Research Article - Biomedical Research (2017) Volume 28, Issue 1
In vitro anticancer effect of hypocrellin B in SiHa human cervical cancer cells through the caspases
Hongli Guo1#, Jianghua Li2#,*, Zi Yin11Department of Pathology, HuBei University of Medicine, Shiyan 442000, Hubei, PR China
2Department of burns and plastic Surgery, Shiyan Taihe Hospital, Shiyan 442000, Hubei, PR China
#Hongli Guo and Jianghua Li contributed equally to this work
Accepted date: June 13, 2016
Abstract
The aim of this study was to determine the in vitro anticancer effects of hypocrellin B (HB) in SiHa human cervical cancer cells by MTT, flow cytometry and RT-PCR assay, as well as to explain the mechanism of this effect through the caspases expression changes by HB. The SiHa cells were treated with 1 and 2 μg/mL HB and meanwhile these cells were also irradiated by the light (LED: light-emitting diodes). The results showed that HB could reduce the growth of SiHa cells, and LED irradiation increased the inhibitory effects. Treatment of 2 μg/mL HB could induce apoptosis in cancer cells (sub-G1 DNA content) at 32.8%, and after LED irradiation, the apoptotic cancer cells raised to 46.8%. The 2 μg/mL HB+LED treated SiHa cells had the highest caspase-3 activity, with 4.61 ± 0.21-fold to control cells. Both LED and 2 μg/mL hypocrellin treated SiHa cells had the strongest caspase-3, caspase-8, caspase-9, Bax, Fas/FasL, TRAIL, DR4, DR5, ROS and weakest Bcl-2, Bcl-xL mRNA expressions. These results proved that HB had a good in vitro anticancer effect, and LED irradiation could raise its anticancer effect, this effect might be derived from the influence of caspases and caspases related gene expressions by HB. HB could be used as a good cancer treatment drug.
Keywords
Hypocrellin B, SiHa human cervical cancer cells, Caspase, Cancer, Gene.
Introduction
Hypocrella bambusae mainly grows in northwest Yunan of China. It is a fungus of Hypocrella bambusesae (B. et. Br) Sacc growing in the alpine region at an altitude of 3000~3500 meters [1]. Hypocrellin B (HB) is a new type of photosensitizer characterized by high yield, definite structure, low toxicity and rapid metabolism. The research in recent years shows it has a lot of good photo sensitivities. Its heat stability also is good. In addition, it can kill cancer cell and suppress HIV virus effectively. The effect of HB for the photosensitive damage of ascetic liver cancer cells is quite obvious [2].
Apoptosis is a procedural biochemical process of cell selfdestruction, which needs 30~60 minutes to completion. It is an important biological phenomenon in multicellular organisms, appearing not only in ontogenetic process but also in normal physiology or disease. Under some physiological or pathological conditions, cells will follow its own course to terminate its own life. At last, cells exfoliate in vitro or disintegrate as some apoptosis body to be devoured by other cells. Generally, apoptosis works by the method that protease caspases mediate protein to disintegrate, which will make cell DNA to break, chromatin to condensate, cell to shrink, mitochondria to swell and apoptosis body to form [3]. In these processes, caspase protease plays an important role. Cell apoptosis generates apoptotic signal inducing from the complicated interaction of internal and external multiple factor. Then the signal is transferred to caspase. The external harmful stimulus signal or death signal are processed by death receptors and mitochondria to determine whether apoptosis pathway is started or not. It starts with caspase activation and finishes with protein substrate cleavage and cytoclasis so that cell apoptosis can be finished [4].
Photodynamic therapy (PDT) combining traditional Chinese medicine to cure cancer has become the top news in recent years, which has been paid more attention by the international biology and medicine [5,6]. Taking advantage of optical excitation to selectively accumulate the photosensitizer in tumor tissue [7]. Then, making photosensitizer to react and generate large amount of ROS so that selectively inducing for apoptosis and necrosis of tumor cell apoptosis . Meanwhile, ROS in cells can cause cell apoptosis [8]. ROS from photosensitizer can increase the activity of caspase-3 and caspase-9 to improve the inducing effect of cancerous cells apoptosis [9].
