Research Article - Biomedical Research (2017) Volume 28, Issue 10
Importance of p53, bcl-2, p21WAF1 and PCNA positivities in renal angiomyolipomas
Metin Budak1*, Omer Yalcin2, Ufuk Usta3 and Burcu Tokuc4
1Department of Biophysics, Trakya University Faculty of Medicine, Edirne, Turkey
2Department of Pathology, Bursa Higher Specialization Training and Research Hospital, Bursa, Turkey
3Department of Pathology, Trakya University Faculty of Medicine, Edirne, Turkey
4Department of Public Health, Trakya University Faculty of Medicine, Edirne, Turkey
- *Corresponding Author:
- Metin Budak
Department of Biophysics
Trakya University Faculty of Medicine, Edirne, Turkey
Accepted on March 09, 2017
Abstract
Angiomyolipomas are tumors of the kidneys which are often benign in character with a potential for malignant transformation. The benign-malign distinction in these tumors can be made only with clinical follow-up as they exhibit the same microscopic appearance. In the present study, immunohistochemical investigation has been performed for p53, p21WAF1, PCNA and bcl-2, which may be markers for the histological distinction of benign and malignant cases. The p53, p21WAF1, PCNA and bcl-2 investigations in 10 tumor tissues with AML revealed significantly higher p53 positivity in 3 patients. The clinical follow-up of these three patients showed malignant progression. In conclusion, we believe that p53 positivity may be an appropriate marker for the benign-malign distinction in AML tumors.
Keywords
Angiomyolipomas, p53, Renal.
Introduction
Angiomyolipoma (AML) is a tumor which usually exhibits benign behaviour. Although it is often detected in the kidney, it may also be located in other tissues such as the liver, lungs, lymph nodes, and retroperitoneal soft tissue [1].
Renal AML is a rare tumor often diagnosed incidentally due to its asymptomatic course. However, it may be associated with hyperthermia, hypertension, retroperitoneal haemorrhage, lumbar pain and mass effect in some cases. It is more frequently reported in females and usually diagnosed in the 2nd-6th decades of life. While often unilateral and solitary, bilateral and multiple AMLs have also been reported. Approximately one third of patients with renal AML suffer from tuberous sclerosis, with a higher incidence in the event of multiple or bilateral tumors. Approximately 80% of the patients with tuberous sclerosis are estimated to have renal AML [2,3].
Although nearly all AMLs behave in a benign fashion, some may cause massive haemorrhage [4]. Bilateral and large AMLs may even lead to renal failure. Mortal invasions to adjacent organs have been reported rarely in AMLs although there are cases with concomitant tumors of distant organs and regional lymph nodes, which is termed as multiple tumors by several authors [3].
These tumors consist of three heterogeneous tissue components of smooth muscle cells, adipocytes and blood vessels with thick-walls and devoid of elastic lamina. The proportion and distribution of these components may vary from tumor to tumor and even from area to area in the same tumor; therefore, their gross appearance is also variable [2-4]. The literature contains reports of cases with AML misdiagnosed as renal cell carcinoma. In addition to the epithelioid and oncocytoma-like variants of AMLs, malignant transformation has also been described in this phenomenon, although rarely [5-7]. Hypercellularity, pleomorphism and mitosis may lead to misdiagnosis of a malignant tumor [2]. Still, these tumors have been often analyzed immunohistochemically and sometimes, genetically. To date, only a few studies have been conducted with immunohistochemical analysis for p53, p21WAF1, PCNA and bcl-2 in AMLs [6,8].
Materials and Methods
We retrospectively investigated formalin-fixed, paraffinembedded tumoral samples of 10 renal AML cases diagnosed during the last ten years at the Pathology Department of Trakya University Faculty of Medicine. Sections of 4 μm in thickness were stained with Hematoxylin-Eosin and re-examined with light microscope. Epithelioid cells, giant cells, atypical cells and atypical mitosis were graded as 0 (not detected), 1 (detected only by rigorous examination), 2 (between 1 and 3) and 3 (easily detected). Subsequently, the sections were stained histochemically with Masson’s-trichrome and periodic acid- Schiff (PAS) and immunohistochemically for pan-cytokeratin, actin, desmin, vimentin and HMB-45. Selected sections, particularly those containing at least one of the criteria, i.e., epithelioid cell, giant cell, atypical cell or atypical mitosis, were stained immunohistochemically for p53, p21WAF1, PCNA and bcl-2. Positivity for p53 was graded as per the aforementioned method. After grading; Percentage values of PCNA, bcl-2 and p21WAF1 were measured with Zeiss Axioplan 2 imaging light microscope and KS 300 Imaging System.
