. Formation of the demarcation line which separates the necrotic tissue from vital tissue was detected. Absence of fibroblasts or new blood vessels was observed. Moreover, vertical section revealed obvious migration of keratinocytes beneath the scab which is the mitotically active layer that divides to form a complete epithelial layer (Figure 7a).
Standard Hematoxylin and Eosin-stained wounds on day 11 showed that the superficial layers were randomly arranged cells. This suggested that the differentiation process of keratinocytes was not completed. In addition, large number of fibroblasts dominated the dermis layer and collagen fibers were haphazardly arranged (Figure 7b). These results were in alignment [38]. Who revealed that after 11th day of wound excision in rats; large number of fibroblasts was present?
Figure 7: Light micrograph of (a) V.S. of healthy wound skin on the 7th day of wound excision showing the demarcation line (arrows), epithelial migration of keratinocytes (*) beneath the scab (S) and dermis (D). (X 200).
(b) V.S. of healthy wound skin on the 11th day of wound excision. Epidermal keratinocytes not completely differentiated into layers (**), higher number of fibroblast (black arrows) as well as disorganized collagen (red arrows), hair follicle (thick black arrow) and sebaceous gland (*). (X 400).
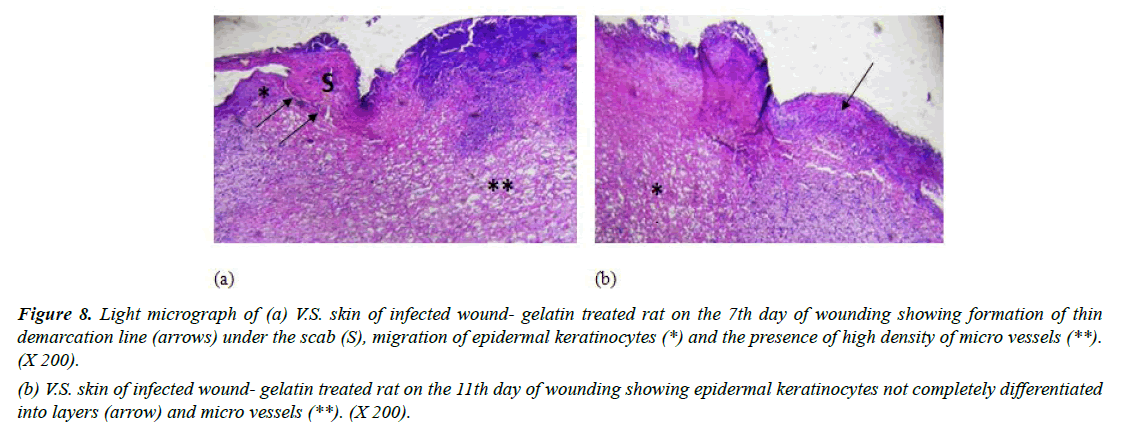
Group III (Infected with MRSA and treated with placebo hydrogel): Gelatin-treated wounds on the 7th day revealed some aspects of epidermal regeneration with migration of epidermal keratinocytes and the appearance of a thin demarcation line in some sections in this group (Figure 8a). On day 11, gelatin treatment enhanced basal layer mitotic activity leading to the formation of some epidermal cell layers; however, they were not completely differentiated into layers and showed some micro vessels (Figure 8b). In accordance with our findings [39]. Reported improved healing of rat wounds infected with MRSA and treated with gelatinsealed Dacron graft after 7 days. They demonstrated decreased neutrophilic infiltrate in fibrous tissue.
Figure 8: Light micrograph of (a) V.S. skin of infected wound- gelatin treated rat on the 7th day of wounding showing formation of thin demarcation line (arrows) under the scab (S), migration of epidermal keratinocytes (*) and the presence of high density of micro vessels (**). (X 200).
(b) V.S. skin of infected wound- gelatin treated rat on the 11th day of wounding showing epidermal keratinocytes not completely differentiated into layers (arrow) and micro vessels (**). (X 200).