Through the experimental method of extracorporeal cell, this paper has discussed the lethal effect of HB for cancer cell under the excitation action of photodynamic PDT. Meanwhile, it further clarifies the mechanism of action that HB adjusts and controls the caspase-related gene and protein under the excitation action of photodynamic PDT to cause the apoptosis of cancer cell and kill cancer cell. The purpose of this experiment and mechanism is to provide the new idea and method for the treatment of surgical oncology.
Materials and Methods
Cell line
SiHa Human cervical cancer cells were purchased from Conservation Genetics CAS Kunming Cell Bank (Kunming, Guangxi, China). The human cervical cancer (SiHa) cells were sustained into RPMI1640 medium (Gibco BRL, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Gibco Co., Birmingham, MI, USA) and cultivated in a 75 cm2 tissue culture flasks under a humidified atmosphere containing 5% CO2 at 37°C. The medium was changed 2-3 times a week, 6-7 d subculture done time.
MTT assay
The growth inhibitory effects were determined by MTT assay. After the SiHa cells were attached, HB was added according to 20 μL per well, the cells were cultured for 24 h. Then the cells were given LED treatment (IR3C08N SOP24 LED multi light source laser instrument, Shenzhen Xinfengshengtai Electronic company, Shenzhen, Guangdong, China), and the light energy was precisely adjusted to 4 J/cm2 at 470 nm by a high energy UV integrating radiometer (EIT UV power puck II, EIT Inc, Sterling, VA, USA) for 24 h, every well were added into 20 μL MTT reagent (Amresco, Solon, OH, USA) with mass concentration of 5 mg/mL and after cultured for another 4 h, supernatant fluid was absorbed and left in each well and DMSO was added according to 150 μL per well and oscillated for 30 min, then determining OD value of each hole and calculating cell proliferation inhibition rate by using enzymelabeled instrument under 540 nm wavelength (model 680, Bio- Rad, Hercules, CA, USA) [10].
Flow cytometry
The apoptotic in SiHa cancer cells (sub-G1 DNA content) were determined by flow cytometry. The cells were treated as the same as above MTT assay treatment for first 48 h, then after the SiHa 24 h cultured by dbcAMP treatment, cancer cells were added with 0.25% pancreatic enzymes to digest and disperse cells. After cells fell off, they were added into the medium with serum to neutralize pancreatic enzymes. Then after 1,500 xg centrifugal for 5 min, supernatant fluid was abandoned and cell precipitation was collected and cleaned with PBS, then 1 mL of 75% ethanol was added to fix cells, and kept under 4oC for whole night. The concentration of cancer cells was adjusted to 5 × 105/mL and the cells were washed with PBS for 3 times, then adding 500 μL PBS containing 50 μg/mL ethidium bromide (EB, ), 100 μg/mL RNase and 0.2% Triton X-100 (CycleTEST™ PLUS kit; Becton Dickinson, Franklin Lakes, NJ, USA) to incubate for 30min under 4oC without light and detecting flow cytometry instrument [11].
Caspase-3 activity determination
Caspase-3 is widely known as a marker of apoptosis and it plays a critical role in apoptosis as an executioner caspase which carries out the mass proteolysis that leads to apoptosis, the caspase-3 activity in cancer cells was determined by the experiment kit. The caspase-3 activity of cells was determined at 360/465 nm using fluorescent photometer with the kit (Clontech Laboratories, Inc., Mountain View, CA, USA) according to manufacturer’s protocol.