Hydrogen-peroxidase and avidin-biotin method was used for the immunohistochemical staining of the samples. The antibodies used in this method are listed in Table 1.
| Source | Code No | Type | |
|---|---|---|---|
| p53 | DAKO | N1581 | M |
| HMB-45 | DAKO | N1545 | M |
| Smooth muscle actin | NeoMarkers | MS-113-R7 | M |
| Desmin | DAKO | M1538 | M |
| Vimentin | NeoMarkers | MS-129-R7 | M |
| Pan-keratin | NeoMarkers | MS-343-R7 | M |
| bcl-2 | Novocastra | 802709 | M |
| Ki-67 | NeoMarkers | 9106R504A | M |
| p21WAF1 Ab-5 | NeoMarkers | MS-387-P | M |
| PCNA-Ab-1(Clone PC10) | NeoMarkers | MS-106-R7 | M |
| M: Monoclonal. | |||
Table 1. The antibodies used for immunohistochemistry staining.
Statistical analysis
Tumor size is expressed as mean ± SD. We used Fisher’s exact test to evaluate the relationship between necrosis or hemorrhage and presence of atypical cells. Mann–Whitney Utest was used for the other comparisons. p<0.05 was considered statistically significant. However, statistical analysis was not possible for some of the comparisons owing to the limited number of our cases.
Results
The study sample consisted of 10 cases between the ages of 23 to 74 years with eight female and two male subjects. Only one subject, a 23-year old female patient suffered from tuberous sclerosis. Furthermore, three patients had other concomitant malignancies; namely, squamous cell carcinoma of the bladder and renal cell carcinoma. All AMLs were unilateral (seven cases in left kidney and the other three in right kidney) (Table 2).
| Cases | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Gender | F | F | F | F | M | F | F | F | M | F |
| Age (Years) | 45 | 51 | 23 | 63 | 48 | 38 | 74 | 63 | 34 | 52 |
| Laterality | Right | Left | Left | Left | Right | Left | Left | Left | Left | Right |
| Tuberous Sclerosis | - | - | + | - | - | - | - | - | - | - |
| Second Tumor | - | - | - | - | - | - | +* | +** | - | +** |
| Diameter (cm) | 3 | 18 | 18 | 4.5 | 21 | 12 | 1.5 | 2 | 17 | 12 |
| Capsular Invasion | - | - | - | - | - | - | - | - | - | - |
| Hemorrhage, Necrosis | + | + | + | + | + | + | - | + | + | - |
| Dominant Smooth Muscle | - | + | + | + | - | - | - | - | - | - |
| Lymphatic Vessel Invasion | - | - | + | - | - | - | - | - | - | - |
| Epitheloid Cell | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | 2 |
| Giant Cell | 0 | 2 | 2 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Atypical Cell | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Atypical Mitosis | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Pan-Keratin | - | - | - | - | - | - | - | - | - | - |
| Vimentin | + | + | + | + | + | + | + | + | + | + |
| Desmin | - | + | + | - | - | - | - | - | - | - |
| Smooth Muscle Actin | + | + | + | + | + | + | + | + | + | + |
| HMB-45 | + | + | + | + | + | + | + | + | + | + |
| p53 | 0 | 3 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| p21WAF1 | 29% | 0% | 0% | 18% | 31% | 21% | 22% | 17% | 54% | 30% |
| PCNA | 18% | 58% | 5% | 7% | 6% | 15% | 66% | 3% | 15% | 60% |
| bcl-2 | 32% | 23% | 90% | 18% | 15% | 12% | 20% | 8% | 32% | 18% |
F: Female, M: Male. +*: Squamous cell carcinoma of bladder/+**: Clear cell variant of renal cell carcinoma. Grade 0: not found, grade 1: detected only by rigorous examination, grade 2: between 1 and 3, grade 3: easily detected. Percentage values of PCNA, bcl-2 and p21WAF1 were measured with Zeiss Axioplan 2 imaging light microscope and KS 300 Imaging System.