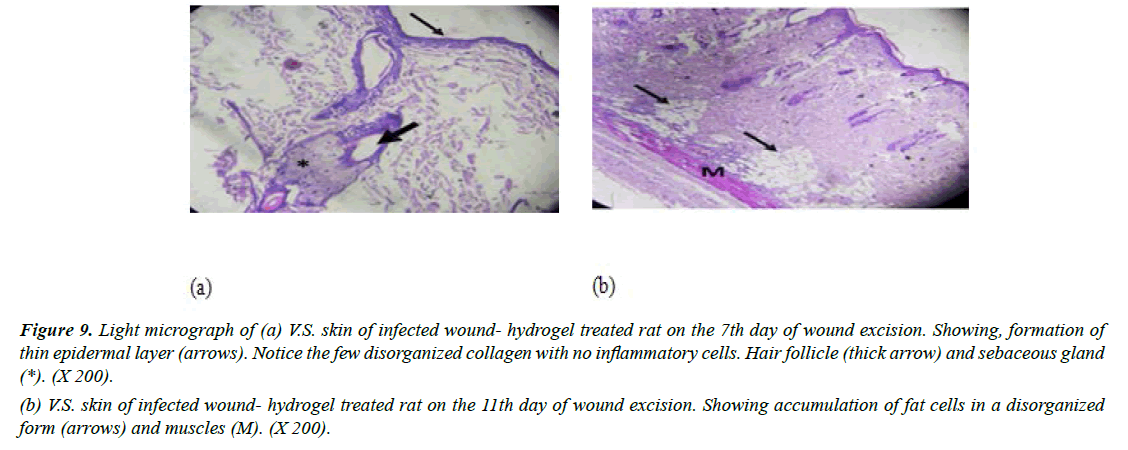
Group IV (Infected with MRSA and treated with CEO hydrogel): Specimens taken after 7 days indicated the formation of new layer of thin epithelial cells, but the differentiation process of keratinocytes into layers was not completed. Examination showed the persistence of a minimal number of inflammatory cells. Preparations also showed that the granulation tissue consisted of a few numbers of newly synthesized non-organized collagen. It was noticed that fibroblasts were not detected in granulation tissue (Figure 9a). Moreover, on day 11, sections revealed an increased number of fat cells which exhibited haphazard organization. It appeared that the presence of numerous fat cells was responsible for delayed formation of granulation tissue that appeared on day 7 (Figure 9b). In accordance with our findings [40]. reported improvements of wound healing by topical application of C. verum hydroethanolic extract in streptozotocin-induced diabetic mice. HPLC data for cinnamon hydroethanolic extract identified cinnamaldehyde (11.26%) as the major components. A significant increase was observed in wound contraction ratio, insulin like growth factor 1 (IGF1), fibroblast proliferation, collagen deposition, re-epithelialization, and keratin biosynthesis in the C. verum-treated groups in comparison to the diabetic non-treated group.
Figure 9: Light micrograph of (a) V.S. skin of infected wound- hydrogel treated rat on the 7th day of wound excision. Showing, formation of thin epidermal layer (arrows). Notice the few disorganized collagen with no inflammatory cells. Hair follicle (thick arrow) and sebaceous gland (*). (X 200).
(b) V.S. skin of infected wound- hydrogel treated rat on the 11th day of wound excision. Showing accumulation of fat cells in a disorganized form (arrows) and muscles (M). (X 200).
Conclusion
In this study, C. verum constituents were evaluated by GC-MS and showed that cinnamaldehyde was the major compound. The results of this study revealed a potential effect of CEO loaded hydrogel in treating MRSA infected wounds. Moreover, the basic polymer of the hydrogel (gelatin) proved to have a major role in wound contraction. Such result can provide evidence that CEO Hydrogel can be used in drug delivery systems and enhance the skin healing process.
References
- Tom IM, Ibrahim MM, Umoru AM, et al. Infection of wounds by potential bacterial pathogens and their resistogram. Open Access Library J. 2019;6(7):1-3.
- Gurung RR, Maharjan P, Chhetri GG. Antibiotic resistance pattern of Staphylococcus aureus with reference to MRSA isolates from pediatric patients. Future sci OA. 2020;6(4):FSO464.
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-41.
- Falagas ME, Karageorgopoulos DE, Leptidis J, et al. MRSA in Africa: filling the global map of antimicrobial resistance. PloS one. 2013;8(7):e68024.
- Borg MA, De Kraker M, Scicluna E, et al. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in invasive isolates from southern and eastern Mediterranean countries. J Antimicrob Chemother. 2007;60(6):1310-5.
- Shakya AK. Medicinal plants: Future source of new drugs. Int J Herb Med. 2016;4(4):59-64.
- El-Baroty GS, Abd El-Baky HH, Farag RS, et al. Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr J Biochem Res 2010;4(6):167-74.
- Doyle AA, Stephens JC. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia. 2019;139:104405.
- Patel HB, Patel HL, Shah ZH, et al. Review on hydrogel nanoparticles in drug delivery. Am J Pharm Res. 2011;1:19-38.
- Mishra B, Upadhyay M, Reddy Adena SK, et al. Hydrogels: an introduction to a controlled drug delivery device, synthesis and application in drug delivery and tissue engineering. Austin J Biomed Eng. 2017;4(1):1037-49.
- Kamoun EA, Kenawy ER, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 2017;8(3):217-33.
- Chen G, Sun F, Wang S, et al. Enhanced extraction of essential oil from Cinnamomum cassia bark by ultrasound assisted hydrodistillation. Chin J Chem Eng. 2021;36:38-46.
- Bauer AW. Antibiotic susceptibility testing by a standardized single disc method. Am J clin pathol. 1966;45:149-58.