RT-PCR assay
The mRNA expression in cancer cells was determined by RTPCR assay. Different groups of cells were collected, and total RNA was isolated by Trizol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized by reverse transcription kit (GE Healthcare, Little Chalfont, and United Kingdom). PCR amplification was modeled with cDNA and took GAPDH as reference gene. The reaction conditions were 50oC for 2 min, 95oC for 30 s, 95oC for 5 s, 60oC for 34 s, for 40 cycles [12].
Statistical analysis
The experiment data were presented as mean ± standard deviation (SD). The significant difference (p<0.05) of different groups were determined by Duncan's multiple range test using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
The growth of hypocrellin B in SiHa human cervical cancer cells
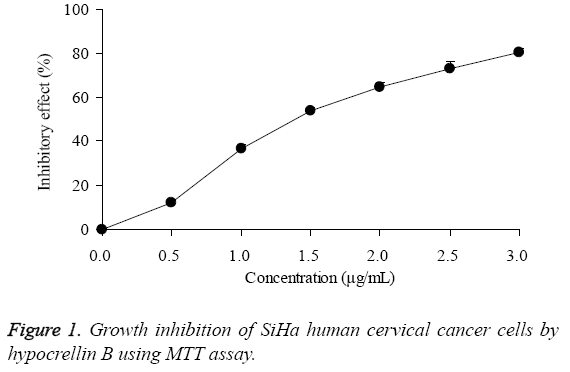
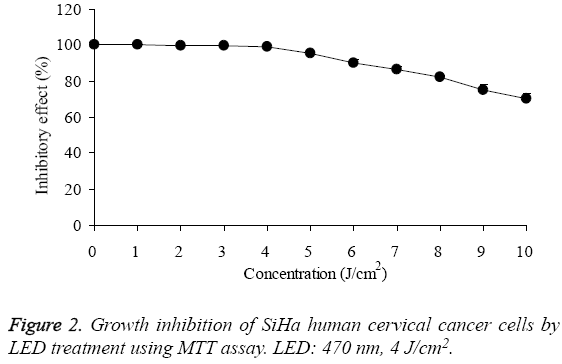
By MTT assay, the growth of SiHa human cervical cancer cells were decreased by hypocrellin B treatment (Figure 1), the IC50 was 1.49 μg/mL. As for the 0-4 J/cm2 of LED treatment, it had no effects on growth of SiHa human cervical cancer cells, under these concentrations, the growth of SiHa human cervical cancer cells were almost 100% (Figure 2).
The concentration of 1 and 2 μg/mL hypocrellin B, and 0-4 J/cm2 LED were chosen for the further experiment. The untreated SiHa cells had the highest OD value (Table 1), after treatment with HB or HB + LED, the OD values were decreased, 1 and 2 μg/mL HB showed 36.7% and 64.7% inhibitory effects. After adding LED treatment, the inhibitory effects of 1 and 2 μg/mL HB treatments were increased to 50.1% and 80.3%.
| Treatment | OD540 value | Inhibitory rate (%) |
|---|---|---|
| Control | 0.451±0.003a | / |
| HB (1 μg/mL) | 0.367±0.010b | 36.7±1.9D |
| HB (1 μg/mL) + LED | 0.225±0.011c | 50.1±1.9C |
| HB (2 μg/mL) | 0.159±0.011d | 64.7±2.2B |
| HB (2 μg/mL) + LED | 0.089±0.011e | 80.3±1.9A |
Table 1: Growth inhibition of SiHa human cervical cancer cells by hypocrellin B by an MTT assay.
DNA content of sub-G1 SiHa human cervical cancer cells
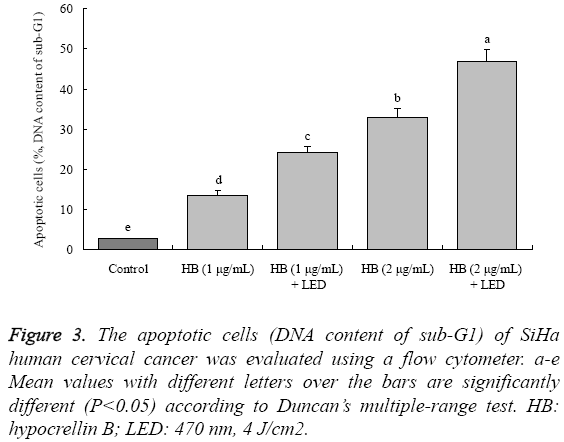
After SiHa cells treatment with HB, the apoptotic cells (sub-G1 DNA content, %) were increased compared to the control cells (2.6 ± 0.3%) (Figure 3). The 1 μg/mL HB had 13.5 ± 1.2% apoptotic cells, and 2 μg/mL HB had the higher apoptotic cells at 32.8 ± 2.2%. Treated with LED, the apoptotic cells were raised, 1 μg/mL HB+LED and 2 μg/mL HB+LED had 24.1% and 46.8% apoptotic cells, respectively.
Caspase-3 activity of sub-G1 SiHa human cervical cancer cells
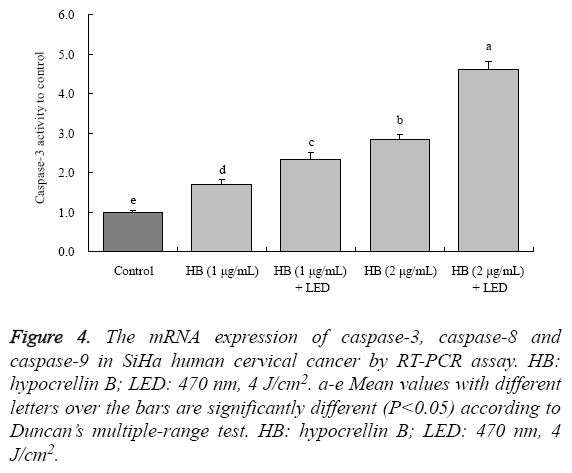
By experiment kit determination, the drug treated cells had the higher caspase-3 activities than control cancer cells (Figure 4). The caspase-3 activities of 1 μg/mL HB, 1 μg/mL HB + LED, 2 μg/mL HB and 2 μg/mL HB + LED treated cells were 1.70 ± 0.12, 2.32 ± 0.17, 2.84 ± 0.14 and 4.61 ± 0.21-fold to control cells, respectively.
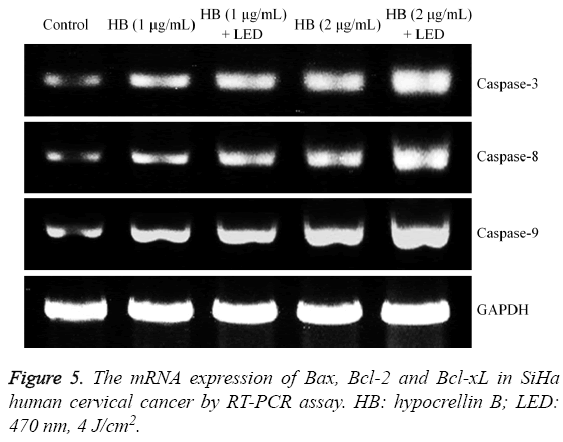
Figure 4: The mRNA expression of caspase-3, caspase-8 and caspase-9 in SiHa human cervical cancer by RT-PCR assay. HB: hypocrellin B; LED: 470 nm, 4 J/cm2. a-e Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan’s multiple-range test. HB: hypocrellin B; LED: 470 nm, 4 J/cm2.
Caspase-3, caspase-8 and caspase-9 mRNA expression in SiHa human cervical cancer cells
By RT-PCR assay, HB could increase the caspase-3, caspase-8 and caspase-9 mRNA expression in SiHa cells (Figure 4). Adding LED treatment showed stronger expressions than with only HB treatment, and high concentration of HB had the stronger expressions than low concentration treatment.