Table 2. Clinical and histopathological characteristics and p53, PCNA, bcl-2 and p21WAF1 positivity of the cases.
Average tumor size was 10.9 ± 7.6 (1.5-21) cm. Tumors were tan-yellow colored with distinct and almost regular margins. Some of the muscle-rich tumors exhibited focal whorled pattern. There was no capsular or perirenal invasion in any of the tissues. Tumoral tissues of eight cases contained necrotic and hemorrhagic areas. Statistical analysis for the comparison between necrosis-hemorrhage and tumor size could not be performed.
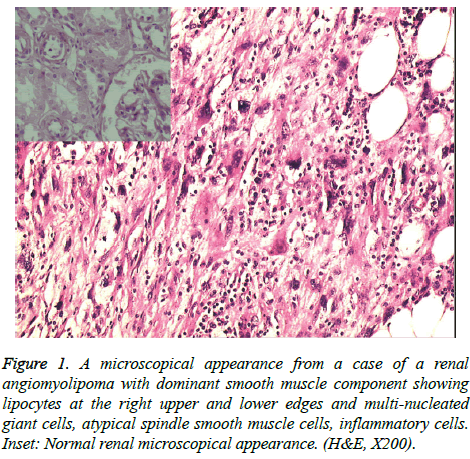
Smooth muscle component was more dominant in three cases compared to others (Figure 1) and lymphatic vessel invasion was detected in one of these three cases (Table 2).
Figure 1: A microscopical appearance from a case of a renal angiomyolipoma with dominant smooth muscle component showing lipocytes at the right upper and lower edges and multi-nucleated giant cells, atypical spindle smooth muscle cells, inflammatory cells. Inset: Normal renal microscopical appearance. (H&E, X200).
Epithelioid cells were detected easily in six tumors, whereas there were little or no epithelioid cells in the other four. While three cases had several multi-nucleated giant cells, they were found rarely in five others and the remaining two cases had no giant cells. In addition, atypical cells were obvious in only three tumors. However, there was no statistically significant relationship between the presence of atypical cells andhemorrhage-necrosis (p>0.05). Rare atypical mitotic figures were detected in tumoral tissues of six cases (Table 2).
PAS staining revealed rare intracytoplasmic crystals in all of the tumor tissues. Immunohistochemically, the spindle cells in all tissues exhibited positive cytoplasmic reaction for smooth muscle actin. However, desmin immunoreactivity was detected only in two cases. HMB-45 immunoreactivity was observed in some epithelioid and spindle cells. All tumor tissues were positive for vimentin and negative for pan-keratin (Table 2).
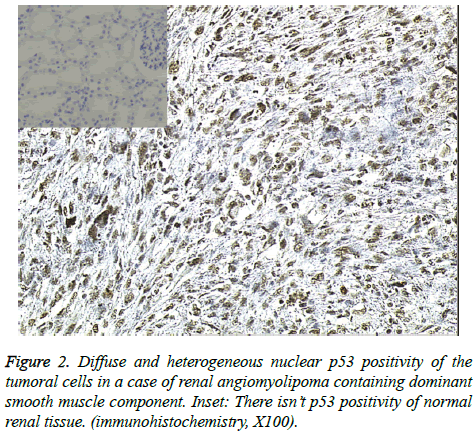
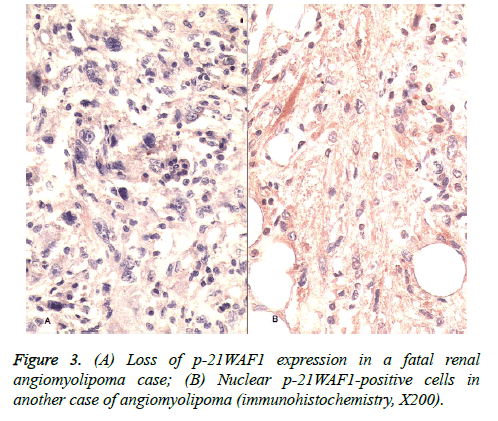
Immunohistochemical staining for p53 revealed no nuclear positivity in three cases while six cases showed rare and focal nuclear immunoreactivity in giant cells, particularly in atypical epithelioid cells. p53 showed diffuse nuclear reaction in one case in which smooth muscle component was dominant and atypical cells were quite prominent (Figure 2). A notable finding was a case with lymphatic vessel tumor invasion among the six cases which showed rare and focal p53 staining. There were two cases the first of which had a high PCNA positivity and loss of p-21WAF1 expression; however, p-21WAF1 expression varied from 17% to 54% in other cases (Figure 3) and the highest level of bcl-2 was detected only in the latter of these two cases. Overall, PCNA positivity ranged from 3% to 66% and bcl-2 expression varied from 8% to 90% in our study sample (Table 2).
