Short Communication - Journal of Biochemistry and Biotechnology (2021) Volume 4, Issue 4
In vitro phytochemical screening, antioxidant potential and in vivo hepatoprotective and renal protective activity of Amaranthus viridis.
Ranjit Thakur*, Rakhi Das, Bijay Kumar Das
Department of Biochemistry, Universal Engineering and Science College, Chakupat, Lalitpur, Nepal
- Corresponding Author:
- Ranjit Thakur
Department of Biochemistry
Universal Engineering and Science College
Chakupat, Lalitpur, Nepal
Tel: + 009779849293396
E-mail: ranjitthakur008@gmail.com
Accepted date: July 15, 2021
Citation: Thakur R, Das R, Das BK. In vitro phytochemical screening, antioxidant potential and in vivo hepatoprotective and renal protective activity of Amaranthus viridis. J Biochem biotech. 2021; 4(4): 22-24.
Abstract
The aim of the present study was to evaluate the antioxidant activity, phytochemical screening, hepatoprotective and renal protective activity of Amaranthus viridis. The leaf, seed and whole plant extracts of these plant were prepared in pure solvent methanol. The Phytochemical tests were performed qualitatively while the antioxidant activity was determined by 2,2-Di-Phenyl- 1-Picrylhydazyl-Hydrate (DPPH) method and the hepato and renal tests were carried out by enzymatic kits method. The study revealed that the methanol extract of seeds of Amaranthus viridis has shown effective antioxidant activity in DPPH assay technique. The study also showed liver and renal protective activity against paracetamol induced liver damage and Aspirin induced renal damage.
Keywords
Phytochemical screening, Antioxidant activity, Hepato, Renal protective
Introduction
Amaranthus virdis (Family: Amarantheceae) is distributed in the warmer parts of the world. The amaranthus represents a horticulturally important group of annual herbaceous plants represtented by total of 60 species in the world flora under amaranthus L., which is the fifth largest genus of the family Amarantheceae. Green amaranthus serve as one of the most delicious leafy vegetables and are rich sources of protein (upto 5.6% on fresh weight basis), requisite vitamins (A,B,C, Folic acid), minerals (Ca, Mg, K, P, Na, N, Fe, Mn, Zn) and fibres (5.25%) [1,2]. Besides, they contain other biologically pro-health compounds such as the antioxidant, squalene, and carotenoids [3] and thus, are recommended as a nutritious food with medicinal properties for young children, lactating mothers and for patients with asthma, hemorrhage, anaemia or kidney complaints. In addition, the whole plant possesses analgesic and anti-pyretic properties and is used for the treatment of pain and fever respectively in traditional system of medicine [4]. Leaves are directly used to eczema, psoriasis and rashes etc., [5] furthermore, the plant possesses anti-proliferative and anti-fungal lactin properties as well as ribosomes inactivating protein beta- carotene [6,7] and anti-viral activities [8]. However, there is not enough scientific reports to support these supposed analgesic and anti-pyretic activities, this has initiated step to conduct the studies to ascertain the authenticity of these important claims of traditional potency [9].
Materials and Methods
Collection of plant material
The plants A. viridis was collected from open fields of Lalitpur district of Nepal. Leaves and seeds of the plants were separated and washed properly with distilled water these plant parts. All Plant parts were dried under shade at room temperature for the prevention of loss of any active constituents. The dried leaves and seeds of the plants were grounded separately to coarse powder using mechanical blenders followed by fine extraction using organic solvents.
Plant extraction: All plant extracts were prepared using the soxhlet extraction method. 20 grams of dry powder from leaves, seed and whole plant of A. viridis were extracted with 200 ml of methanol separately in soxhlet extractor until the final extraction was colorless. These methanol extracts were then subjected to rotary evaporator at 64.7ºC respectively. The crude extracts obtained were stored at 4ºC in airtight glass bottles for further use.
Preliminary phytochemical screening: The methanol extract of A. viridis was screened for the presence of various phytoconstituents like flavonoids, saponins, glycosides, terpenoids amino acids, alkaloids, carbohydrates, phenolic compounds proteins etc [10,11].
Antioxidant activity: [2, 2-Di-Phenyl-1-Picryl- Hydrazylhy (DPPH) assay]: The antioxidant activities of A. viridis extracts obtained by soxhlet extraction were determined by using DPPH free radical assay as described by Molyneux (2004). The working dilutions of plant extracts were prepared by dissolving 2,2-Di-Phenyl-1- Picryl-Hydrazylhy (DPPH) in methanol. Ascorbic acid was used as positive control. One milliliter of plant extract was added to 3 ml of DPPH solution. All dilutions were used in triplicates. The absorbance was recorded at 517 nm by UV-Spectrophotometer against ascorbic acid.
Animal management and grouping: Thirty Swiss Albino mice with weight ranging from 25-30 gram of both sexes were obtained from Natural Product Research Laboratory, Thapathali, Kathmandu. The animals were handled humanely, kept in well ventilated cage under suitable conditions of temperature and humidity. They were provided food of composition as prescribed by the animal house and served water soaked in cotton. Animals were kept in 12 hrs dark/light cycle and allowed to acclimatize for 1 week in laboratory condition.
Group I: Control
Group II: Paracetamol Induced
Group III: Paracetamol+methanolic extract of whole plant
Group IV: Aspirin induced
Group V: Aspirin+methanolic extract of whole plant
Group VI: Those given methanolic extract of whole plant only
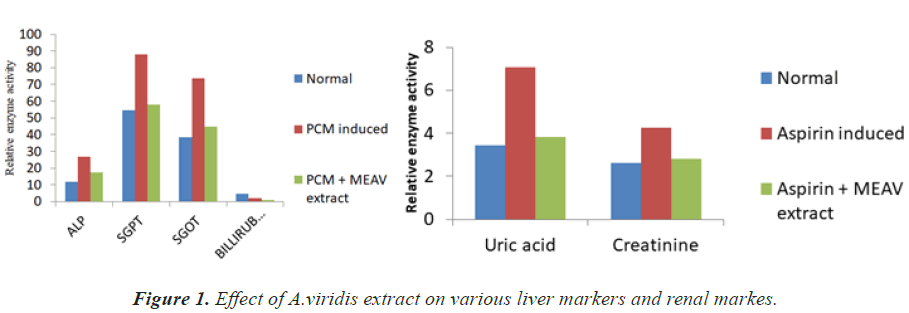
Blood sample collection: After one week of feeding drugs and plant extracts, blood sample was collected from neck region after cutting with blade under chloroform anesthesia. Blood obtained was kept in small sized test tube which can be fitted in the centrifuge machine and allowed to clot and stored in ice bath. Then when all blood samples were withdrawn they are centrifuged at 3000 rpm for 10-15 mins to obtain serum. The clear serum obtained was kept in microfuge tube and stored at 4ºC for further biochemical tests like ALP, SGOT, SGPT, Billirubin (for Liver Test), Uric acid and Creatinine ( For Renal Test) (Table 1) [12-17].
| Biochemical parameter | Normal Value |
|---|---|
| Alkaline Phosphate (ALP) (U/I) | 11.72 ± 8.63 |
| SGOT (U/I) | 38.56 ± 6.21 |
| SGPT (U/I) | 54.61 ± 2.02 |
| Bilirubiun (mg/I) | 0.806 ± 0.09 |
| Uric acid (mg/dl) | 3.42 ± 1.85 |
| Creatinine (mg/I) | 2.63 ± 0.95 |
Table 1: Compositions of gases in the six gas groups.
Results and Discussion
On preliminary phytochemical anlaysis of methanolic extract of leaves, seed and whole plant of A. viridis showed the presence of flavonoids, saponins, glycosides, terpenoids aminoacids, alkaloids, carbohydrates, phenolic compounds and proteins. These plants were also assessed for their antioxidant activity by DPPH assay. The DPPH inhibition of leaf, seed and whole plant extracts of A. viridis are recorded in Table 2. The free radical scavenging activity of positive control (ascorbic acid) was 89.26 ± 0.957 (40 mg/ml). Percentage scavenging activity of A. Viridis extracts ranged from 59.03 ± 1.916 to 85.75 ± 3.421.
| Concentration mg/ml | % Inhibition | |||
|---|---|---|---|---|
| Leaf | Seed | Whole plant | Ascorbic acid | |
| 20 | 63.37 ± 5.874 | 68.97 ± 4.032 | 59.03 ± 1.916 | 84.79 ± 1.967 |
| 30 | 75.17 ± 2.056 | 82.19 ± 4.863 | 71.68 ± 2.731 | 87.63 ± 1.053 |
| 40 | 75.89 ± 2.589 | 85.75 ± 3.421 | 73.59 ± 2.024 | 89.26 ± 0.957 |
Table 2: DPPH radical scavenging activity of A.viridis.
Paracetamol administration to mice produced hepatotoxicity showed by significant increase in the levels of SGOT, SGPT, ALP, and Bilirubin in comparsion to control group. Likewise aspirin administration to mice produced renal toxicity showed by significant increase in the levels uric acid and creatinine. The methanol extract of whole plant of A. viridis showed significant decrease in the SGOT, SGPT, ALP, Bilirubin when compared to paracetamol group (Figure 1) and also uric acid and creatinine when compared to aspirin group.
Conclusion
Our investigation suggests that methanolic extract of whole plant of Amaranthus viridis Linn, possess liver and renal protective activity against paracetamol and aspirin induced Hepato and renal toxicity in mice. The study also revealed that the methanol extract of Amaranthus viridis has shown effective antioxidant activity in DPPH assay technique. Therefore, further work could be done on isolation of active constituents and study of its liver and renal protective activity.
References
- Elias. J. Proceedings of first amaranth seminar. Rodalle Press Inc, Emmaus, 1977: 16.
- Flores HE, Teutonico RE. Amaranthus (Amaranthus spp.): potential grain and vegetable crops. In: Bajaj YPS (eds) Biotechnology in Agriculture and Forestry Crops I. (2ndedn) Springer-Verlag, Berlin, Heidelberg, 1986; pp.568-578.
- Kumari N, Prakash D. Carotenoids: do they defend against cancer and heart diseases. Inov Intel. 2005;40:24-30.
- Eluwa MC. Studies on Gasteroclisus rhomboidalis (Boheman.)(Coleoptera: Curculionidae)-a pest of the African ‘spinach’. J Nat Hist. 1977;11(4):417-24.
- Kiritikar KR, Basu BD. Indian Medicinal Plants. In: Kirtikar KR, Basu BD (eds). International book distributors (2ndedn). India:Dehra Dun, 1987; pp.2061-2.
- Sena LP, Vanderjagt DJ, Rivera C, et al. Analysis of nutritional components of eight famine foods of the Republic of Niger. Plant Foods Hum Nutr. 1998;52(1):17-30.
- Gallagher RS, Cardina J. Phytochrome-mediated Amaranthus germination I: Effect of seed burial and germination temperature. Weed Sci. 1998;46(1):48-52.
- Nordeide MB, Hatloy A, Folling M, et al. Nutrient composition and nutritional importance of green leaves and wild food resources in an agricultural district, Koutiala, in southern Mali. Int J Food Sci Nutr. 1996;47(6):455-68.
- Kumar BS, Lakshman K, Jayaveera KK, et al. Antinociceptive and antipyretic activities of amaranthus viridis linn in different experimental models. Avicenna J Med Biotechnol. 2009;1(3):167.
- A Text book of pharmacognosy by M.K.Gupta, P.K.Sharma. 120-135
- Rao SK, Andrade C, Reddy K, et al. Memory protective effect of indomethacin against electroconvulsive shock-induced retrograde amnesia in rats. Biol Psychiatry. 2002;51(9):770-3.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56-63.
- Tietz NW. Fundamentals of Clinical Chemistry. 1970:447-9.
- Kind PR, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7(4):322.
- Varely H. Practical Clinical Biochemistry, 4th edition. 1975
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6(1):24-7.
- Schettler Gand E. Inflammatory biomarkers in Asian Indian women with metabolic syndrome. Nussel Arbeitsmed Sozialmed Praventivmed. 1975;10:25.