Bax, Bcl-2 and Bcl-xL mRNA expression in SiHa human cervical cancer cells
The Bax expression in untreated SiHa cells was weakest, but Bcl-2 and Bcl-xL expression were strongest (Figure 5). HB (2 μg/mL) + LED treated cells had the stronger Bax expression and weaker Bcl-2 and Bcl-xL expression than HB (1 μg/mL) +LED, 1 and 2 μg/mL HB treated cells.
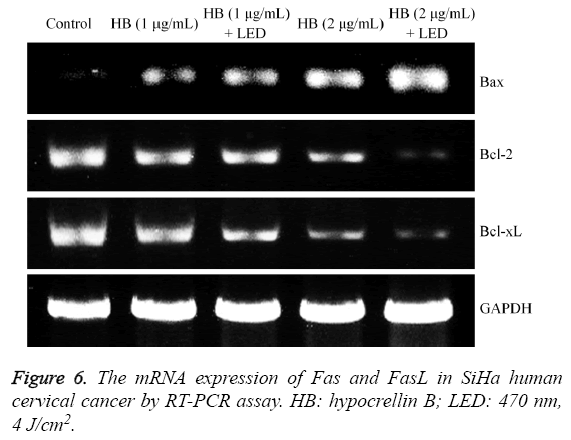
Fas and FasL mRNA expression in SiHa human cervical cancer cells
Fas and FasL expression were raised after HB treatment compared to the control cells (Figure 6), and the 2 μg/mL HB treated cells had higher Fas and FasL expression than 1 μg/mL HB treated cells. After adding the LED treatment, these expressions were increased compared to only HB treatment.
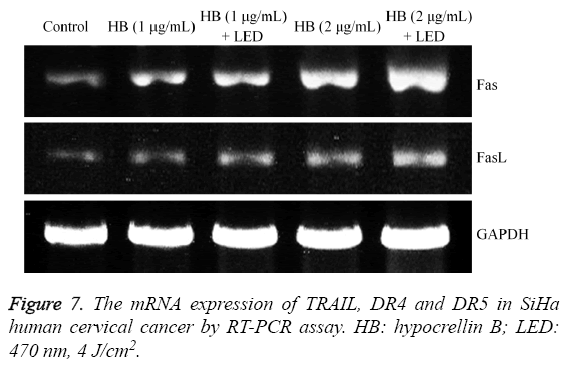
TRAIL, DR4 and DR5 mRNA expression in SiHa human cervical cancer cells
The HB treatment could raise TRAIL, DR4 and DR5 mRNA expression in SiHa cells compared to the control cells (Figure 7). High concentration HB (2 μg/mL) showed the stronger TRAIL, DR4 and DR5 mRNA expression than low concentration HB (1 μg/mL). Both HB and LED treatment can make the cells have stronger TRAIL, DR4 and DR5 expression than only treated with the same concentration of HB.
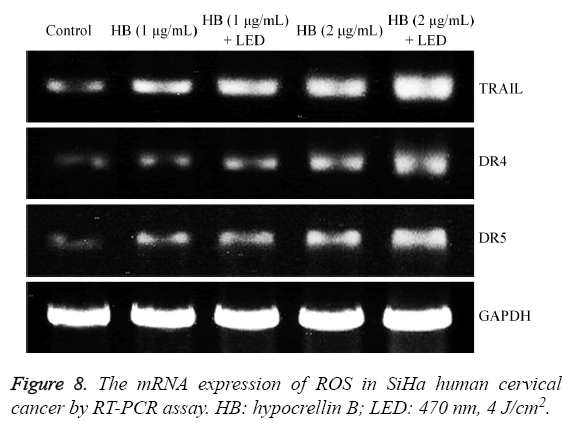
ROS mRNA expression in SiHa human cervical cancer cells
The ROS mRNA expression of SiHa human cervical cancer cells without drug treatment was weakest (Figure 8), after treatment with HB, the expressions were increased, the higher HB could make the ROS expression stronger, and both HB and LED treated SiHa cells had the stronger expressions than with only HB treated cells.
Discussion
At present, cell apoptosis is taken as a death process through a series of cell initiated cascade-activity, after cells are stimulated by various death signals. Caspase plays a key role in the molecular mechanism inducing cell apoptosis [13]. It is the rendezvous point of multiple apoptosis pathways. It is the final pathway to implement apoptosis. Most members of caspase are the promoter or effector of apoptosis, who play an important role in the process of cell apoptosis [14]. According to different position and function of caspase at the upstream and downstream of cascade reaction, it can be classified into three. First one is the promoter of apoptosis, located at the upstream of cascade reaction, including caspase-2, caspase-8, caspase-9 and caspase-10 etc., who can activate themselves and activate the caspase of downstream under the participation of other protein [15]. The second one is the effector of apoptosis, located at the downstream of cascade reaction, including caspase-3, caspase-6and caspase-7. They can be activated by the upstream promoter. The caspase after activation is used for substrate specificity to make cell to realize biochemical reaction and morphological changes. Then cell apoptosis is generated. The last one includes caspase-1, caspase-4, caspase-5, caspase-13 and caspase-14, who mainly participate in the mediated inflammatory reaction of cell factor [16]. They also play the supporting role in the apoptosis pathway of cell mediated by death receptors. But caspase-3 plays the key role in this pathway, which is taken as the key protease of apoptosis. Once activated, the cascade reaction of downstream will occur immediately to make apoptosis unavoidable. Consequently, caspase-3 is known as “death protease” [17]. Under normal condition, caspase-3 in cytoplasm exists in the form of inactive enzyme archetype. The appearing of cell apoptosis signal may result in the cleavage and then activation of caspase-3 under the function of multiple proteolytic enzymes. Caspase-3 is the proteasome system that directly results in the disintegration of apoptotic cell. It plays the core role in the mechanism network of cell apoptosis. Till now, great deals of researches have suggested that caspase-3 is a key protease in the apoptosis of mammalian cell. It makes the cell to disintegrate after activation [18].
The apoptosis pathway related to caspase includes mitochondria/cytochrome C pathway, death receptors pathway and endoplasmic reticulum pathway. In mitochondria/ cytochrome C pathway, after the inhibitor of zymolytic inactivated apoptosis, Bcl-2 is enzymolysised and inactivated, the function and form of cell will change, which is reflected by cell shrinkage, separation with adjacent cells, aggregation of chromatin and nuclear fragmentation [19]. Members of Bcl-2 regulate the activity of Apaf-1. Bcl-xL can inhibit the interaction of Apaf-1/caspase-9 so as to hinder the activation of caspase-9. Bik can antagonize the function of Bcl-xL [20]. The proportion of Bcl-2/Bax protein in cell is one of important factors to determine the sensitivity of cell apoptosis. When Bcl-2 is higher than Bax, cell is not sensitive to apoptosis [21]. Otherwise, it is easy for cell to generate apoptosis. Death receptors pathway includes Fas, DR4 and DR5 etc. Fas, FADD and caspase-8 have formed death-inducing signaling complex (DISC). The ligand of Fas molecule is FasL, which works through the death receptors of membrane. Caspase can be activated by the external factor of cell such as FasL or Fas antibody [22]. The combination of ADD and DED of recruitment procaspase-8 forms the signaling complex to make procaspase-8 to hydrolyze by itself and activate. Then the active caspase-8 is formed. The activated caspase-8 activates the downstream caspase to cause cell apoptosis. Caspase-8 also can cut the cytoplasmic Bcl-2 member, Bid precursor to form the truncated BidtBid while it can activate caspase-3 directly. The activated tBid is translocated to mitochondria to trigger the homologous and oligomeric function of Bak and Bax [23]. Then start the release of cytochrome C. Consequently, Bid transfers the apoptosis signal to the mitochondrial pathway from death receptors pathway. It also combines the mitochondrial pathway with the death receptors pathway, which amplifies the apoptosis signal effectively. Bcl-2 protein is the antagonism for caspase-3 [24].
DR4 and DR5 have the similar structure and function with Fas. Cell apoptosis is mediated through their ligands, TRAIL/ Apo2L. Signaling pathway also is similar with Fas pathway [25]. Both of them need to recruit FADD and activate caspase. However, the distribution of their ligands is different. Fas ligand is only limited to the activated T cell and NK cell as well as immunologically privileged sites. In many tissues, the expression of mRNA of TRAIL is constructive [26].
Photosensitizer could raise ROS level in cancer cells, and the high level ROS could make strengthen the cancer cells apoptotic inducing effect, increasing ROS level could also increase caspase-3 induced apoptosis effect [8,9]. ROS might be an important factor for raising caspase-3 activity.
In conclusion, through experiment and research, we found that the combination of HB and PDT can obviously enhance their functions for inhibiting cancer cell. The caspase-3 may be a marker of the action by HB and PDT treatment, HB and PDT can induce apoptosis through the change activity of caspase-3. Meanwhile, their combination also can induce the apoptosis of cancer cell and kill cancer cell finally. Consequently, it suggests us that the application of combined treatment by photodynamic medicine HB and PDT could become one effective treatment method in the treatment of surgical oncology. It has unique advantage and prospect. In addition, its mechanism of action may be originated from its regulating effect for the caspase-related gene. The mechanism can provide the theoretical support for in-depth use of HB.
References
- Xu H, Fang WS, Chen XG, He WY, Cheng KD. Cytochalasin D from Hypocrellabambusae. J Asian Nat Prod Res 2001;3:151-155.
- Wang X, Luo J, Leung AW, Li Y, Zhang H, Xu C.Hypocrellin B in hepatocellular carcinoma cells: Subcellular localization and sonodynamic damage.Int J RadiatBiol 2015;91:399-406.
- Jeong CH, Joo SH.Downregulation of reactive oxygen species in apoptosis.J Cancer Prev 2016;21:13-20.
- Shang LH, Yu Y, Che DH, Pan B, Jin S, Zou XL.LuffaechinataRoxbinduced apoptosis in human colon cancer cell (SW-480) in the caspase-dependent manner and through a mitochondrial apoptosis pathway.Pharmacogn Mag 2016;12:25-30.
- Lin S, Lei K, Du W, Yang L, Shi H, Gao Y, Yin P, Liang X, Liu J.Enhancement of oxaliplatin sensitivity in human colorectal cancer by hypericin mediated photodynamic therapy via ROS-related mechanism.Int J Biochem Cell Biol 2016;71:24-34.
- Jiang Y, Leung AW, Wang X, Zhang H, Xu C.Inactivation of Staphylococcus aureus by photodynamic action of hypocrellin B.Photodiagnosis Photodynamic Therapy 2013;10:600-606.
- Li W, Tan G, Cheng J, Zhao L, Wang Z, Jin Y.A novel photosensitizer 3¹,13¹-phenylhydrazine -Mppa (BPHM) and it’s in vitro photodynamic therapy against HeLa cells.Molecules 2016;21: E558.
- Wachowska M, Muchowicz A, Demkow U.Immunological aspects of antitumor photodynamic therapy outcome.Cent Eur J Immunol 2015;40:481-485.
- Zhu X, Wang H, Zheng L, Zhong Z, Li X, Zhao J, Kou J, Jiang Y, Zheng X, Liu Z, Li H, Cao W, Tian Y, Wang Y, Yang L.Upconversion nanoparticle-mediated photodynamic therapy induces THP-1 macrophage apoptosis via ROS bursts and activation of the mitochondrial caspase pathway.Int J Nanomedicine 2015;10:3719-3736.
- Zhao X, Wang Q, Li GJ, Chen F, Qian Y, Wang R. In vitro antioxidant, anti-mutagenic, anti-cancer and anti-angiogenic effects of Chinese Bowl tea. J Funct Food 2014; 7: 590-598.
- Zhao X, Qian Y, Zhou YL, Wang R, Wang Q, Li GJ. Pu-erh tea has in vitro anticancer activity in TCA8113 cells and preventive effects on buccal mucosa cancer in U14 cells injected mice in vivo. Nutr Cancer An Int J 2014; 66: 1059-1069.
- Zhang J, Zhang G, Yang S, Qiao J, Li T, Yang S, Hong Y.Macrophage migration inhibitory factor regulating the expression of VEGF-C through MAPK signal pathways in breast cancer MCF-7 cell.World J SurgOncol 2016; 14:51.
- Hsieh MJ, Chen MK, Chen CJ, Hsieh MC, Lo YS, Chuang YC, Chiou HL, Yang SF.Glabridin induces apoptosis and autophagy through JNK1/2 pathway in human hepatoma cells.Phytomedicine 2016;23:359-366.
- Afsar T, Trembley JH, Salomon CE, Razak S, Khan MR, Ahmed K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways.Sci Rep 2016;6:23077.
- Ekici OD, Li ZZ, Campbell AJ, James KE, Asgian JL, Mikolajczyk J, Salvesen GS, Ganesan R, Jelakovic S, Grütter MG, Powers JC.Design, synthesis, and evaluation of aza-peptide Michael acceptors as selective and potent inhibitors of caspases-2, -3, -6, -7, -8, -9, and -10.J Med Chem 2006;49:5728-5749.
- Humke EW, Ni J, Dixit VM.ERICE, a novel FLICE-activatablecaspase.J BiolChem 1998;273:15702-15707.
- Wier EM, Fu K, Hodgson A, Sun X, Wan F.Caspase-3 cleaved p65 fragment dampens NF-κB-mediated anti-apoptotic transcription by interfering with the p65/RPS3 interaction.FEBS Lett 2015;589:3581-3587.
- Ding AX, Sun G, Argaw YG, Wong JO, Easwaran S, Montell DJ.CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo.Elife 2016;5: e10936.
- Shao Y, Gao Z, Marks PA, Jiang X.Apoptotic and autophagic cell death induced by histone deacetylase inhibitors.ProcNatlAcadSci U S A 2004;101:18030-18035.
- Newmeyer DD, Bossy-Wetzel E, Kluck RM, Wolf BB, Beere HM, Green DR.Bcl-xL does not inhibit the function of Apaf-1.Cell Death Differ 2000;7:402-407.
- Sadek K, Abouzed T, Nasr S.Lycopene modulates cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in a rat model.Can J PhysiolPharmacol 2016;94:394-401.
- Galski H, Oved-Gelber T, Simanovsky M, Lazarovici P, Gottesman MM, Nagler A.P-glycoprotein-dependent resistance of cancer cells toward the extrinsic TRAIL apoptosis signaling pathway.BiochemPharmacol 2013;86:584-596.
- Schwarzer C, Fu Z, Shuai S, Babbar S, Zhao G, Li C, Machen TE.Pseudomonas aeruginosahomoserine lactone triggers apoptosis and Bak/Bax-independent release of mitochondrial cytochrome C in fibroblasts.Cell Microbiol 2014;16:1094-1104.
- Gao CK, Liu H, Cui CJ, Liang ZG, Yao H, Tian Y. Roles of MicroRNA-195 in cardiomyocyte apoptosis induced by myocardial ischemia-reperfusion injury.J Genet 2016;95:99-108.
- Jazirehi AR, Arle D.Epigenetic regulation of the TRAIL/Apo2L apoptotic pathway by histone deacetylase inhibitors: an attractive approach to bypass melanoma immunotherapy resistance.Am J ClinExpImmunol 2013;2:55-74.
- Amarante-Mendes GP, Griffith TS.Therapeutic applications of TRAIL receptor agonists in cancer and beyond.PharmacolTher 2015;155:117-131.