It was not possible to perform a statistical analysis for the comparison between necrosis-hemorrhage and the values of p53, p21WAF1, PCNA and bcl-2. There was no statistically significant relationship between tumor size and the values of p53, p21WAF1, PCNA and bcl-2 (p>0.05).
Statistical analysis could not be performed for the comparison between dominant smooth muscle component and p53 value. However, Mann-Whitney U-test was performed for the statistical analysis of the comparison between dominant smooth muscle component and values of p21WAF1, PCNA and bcl-2. While there was a statistically significant association between dominant smooth muscle component and loss of p21WAF1 expression (p<0.05), this was not the case for the other two markers (p>0.05) (Table 3).
| Dominant smooth muscle content | p21WAF1 | PCNA | bcl-2 |
|---|---|---|---|
| +(n=3) | 0.0 (0.0-18.0) | 7.0 (5.0-58.0) | 23.0 (18.0-90.0) |
| -(n=7) | 29.0 (17.0-54.0) | 15.0 (3.0-66.0) | 18.0 (8.0-32.0) |
| P | 0.033 | 0.667 | 0.267 |
Table 3. Comparison of dominant smooth muscle content and expression values of PCNA, bcl-2 and p21WAF1 of the cases.
It was not possible to conduct a statistical analysis for the comparison between lymphatic vessel invasion and the values of p53, p21WAF1, PCNA and bcl-2. The limited number of samples also did not allow a statistical analysis for the comparison between the presence of epithelioid cells and p53 values. On the other hand, there was no statistically significant association between the presence of epithelioid cells and values of p21WAF1, PCNA and bcl-2 (p>0.05).
Statistical analysis could not be performed for the comparison between the presence of giant cells and values of p53, p21WAF1, PCNA and bcl-2.
Statistical analysis was also not possible for the comparison between the presence of atypical cells or atypical mitosis and p53 values. While there was a statistically significant association between the presence of atypical cells or atypical mitosis and loss of p21WAF1 expression (p<0.05), such a difference was not observed for the other two markers (p>0.05) (Tables 4 and 5).
| Atypical cells | p21WAF1 | PCNA | bcl-2 |
|---|---|---|---|
| 1 (n=7) | 29 (17-54) | 15.0 (3-66) | 18.0 (18-90) |
| 2 (n=3) | 0.0 (0-18) | 7.0 (5-58) | 23.0 (18-90) |
| P | 0.033 | 0.667 | 0.267 |
Table 4. Comparison of atypical cells and expression values of PCNA, bcl-2 and p21WAF1 of the cases.
| Atypical mitosis | p21WAF1 | PCNA | bcl-2 |
|---|---|---|---|
| 0 (n=4) | 30.5 (22-54) | 37.5 (6.0-66.0) | 19.0 (15-32) |
| 1 (n=6) | 17.5 (0-29) | 11.0 (3-58) | 20.5(8-90) |
| P | 0.019 | 0.257 | 1.000 |
Table 5. Comparison of atypical mitosis and expression values of PCNA, bcl-2 and p21WAF1 of the cases.
Discussion
Angiomyolipomas are benign renal tumors which may also be seen in other tissues or organs such as the liver, lymph nodes and retroperitoneal tissues (19.3%). Although very rarely, malignant differentiation has been reported in AMLs [5,6].
Renal AMLs often affect females and it is usually seen between the 2nd and 6th decades of life [2,3]. Most of the cases included in the present study were female subjects and their age distribution was as described above. Furthermore, one of our cases with tuberous sclerosis was younger compared to the other subjects.
Angiomyolipomas are composed of smooth muscle, adipose tissue and vascular structures. Their color and degree of density vary depending on the percentage of these components. The adipose tissue-rich angiomyolipomas may be misdiagnosed as well-differentiated liposarcomas, whereas the tumors rich in smooth muscle may be misdiagnosed as leiomyosarcomas [3]. Three of our cases had smooth musclerich tumors; and one of them had a nodule similar to leiomyoma. Furthermore, these three cases had highly limited amounts of adipose tissue and vascular structures.
Structurally, adipose tissue composed of mature fat cells is present in AMLs with infrequent fat cells similar to lipoblasts. The smooth muscle cell component displays a range from uniform, ordinary cells to epithelioid cell layers with eosinophilic, granular, wide cytoplasm. These smooth muscle cells originate from vascular walls, and the morphological features of vascular components tend to be variable. Thick and hyalinized vessels with an eccentric lumen may be seen in most cases. Tumors composed of predominantly spindleshaped smooth muscle cells often contain vessels with thin walls which resemble hemangiopericytoma. Pronounced epithelioid cells often surround vascular structures and provide particularly important diagnostic clue for adipose tissue-rich tumors. Mitosis is rare [2,3]. Epithelioid variant is dominantly or partially composed of polygonal cells with dense, eosinophilic cytoplasm. Various degrees of atypia may be seen in these cells which may also be multilobulated and multinucleated. Smooth muscle may be challenging for pathologists owing to the fact that it may display hypercellularity, significant pleomorphism and moderate mitotic activity. These features may lead to a misdiagnosis of leiomyosarcoma [2]. Masson’s-trichrome staining has shown that three of our cases had smooth muscle-rich tumors. The presence of atypical cells in three cases, epithelioid cells in two cases and atypical mitotic figures detected with difficulty in all three cases were important findings.
PAS-positive cytoplasmic granules and stick-shaped crystals are primarily seen in perivascular epithelioid cells of AMLs [3]. Immunohistochemical studies have reported that particularly epithelioid and spindle-shaped cells in tumors display positive reactivity for HMB-45, S-100 protein, smooth muscle actin, and vimentin with negative reactivity for epithelial markers such as cytokeratin [9,10]. On the other hand, there are also studies which report negativity for S-100 protein [1,11-13]. PAS-positive cytoplasmic crystals were observed on the slides of our cases, although rarely. The spindle-shaped cells of all cases were positive for smooth muscle actin, whereas only two cases showed cytoplasmic positivity for desmin. Some epithelioid and/or spindle-shaped cells exhibited HMB-45 immunoreactivity. There was no positivity with pan-keratin, whereas vimentin immunoreactivity was observed.
p53, which controls normal cell growth, is present in normal cells and it is accepted as a major tumor suppressor gene. The production of non-mutant p53 protein induces p21WAF1 protein, a cyclin-dependent kinase inhibitor. The inhibited kinase activity arrests G1, thereby stopping cell cycle progression, and it can also inhibit the activity of PCNA [14]. Contrary to this, mutant p53 protein may reduce the p21WAF1 protein and increase PCNA activity. PCNA is an essential protein for DNA replication and damage repair [15]. Some authors have reported that positivity for p53 and PCNA is associated with various malignant tumors [16,17]. p53 mutations are the most frequent genetic abnormalities encountered in cancers. These mutations display variations among different tumors. Moreover, they may be present with different mutation patterns in the same tumor depending on several etiologic factors. p53 may be inactivated by proteins which are in bound-form. Additionally, bcl-2 inhibits apoptosis. Therefore, the over expression of this protein may prevent apoptosis in damaged cells. This may lead to continued division of mutated cells lines, and eventually, cancer. However, we found a limited number of studies investigating p53 immunohistochemical staining for PCNA, p21WAF1 and bcl-2 in AMLs [6,18].
Distribution of nuclear p53 positivity was widespread in one of our cases with abundant smooth muscle content. The presence of giant cells, epithelioid cells and atypical cells in this case led us to considering malignant transformation. Although p53 positivity in AML has been scarcely encountered in recent studies, this p53 positive case led to a close clinical follow-up because of its suspicious malignant transformation. After radical nephrectomy, liver capsule invasion was present on radiological analysis. Despite medical oncologic therapy, the patient died at 6 months due to complications of tumoral recurrence.
Interestingly, squamous cell carcinoma of the bladder was present in one of the other two cases and renal cell carcinoma of the opposite kidney was observed in the other case in addition to AMLs of the kidney, and p53 was positive in the AML mass. While postoperative status of the former patient remains unknown, the latter patient is known to be alive.
Another interesting case in our study was the young female with tuberous sclerosis. We detected both lymphatic vessel invasion and local p53 positivity in her tumor. Postoperative status of this patient remains unknown.
A diagnosis of malignant transformation is quite difficult in AMLs. Presence of pleomorphism, giant cells and mitosis in AMLs may lead a pathologist to misdiagnose the entity as malignant. Even multiple tumoral masses often detected in tuberous sclerosis may be inaccurately diagnosed as metastasis. Also, p53 positivity may provoke a suspicion of malignant transformation in such cases and require clinical follow-up. However, the limited number of recent studies about p53 positivity in AMLs is currently unsatisfactory to draw a conclusion. Some authors have indicated no definite link between p53 abnormalities and the atypical appearance of AML [19]. In our study, it was not possible to conduct a statistical analysis for p53.
The bcl-2 protein and related cytoplasmic proteins are key regulators of apoptosis, the cell suicide program critical for development, tissue homeostasis, and protection against pathogens. The proteins most similar to bcl-2 promote cell survival by inhibiting the adapters needed for activation of proteases (caspases) which dismantle the cell. More distant relatives of this protein promote apoptosis instead, apparently through mechanisms which include displacing the adapters from pro-survival proteins. Thus, the balance between these competing activities determines cell fate for most, but not all, apoptotic signals. Members of the bcl-2 family are essential for the maintenance of major organ systems, and mutations affecting these proteins are implicated in cancer. Increased amounts of bcl-2 protein have been shown in several different types of cancers. Some authors have also demonstrated that bcl-2 positivity is localized around dysmorphic cells in epithelioid AML [8].
The highest positivity of bcl-2 seen in one of our cases suggested possibility of malignant transformation; however, the postoperative status of the patient in question remains unknown.
p21 expression has been investigated in various tumoral and non-tumoral tissues. In one study, authors showed that a reduction in the frequency of p21 expression was associated with colorectal neoplastic development in sporadic patients [14]. Similarly, we found loss of p21WAF1 expression in two cases, one of which had high PCNA positivity and resulted in a fatal outcome. PCNA is a homotrimeric ring-shaped protein which plays a fundamental role in DNA replication and repair, but is also involved in post-replication events such as DNA methylation, chromatin assembly and remodeling, and sister chromatid cohesion by coordinating these activities through cell cycle control. However, relevant aspects of the PCNA function remain poorly understood [20]. Some authors have showed that loss of p21 restores PCNA abundance and results in G1 checkpoint bypass [21]. On the other hand, the PCNAlabelling index and p53-positive cases have been significantly correlated with histological grading of malignancy in some studies [22].
Additionally, our study has shown statistically significant links not only between loss of p21WAF1 expression and atypical cells or atypical mitosis, which are malignancy criteria for different tumors, but also between loss of p21WAF1 expression and dominant smooth muscle component. The latter correlation supports that AML with dominant smooth muscle may have a predisposition to malignant transformation. Therefore, we found that loss of p21WAF1 expression may be an acceptable and strong marker for the malignancy in AMLs.
In conclusion, the presence of atypical cells and atypical mitosis in angiomyolipoma must alert the pathologist in terms of malignant transformation regardless of traditional principles and immunohistochemical staining must be performed for p53, p21WAF1, PCNA and bcl-2 during the investigation of such tissues.
Further studies with a larger number of patients, particularly those with malignant AMLs, may help elucidate the roles of p53, p21WAF1, PCNA and bcl-2 in the malignant transformation of AML. Other approaches such as comparative genome hybridization may also be useful to investigate the molecular pathogenesis of malignant AMLs.
References
- Ito M, Sugamura Y, Ikari H, Sekine I. Angiomyolipoma of the lung. Arch Pathol Lab Med 1998; 122: 1023-1025.
- Ordóñez NG, Rosai J. Anjiomyolipoma. In: Rosai J (ed) Rosai and Ackerman’s Surgical Pathology, Vol 1. Mosby Missouri 2004; 1266-1270.
- Reuter VE. Gaudin: Angiomyolipoma. In: Sternberg SS (eds) Diagnostic Surgical Pathology, Lippincott Williams & Wilkins, Philadelphia 1999; 1812-1816 .
- Nonomura A, Mizukami Y, Takayanagi N, Masuda S, Ishii K. Immunohistochemical study of hepatic angiomyolipoma. Pathol Int 1996; 46: 24-32.
- Cibas ES, Goss GA, Kulke MH, Demetri GD, Fletcher CD. Malignant epithelioid angiomyolipoma ('sarcoma ex angiomyolipoma') of the kidney: a case report and review of the literature. Am J Surg Pathol 2001; 25: 121-126.
- Kawaguchi K, Oda Y, Nakanishi K, Saito T, Tamiya S. Malignant transformation of renal angiomyolipoma: a case report. Am J Surg Pathol 2002; 26: 523-529.
- Ovcak Z, Zidar N, Masera A. Angiomyolipoma of the kidney--an immunohistochemical study. Acta Med Croatica 1999; 53: 111-114.
- Cho NH, Shim HS, Choi YD, Kim DS. Estrogen receptor is significantly associated with the epithelioid variants of renal angiomyolipoma: A clinicopathological and immunohistochemical study of 67 cases. Pathol Intern 2004; 54: 510-515.
- Barnard M, Lajoie G. Angiomyolipoma: immunohistochemical and ultrastructural study of 14 cases. Ultrastruct Pathol 2001; 25: 21-29.
- Lebe B, KoyuncuoÄŸlu M, Tuna B, Tuncer C. Epithelioid angiomyolipoma: a case report. Tumori 2001; 87: 196-199.
- Chandrasoma S, Moatamed N, Chang A, Daneshmand S, Ma Y. Angiomyolipoma of the Kidney: Expanding Disease Spectrum Demonstrated by 3 Cases. Case Report. Appl Immunohistochem & Mol Morphol 2004; 12: 277-283.
- Martignoni G, Pea M, Bonetti F, Brunelli M, Eble JN. Oncocytoma-like angiomyolipoma. A clinicopathologic and immunohistochemical study of 2 cases. Arch Pathol Lab Med 2002; 126: 610-612.
- Stone CH, Lee MW. Renal angiomyolipoma: Further immunophenotypic characterization of an expanding morphologic spectrum. Arch Pathol Lab Med 2001; 125: 751-758.
- Sinicrope FA, Roddey G, Lemoine M, Ruan S, Stephens LC, Frazier ML. Loss of i21WAF1/CiP1 Protein Expression Accompanies Progression of Sporadic Colorectal Neoplasms but not Hereditary Nonpolyposis Colorectal Cancers. Clinical Cancer Research 1998; 4: 1251-1261.
- Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC. Tyrosine phosphorylation controls PCNA function through protein stability. Nature Cell Biology 2006; 8: 1359-1368.
- Kumar V, Abbas AK, Fausto N, Robbins SI, Cotran RS. Robbins and Cotran Pathologic Basis of Disease. Elsevier Saunders 2005; 269-342.
- Malkas LH, Herbert BS, Abdel-Aziz W, Dobrolecki LE, Liu Y. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc Natl Acad Sci 2006; 103: 19472-19477.
- Acikalin MF, Tel N, Oner U, Pasaoglu O, Dönmez T. Epithelioid angiomyolipoma of the kidney. Int J Urol 2005; 12: 204-207.
- Ma L, Kowalski D, Javed K, Hui P. Atypical angiomyolipoma of kidney in a patient with tuberous sclerosis: a case report with p53 gene mutation analysis. Arch Pathol Lab Med 2005; 129: 676-679.
- Prosperi E. The fellowship of the rings: distinct pools of proliferating cell nuclear antigen trimer at work. The FASEB Journal 2006; 20: 833-837.
- Gehen SC, Vitiello PF, Bambara RA, Keng PC, O'Reilly MA. Downregulation of PCNA potentiates p21-mediated growth inhibition in response to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2007; 292: L716-724.
- Kurokawa H, Yamashita Y, Takeda S, Miura K, Murata T. The expression of proliferating cell nuclear antigen (PCNA) and p 53 protein correlate with prognosis of patients with oral squamous cell carcinoma. Fukuoka Igaku Zasshi 1999; 90: 6-13.