- Joe MM, Jayachitra J, Vijayapriya M. Antimicrobial activity of some common spices against certain human pathogens. J Med Plants Res. 2009;3(11):1134-6.
- Wong YC, Ahmad-Mudzaqqir MY, Wan-Nurdiyana WA. Extraction of essential oil from cinnamon (Cinnamomum zeylanicum). Orient J Chem. 2014;30(1):37.
- Mekonnen A, Yitayew B, Tesema A, et al. In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int J Microbiol. 2016;2016.
- Jham GN, Dhingra OD, Jardim CM, et al. Identification of the major fungitoxic component of cinnamon bark oil. Fitopatologia Brasileira. 2005;30:404-8.
- Kasim NN, Ismail SN, Masdar ND, et al. Extraction and potential of cinnamon essential oil towards repellency and insecticidal activity. Int j sci res publ. 2014;4(7):2250-3153.
- Oliveira MM, Brugnera DF, Piccoli RH. Essential oils of thyme and Rosemary in the control of Listeria monocytogenes in raw beef. Braz J Microbiol. 2013;44:1181-8.
- Liu P, Peng J, Li J, et al. Radiation crosslinking of CMC-Na at low dose and its application as substitute for hydrogel. Radiat Phys Chem. 2005;72(5):635-8.
- Kamoun EA, Omer AM, Abu-Serie MM, et al. Photopolymerized PVA-g-GMA hydrogels for biomedical applications: factors affecting hydrogel formation and bioevaluation tests. Arab J Sci Eng. 2018;43(7):3565-75.
- Pourjavadi A, Kurdtabar M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur Polym J. 2007;43(3):877-89.
- Jahandideh M, Hajimehdipoor H, Mortazavi SA, et al. Evaluation of the wound healing activity of a traditional compound herbal product using rat excision wound model. Iran J Pharm Res: IJPR. 2017;16(Suppl):153.
- Belachew TF, Asrade S, Geta M, et al. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of Hagenia abyssinica (Bruce) JF Gmel in mice. Iran J Pharm Res. 2020;2020.
- Elnahas RA, Elwakil BH, Elshewemi SS, et al. Egyptian Olea europaea leaves bioactive extract: Antibacterial and wound healing activity in normal and diabetic rats. J Tradit Complement Med. 2021;11(5):427-34.
- Vemula VR, Lagishetty V, Lingala S. Solubility enhancement techniques. International Int J Pharm Sci Rev Res. 2010;5(1):41-51.
- Zhang Y, Liu X, Wang Y, et al. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282-9.
- Rakshit M, Ramalingam C. In-vitro antibacterial and antiodixant activity of Cinnamomum verum (Cinnamon) aqueous bark extract in reference to its total phenol content as natural preservative to food. Int J Biol Biotech. 2011;8(4):529-37.
- Taguchi Y, Hasumi Y, Abe S, et al. The effect of cinnamaldehyde on the growth and the morphology of Candida albicans. Medical molecular morphology. 2013;46(1):8-13.
- Buru AS, Pichika MR, Neela V, et al. In vitro antibacterial effects of Cinnamomum extracts on common bacteria found in wound infections with emphasis on methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 2014;153(3):587-95.
- Jia P, Xue YJ, Duan XJ, et al. Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin?resistant Staphylococcus aureus. Lett Appl Microbiol. 2011;53(4):409-16.
- Ooi LS, Li Y, Kam SL, et al. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am J Chin Med. 2006;34(03):511-22.
- Hussein HA, Abaas IS, Ali RH. Antibacterial activities of cinnamon zelanicum syzygium aromaticum essential oil. Int J Pharm Pharm Sci. 2014;6(5):165-8.
- Shetye SP, Godbole A, Bhilegaokar S, et al. Hydrogels: Introduction, preparation, characterization and applications. Hum J. 2015;1:47-71.
- Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. Journal of advanced research. 2015;6(2):105-21.
- Julianti E, Rajah KK, Fidrianny I. Antibacterial activity of ethanolic extract of cinnamon bark, honey, and their combination effects against acne-causing bacteria. Scientia pharmaceutica. 2017;85(2):19.
- Shi CM, Nakao H, Yamazaki M, et al. Mixture of sugar and povidone-iodine stimulates healing of MRSA-infected skin ulcers on db/db mice. Arch Dermatol. 2007;299(9):449-56.
- Carvalho MD, Pereira CS, Fregnani JH, et al. Comparative histological study on wound healing on rat's skin treated with Mitomycin C or Clobetasol propionate. Acta Cir. Bras. 2015;30:593-7.
- Yasim A, Gul M, Ciralik H, et al. Gelatin-sealed dacron graft is not more susceptible to MRSA infection than PTFE graft. Eur J Vasc Endovasc Surg. 2006;32(4):425-30.
- Daemi A, Lotfi M, Farahpour MR, et al. Topical application of Cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharm Biol. 2019;57(1):799-806.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref