Research Article - Biomedical Research (2017) Volume 28, Issue 6
In vitro evaluation of anti-caries effect of cinnamon extracts on oral pathogens
Hye-Young Kim1 and Jun-Beom Park2*
1Department of Dental Hygiene, College of Health Science, Kangwon National University, Samcheok-si, Republic of Korea
2Department of Periodontics, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- *Corresponding Author:
- Jun-Beom Park
Department of Periodontics
College of Medicine
The Catholic University of Korea
Seoul, Republic of Korea
Accepted on November 23, 2016
Abstract
Cinnamon has been used for neuralgia on the knees and waist, and it is shown to have a wide antibacterial spectrum in both Gram-positive bacteria and Gram-negative bacteria. In this study, glucosyltransferase adhesive ability, acid-production suppression effect, and antibacterial activity were measured to identify the anti-caries effect of cinnamon extract. It was shown that cinnamon extract effectively suppressed acid production and bacterial adhesion for Streptococcus mutans and Streptococcus sanguinis . Additionally, the measurement result of the minimum inhibitory concentration of the cinnamon extract showed that the bacterial growth and development was inhibited at a 4 mg/ml concentration for anti-caries bacteria. In terms of the minimum bactericidal concentration, 99.99% bactericidal action was also confirmed at 4 mg/ml, and a high bactericidal action was shown with the smallest concentration for cariogenic bacteria. With regard to the adhesion-inhibition of glucosyltransferase, which is an oral pathogen for Streptococcus mutans that typically causes dental, caries among oral pathogens, the inhibitory effect appeared to be based on the concentration of the cinnamon extract, thereby showing that cinnamon extract has the ability to inhibit the fraction’s adhesion. Based on the results, it is concluded that cinnamon extract has the potential to be used as a natural antibacterial agent for oral pathogens, as it possesses acid-production suppression ability, the bactericidal action of the minimum bactericidal concentration, adhesion-inhibition ability, and anticaries effect.
Keywords
Cinnamomum zeylanicum, Dental caries, Herbal medicine, Streptococcus mutans, Streptococcus sanguis.
Introduction
The World Health Organization (WHO) has selected oral diseases, including dental caries, periodontal disease, halitosis, and oral pain, as international health problems that need to be solved in the future [1]. In a worldwide scope, 60% to 90% of school children and almost 100% of adults have dental caries, while 15% to 20% of middle-aged (35 to 44 years old) adults have severe periodontal disease [1]. These oral diseases become risk factors of tooth loss and potential risk factors of systematic diseases, such as diabetes complications, hypertension, pneumonia, osteoporosis, and low birth weight [2,3]. Oral disease is a global health concern that needs to be solved, and it requires more study and the development of an effective and strong oral bactericide without the risk of side effects [4].
The intraoral environment with good humidity and a good temperature may serve as a breeding ground for bacteria [5]. The use of antibiotics, fluorine compounds, and chlorhexidine, among others, can prevent biofilm-related diseases, however, resistance to antibiotics, fluoride compounds, and chlorhexidine causes side effects in the human body [6]. Chlorhexidine, which is well-established active ingredient in mouthwashes, can decrease the amount of bacteria or suppress the formation of oral biofilm [7]. However, the regular use of chlorhexidine may cause side effects, such as a burning sensation, the staining of mucous membranes, and pigmentation on the tongue and teeth [8,9].
The above mentioned issues sparked the conducting of a study on using natural materials for commuting antibiotics and averting the side effects of chemicals [10,11]. Cinnamon bark belongs to the tall evergreen tree and contains 1% volatile oil ingredients, including phellandrene, eugenol, and methyleugenol, and it has a spicy and sweet taste as well as a recessive nature [12]. Cinnamon is widely used for neuralgia on the knees and waist, along with joint pathologies and nasogastric-tube revitalization [13]. Its benefits include food preservation, the antibacterial activity of synthetic food preservatives, and anti-cancer effects [14]. Cinnamon extracts showed a wide antibacterial spectrum in Gram-positive bacteria and in Gram-negative bacteria, particularly showing a strong genesistasis for Streptococcus iniae, Edwardsiella tarda, and Listonella anguillarum [15]. It was shown that cinnamon has a stronger sterilizing action in Gram-negative bacteria than in Gram-positive bacteria. However, limited antibacterial study of cinnamon has been conducted on the oral pathogen. As a result, this study tests the antibacterial effect of bacillus on dental caries by using cinnamon extract to contribute to the development of an effective intervention for the prevention of oral disease. This study evaluated the inhibition of glucosyltransferase adhesive ability, the suppression effect of acid production, and the antibacterial activity of cinnamon.
Materials and Methods
Extract preparation and fraction
Cinnamon was obtained from the College of Biomedical Science, Kangwon National University, Chuncheon-si, Republic of Korea, and the material was identified by M.W. Cinnamon was chopped up and powdered in a mechanical grinder. Ethanol extract was obtained via a rotary vacuum evaporator (CCA-1110; Eyela, Tokyo, Japan) after filtration through filter paper (100 mm; Whatman, Maidstone, United Kingdom (UK)) by adding 80% ethanol to the pulverized cinnamon and doubling the weight. The remaining specimens were deposited at the Department of Medical Biotechnology, College of Biomedical Science, Kangwon National University.
Used strain
To measure the antibacterial activity among oral pathogens, bacteria-causing dental caries (Streptococcus mutans KCTC 3065 (brain heart infusion agar, 37°C), Streptococcus sanguinis KCTC 3284 (tryptic soy broth agar, 37°C), and Streptococcus sanguinis KACC 11301 (tryptic soy broth agar, 37°C)), and general bacteria (Bacillus cereus ATCC14579 (Nutrient Agar (NA), 37°C), Staphylococcus aureus CCARM 3090 (Luria- Bertani (LB) agar, 37°C), Escherichia coli ATCC 10798 (Luria-Bertani (LA) agar, 37°C, and Shigella sonne ATCC 2531i (Nutrient Agar (NA), 37°C)) were used.
Glucosyltransferase adhesive ability
First, after the seed culture for Streptococcus mutans KCTC 3065, which is the glucosyltransferase-producing strain, in the Brain-Heart Infusion (BHI) medium at a temperature of 37°C for 24 hours, the main culture was carried out by inoculating 2% (v/v) into a 450 ml BHI medium in the same conditions. For the supernatant, which was obtained via centrifugation at 6,000 rpm for 20 minutes at room temperature (Centrifuge 5427 R. Eppendorf, Germany), a 300 ml cooled ethanol was added to precipitate the protein. After being kept overnight at a temperature of 4°C, the precipitated protein that was obtained via centrifugation at 8,000 rpm for 30 minutes was suspended in 1 ml 0.05 M potassium phosphate buffer (pH 6.8, Sigma- Aldrich Co. LLC, Germany). It was set to be crude glucosyltransferase and kept in a freezer at a temperature of-20°C.
By adding a 0.8 ml substrate solution (12.5 g sucrose and 0.25 g NaN3 are contained in 1 L 0.0625 M potassium phosphate buffer (pH 6.5)), 0.025 ml crude glucosyltransferase, and 0.175 ml cinnamon at a 250 mg/ml concentration to a test tube, the final solution was adjusted to 1 ml. For the control group, 0.175 ml distilled water was added. After making the test tube stand at approximately a 30° angle against the horizontal plane, the solution reacted at a temperature of 37°C for 16 hours. After the reaction, the supernatant was discarded, and after a 5 s ultrasound treatment by adding 3 ml distilled water, the formed glucan was dispersed. Right after dispersion, the absorbance was measured at 550 nm with a spectrophotometer (UV-1601. Shimadzu, Japan) and the degree of the cinnamon extract’s adhesion-inhibition was calculated. The adhesiveinsoluble glucan-synthesis inhibition rate was calculated by using the following equation. The adhesive-insoluble glucansynthesis inhibition rate (%)=A-B/A × 100 (A is the absorbance value of adhesive glucans that are formed in the control group, while B is the absorbance value of adhesiveinsoluble glucans that are formed in cinnamon).
Measurement of acid-production suppression effect
The effect of cinnamon extracts on acid-production ability in Streptococcus mutans, Streptococcus sanguinis KCTC 3284, and Streptococcus sanguinis KACC 11301 was examined. After mixing by adding 0.5 ml fractions prepared by concentration (2 mg/ml and 4 mg/ml), which served as a control group, 1 ml of a 2% glucose solution was added to 2 ml bacterial suspension (OD600=1.2) in each test tube. The pH that changes overtime was measured for 4 hours with a onehour interval, while the solutions were cultured at the optimal incubation temperature to examine the degree of acidproduction suppression.
Measurement of antibacterial activity
For the minimum-inhibitory-concentration measurement of the cinnamon extract, after diluting the concentration of cinnamon extract by stage and setting the final dilution concentration of bacteria to OD600=1.2, it was cultured at a temperature of 37°C for 24 hours, and then, absorbance was measured and read at 600 nm. The culture medium, which was not proliferated based on the minimum inhibitory concentration, was diluted in 0.9% NaCl, and afterward, with the culture at a temperature of 37°C for 24 hours by uniformly applying it into the optimal medium, the minimum bactericidal concentration was read at the concentration where 99.9% sterilization was done.
Results
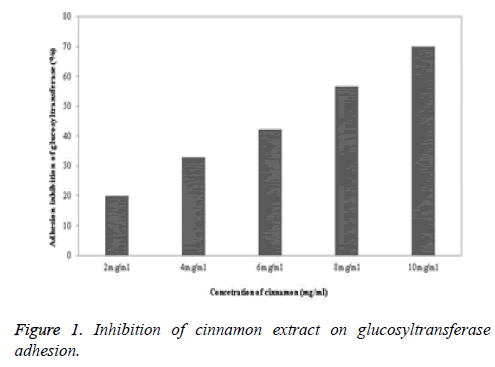
Glucosyltransferase adhesion-inhibition effect of cinnamon extract
The glucosyltransferase adhesion-inhibition effect of the cinnamon extract was highest at the 10 mg/ml concentration, which was 70%, followed by 56.6% at 8 mg/ml, 42.1% at 6 mg/ml, 33% at 4 mg/ml, and 20% at 2 mg/ml (Figure 1). It was confirmed that the cinnamon extract had fraction adhesioninhibition ability.
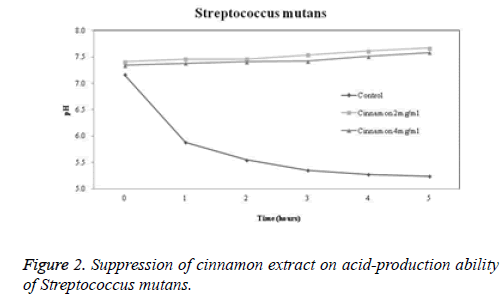
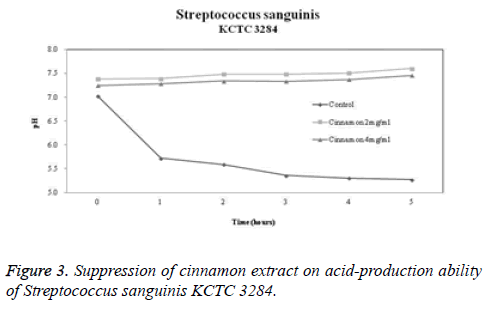
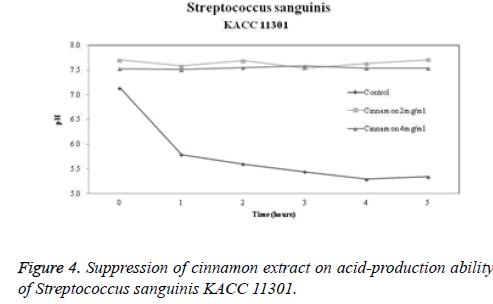
Acid-production suppression ability
The acid-production suppression ability of cinnamon extract is shown in Figures 2-4. The pH of the control group in which glucose was added to Streptococcus mutans, Streptococcus sanguinis KCTC 3284, and Streptococcus sanguinis KACC 11301 at 0 hour was 7.17, 7.02, and 7.14, respectively. When 2 mg/ml cinnamon extract was added to Streptococcus mutans, Streptococcus sanguinis KCTC 3284, and Streptococcus sanguinis KACC 11301, the pH was 7.41, 7.38, and 7.7, respectively. When 4 mg/ml cinnamon extract was added to Streptococcus mutans, Streptococcus sanguinis KCTC 3284, and Streptococcus sanguinis KACC 11301, the pH was 7.34, 7.25, and 7.53, respectively. There was a slight difference. After 5 hours, the pH in the control group dropped to 5.24, 5.27, and 5.34, respectively. However, in terms of the pH change of the cinnamon extract, the pH at 2 mg/ml was 7.67, 7.6, and 7.71, respectively. In terms of the pH change of the cinnamon extract, the pH at 4 mg/ml was 7.58, 7.46, and 7.54, respectively. It was confirmed that the cinnamon extract inhibited more acid production than did the control group.
Measurement of antibacterial activity of the cinnamon extract
As a result of measuring the minimum inhibition concentration of the cinnamon ethanol extract, the growth and development of Streptococcus mutans, Streptococcus sanguinis KCTC 3284, and Streptococcus sanguinis KACC 11301 were inhibited at a 4 mg/ml concentration (Table 1). As for the minimal bactericidal concentration, 99.99% bactericidal action was achieved at a 4 mg/ml concentration.
| Microorganisms | Growth at concentration (mg/ml) | Minimal bactericidal concentration (mg/ml) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Streptococcus mutans | + | + | + | + | - | 4 |
| Streptococcus sanguinis KCTC 3284 | + | + | + | + | - | 4 |
| Streptococcus sanguinis KACC 11301 | + | + | + | + | - | 4 |
Table 1. Measurement of minimal inhibition concentration and minimal bactericidal concentration (mg/ml).
Discussion
This study used the antimicrobial susceptibility test to learn about the antibacterial effect of cinnamon on oral pathogens, and it clearly showed that cinnamon extract showed excellent antibacterial activity on Streptococcus mutans, Streptococcus sanguinis KCTC 3284, and Streptococcus sanguinis KACC 11301.
Current antimicrobial susceptibility tests include the disk diffusion test, agar dilution method, broth dilution method, and minimum inhibitory concentration. The broth dilution method includes the macrodilution method and microdilution method. These methods are fast and straightforward, but they cannot be applied to drugs with poor diffusion among slow-growing bacterial species, anaerobic bacteria, and an agar medium [16,17]. However, minimum inhibition concentration has recently been carried out in bacteriology laboratories across the world and is suitable for performing systematic and reproducible antimicrobial tests for antibacterial agents [18]. In this study, the antibacterial activity of cinnamon was measured by using minimum inhibitory concentration, which is used for antibiotic substances to test for the ability to inhibit bacterial growth.
Many essential oils have been advocated for use in complementary medicine for bacterial and fungal infections [19]. Cinnamon oil was found to be more effective than clove oil, exhibiting a broad spectrum of antibacterial activity [20]. Cinnamon oil produced the maximum inhibition zone of a diameter of 24.0 mm against the major causative bacteria of dental plaque of Streptococcus mutans, which had a maximum inhibition zone of a diameter of 13.0 mm [20]. Cinnamon oil showed the highest activity against Streptococcus mutans, followed by lemongrass oil and cedarwood oil. Wintergreen oil, lime oil, peppermint oil, and spearmint oil showed no antibacterial activity [19].
Moreover, cinnamon extract showed intracanal bacterial reduction against Enterococcus faecalis with 80 to 85% intracanal bacterial reduction [21]. This suggested that cinnamon oil has the potential to be used as an antimicrobial agent in root canal treatment [21]. The use of the cinnamon extract decreased plaque and gingival scores, suggesting the ability to reduce plaque levels and gingivitis [22]. The functionalization of different dental implant material surfaces with cinnamon oil resulted in immediate and on-going antibacterial and antiplaque activities [23]. Toothpastes featuring natural compounds with cinnamon are commercially available, and they showed antimicrobial activity [24]. Additionally, commercially available sugar-sweetened cinnamon chewing gum may benefit halitosis by reducing volatile sulphur compounds producing anaerobes in the oral cavity [25].
This study reports that the glucosyltransferase adherence inhibition of the odontopathogenic Streptococcus mutans was the highest in 10 mg/ml and sequentially appeared to decrease 56.6% in 8 mg/ml, 42.1% in 6 mg/ml, 33% in 4 mg/ml, and 20% in 2 mg/ml, therefore, cinnamon extract has the ability to prevent sticking fraction. In the previous study, the glucosyltransferase adherence-inhibition effect of Lonicera flower extract on Streptococcus mutans was effective at a rate of 11.4% in 25 mg/ml, 20% in 50 mg/ml, 28% in 100 mg/ml, and 71.4% in 200 mg/ml [26]. Cinnamon extract showed a similar glucosyltransferase adherence-inhibition effect in a twenty times lower concentration when compared with the previous work.
The results showed that the acid product inhibition of cinnamon is significantly higher in the control group. Meanwhile, the result of adding 2 mg/ml and 4 mg/ml cinnamon extract after 5 hours for each group showed that the pH had been maintained over 7.4, and there was only a low concentration of the acid product inhibition of the cinnamon extract. The pH result was higher than in the previous study showing that the Lonicera flower can inhibit acid production in its pH with 6.57 and 6.16 in 2 mg/ml and 4 mg/ml [26]. A previous report also showed that the Lonicera flower showed antibacterial effects on Staphylococcus aureus [27]. Camellia japonica extract's antibacterial activity is notable for suppressing as much as 18 mm in concentration of 250 mg/disc with the high inhibition of acid production [26].
In the previous report evaluating the antibacterial effect of cinnamon extract against fish pathogenic bacteria, the minimum growth inhibitory concentration of the Gram-positive bacteria of cinnamon extract was 75.8~189.6 μg/ml, while the minimum growth inhibitory concentration of the Gramnegative bacteria of cinnamon extract was 75.8~113.8 μg/ml [15]. In this report, the results for cinnamon extract’s minimum growth inhibitory concentration showed 99.99% of germicidal effect in 4 mg/ml. In the previous report, the minimum inhibitory concentration of cinnamon was 65 μg/ml, and this was also the minimum fungicidal concentration value [28]. The minimum inhibitory concentration on Streptococcus mutans with 70% ethanol extract of pine needles was 20 mg/ml [29]. This result revealed that the cinnamon extract was better than the pine needle extract in terms of germicidal effect with the minimum growth inhibitory concentration and antibiotic properties on Streptococcus mutans even with a low concentration.
A satisfactory level of the safety and tolerability of cinnamon oil was reported [30], and cinnamon oil was reported to be cytocompatible with fibroblast [31]. However, cinnamoninduced contact stomatitis has been reported, and the most commonly affected site was the gingiva, which showed diffuse or generalized erythema and epithelial sloughing [32,33]. The resolution of symptoms occurred after discontinuing the product containing cinnamon [34,35].
Based on the results of this study, cinnamon extract showed high effects on acid-production inhibition and the minimum growth inhibitory concentration. In addition, the inhibition of glucosyltransferase adherence can be effectively used for the prevention of dental disease and contribute to the enhancement of oral health. Further clinical trials seem necessary to establish their safety and efficacy.
Conflict of Interest
The authors report no conflicts of interest related to this study. The author does not have any financial interest in the companies whose materials are included in the article.
References
- World Health Organization, Oral health. http://www.who.int/mediacentre/factsheets/fs318/en/2016.03.13.
- Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. ClinMicrobiol Rev 2000; 13: 547-558.
- Choi HM, Han K, Park YG, Park JB. Associations between the number of natural teeth and renal dysfunction. Medicine (Baltimore) 2016; 95: e4681.
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ 2005; 83: 661-669.
- Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 2006; 42: 80-87.
- Baygin O, Tuzuner T, Kusgoz A, Senel AC, Tanriver M. Antibacterial effects of fluoride varnish compared with chlorhexidine plus fluoride in disabled children. Oral Health Prev Dent 2014; 12: 373-382.
- Eick S, Goltz S, Nietzsche S, Jentsch H, Pfister W. Efficacy of chlorhexidinedigluconate-containing formulations and other mouthrinses against periodontopathogenic microorganisms. Quintessence Int 2011; 42: 687-700.
- Seymour RA, Rudralingham M. Oral and dental adverse drug reactions. Periodontol 2008; 46: 9-26.
- Korstanje MJ. Drug-induced mouth disorders. ClinExpDermatol 1995; 20: 10-18.
- Kim KJ, Yu HH, Jeong SI, Cha JD, Kim SM, You YO. Inhibitory effects of Caesalpiniasappan on growth and invasion of methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 2004; 91: 81-87.
- Wang MH, Jeong SH, Guo H, Park JB. Anti-inflammatory and cytotoxic effects of methanol, ethanol, and water extracts of AngelicaeDahuricae Radix. J Oral Sci 2016; 58: 125-131.
- Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003; 26: 3215-3218.
- Baur MB. The treatment of herpes zoster and post zoster neuralgia with acupuncture and Western herbs in Chinese medicine. J HomeopAyurv Med 2015; 4: 179.
- Hintz T, Matthews KK, Di R. The use of plant antimicrobial compounds for food preservation. Biomed Res Int 2015; 2015: 246264.
- Mok JS, Song KC, Choi NJ, Yang HS. Antibacterial effect of cinnamon (Cinnamomum cassia) bark extract against fish pathogenic bacteria. Korean J Fish AqutSci 2001; 34: 545-549.
- Koch AL. Diffusion through agar blocks of finite dimensions: a theoretical analysis of three systems of practical significance in microbiology. Microbiol 1999; 145: 643-654.
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008; 3: 163-175.
- Jenkins SG, Schuetz AN. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo ClinProc 2012; 87: 290-308.
- Chaudhari LK, Jawale BA, Sharma S, Sharma H, Kumar CD, Kulkarni PA. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J Contemp Dent Pract 2012; 13: 71-74.
- Gupta C, Kumari A, Garg AP, Catanzaro R, Marotta F. Comparative study of cinnamon oil and clove oil on some oral microbiota. Acta Biomed 2011; 82: 197-199.
- Gupta WA, Wadhwa J, Duhan J. Comparative evaluation of antimicrobial efficacy of three herbal irrigants in reducing intracanal E. faecalis populations: An in vitro study. J ClinExp Dent 2016; 8: e230-235.
- Gupta D, Jain A. Effect of cinnamon extract and chlorhexidinegluconate (0.2%) on the clinical level of dental plaque and gingival health: A 4-week, triple-blind randomized controlled trial. J IntAcadPeriodontol 2015; 17: 91-98.
- Al-Radha AS, Younes C, Diab BS, Jenkinson HF. Essential oils and zirconia dental implant materials. Int J Oral Maxillofac Implants 2013; 28: 1497-1505.
- de Camargo Smolarek P, Esmerino LA, Chibinski AC, Bortoluzzi MC, Dos Santos EB. In vitro antimicrobial evaluation of toothpastes with natural compounds. Eur J Dent 2015; 9: 580-586.
- Zhu M, Carvalho R, Scher A, Wu CD. Short-term germ-killing effect of sugar-sweetened cinnamon chewing gum on salivary anaerobes associated with halitosis. J Clin Dent 2011; 22: 23-26.
- Choung MG, Nam SH, Kim HY. The antibacterial effect of Camellia japonica extract on oral microorganisms. Int J Applied Eng Res 2015; 10: 33668-33670.
- Cho MJ, Lee HN, Kim EM. Antibacterial activity and artificial plaque formation inhibition effects of herbal extracts. J Korean Acad Dent HygEdu 2005; 5: 123-130.
- Almeida Lde F, Paula JF, Almeida RV, Williams DW, Hebling J. Efficacy of citronella and cinnamon essential oils on Candida albicans biofilms. ActaOdontolScand 2016; 74: 393-398.
- Cho HD, Koh YJ, Choi IW, Kim YS, Park YK. Anti-cariogenic activity and glucosyltransferase inhibitory effects of extracts from pine needle and twig. Korean J Food SciTechnol 2007; 39: 336-341.
- Oliveirajde A, da Silva IC, Trindade LA, Lima EO, Carlo HL, Cavalcanti AL. Safety and tolerability of essential oil from Cinnamomumzeylanicumblume leaves with action on oral candidosis and its effect on the physical properties of the acrylic resin. EvidCompl Alt Med eCAM 2014; 2014: 325670.
- Abbaszadegan A, Dadolahi S, Gholami A, Moein MR, Hamedani S, Ghasemi Y. Antimicrobial and cytotoxic activity of Cinnamomumzeylanicum, calcium hydroxide, and triple antibiotic paste as root canal dressing materials. J Contemp Dent Pract 2016; 17: 105-113.
- Endo H, Rees TD. Clinical features of cinnamon-induced contact stomatitis. CompendContinEduc Dent 2006; 27: 403-409.
- Miller RL, Gould AR, Bernstein ML. Cinnamon-induced stomatitis venenata, Clinical and characteristic histopathologic features. Oral Surg Oral Med Oral Pathol 1992; 73: 708-716.
- Allen CM, Blozis GG. Oral mucosal reactions to cinnamon-flavoured chewing gum. J Am Dent Assoc 1988; 116: 664-667.
- Cohen DM, Bhattacharyya I. Cinnamon-induced oral erythema multiforme like sensitivity reaction. J Am Dent Assoc 2000; 131: 929-934.