Research Article - Journal of Infectious Diseases and Medical Microbiology (2018) Volume 2, Issue 3
Implementation and evaluation of the impact of a "ventilator-bundle" at Kinshasa University Clinics: Before and after study
Mavinga NJ1*2, Nsiala MJ1,2, Mafuta ME3, Yanga MY1, Amisi BE1, Ilunga JP1, Kilembe MA1
1Department of Anesthesia and Resuscitation, University Clinics of Kinshasa, DRC
2Department of Anesthesia and Resuscitation, Clinique Caron, France
3Department of Statistics, School of Public Health, University Clinics of Kinshasa, DRC
- *Corresponding Author:
- Jose Mavinga Nyombo
Department of Anesthesia and Resuscitation
University Clinics of Kinshasa
Democratic Republic of the Congo
Tel: +243 815992878; +243898075771
E-mail: joicemav@yahoo.fr
Accepted date: August 21, 2018
Citation: Mavinga NJ, Nsiala MJ, Mafuta ME, et al. Implementation and evaluation of the impact of a "ventilator-bundle” at Kinshasa University Clinics: Before and after study. J Infectious Disease Med Microbiol. 2018;2(3):21-5.
Abstract
Objective: To set up a program of VAP prevention (ventilator-bundle) then to evaluate its impact on morbidity and mortality of the patients under mechanical ventilation in our service. Patient and Methods: Prospective, mono-centric, quasi-experimental study, before-after type; performed in the intensive care unit of University Clinics of Kinshasa in the Democratic Republic of Congo. The study was conducted in two phases, from February 1st, 2014 to February 15th, 2016. All consecutive patients intubated and mechanically ventilated for more than 48 hours were included. Five preventive measures were implemented. In patients suspected of having VAP (CPIS>6), the diagnosis was made when isolating a pathogen at ≥ 104 CFU/mL in the tracheobronchial aspiration culture. The main outcome measures were the incidence of VAP and the mortality rate. Results: We included 44 patients in phase 1 and 58 patients in phase 2. The basic characteristics of patients were similar in both groups. Compliance with all the measures put in place was improved between the two phases from 0 to 32.75%. The incidence density decreased from 33.74 to 18.05 VAP for 1000 day ventilator, but the mortality was similar in both groups (88.6% vs. 86.0%). Conclusion: The implementation of a "ventilator bundle" has significantly reduced the impact of VAP in our service. However, our study failed to show a decline in mortality.
Keywords
Ventilator acquired pneumonia, Ventilator-bundle, Intensive care unit.
Introduction
Based on alarming accumulated facts in the previous few years, antimicrobial resistance is an increasingly 8-28% of patients under mechanical ventilation develop pneumonia [1]. This nosocomial infection called ventilator acquitted pneumonia (VAP) is associated with a prolongation of the duration of mechanical ventilation, the length of stay in intensive care unit and an excess mortality of about 6% [2]. It is therefore a frequent and serious condition for which the prevention is essential in order to reduce this morbidity and mortality.
Thus, many measures to prevent the occurrence of these pneumopathies have been proposed by learned societies [3,4]. Most often grouped under what the Anglo-Saxons call "bundle", these measures have demonstrated their effectiveness in several studies [5,6]. However, compliance with the different preventive measures evaluated in these studies varies considerably from one service to another [7]. This situation highlights the importance of the dissemination of procedures, the establishment of recall processes and the regular conduct of evaluation audits.
In our department, there is no written protocol for the prevention of VAP. The aim of our study was to set up a program to prevent VAP and then evaluate its impact on the morbidity and mortality of patients placed under mechanical ventilation in our context.
Patients and Methods
It is a prospective, mono-centric, quasi-experimental study of the before-after type, carried out in the Multipurpose Resuscitation Service of the University Clinics of Kinshasa (CUK), in the Democratic Republic of Congo (DRC). Our study received the approval of the Ethics Committee of the School of Public Health of the University of Kinshasa, under the approval number: ESP/ CE/015/2015. It took place in two phases. The observational phase (phase 1) was conducted from February 1st to December 31st, 2014 (11 months) and the interventional phase (phase 2) from February 15th, 2015 to February 15th, 2016 (12 months). All consecutive patients of 16 years or older admitted in our department and ventilated for more than 48 hours, during the study period were included. Patients with prior pneumonia or immunosuppression and non-invasively ventilated, were excluded from this study.
After the first phase, a program for the prevention of pneumopathies was set up for caregivers to raise awareness. This program included: several presentations in the service about VAP, hospital hygiene, training for nurses and nursing assistants and an information meeting on the study protocol. The five preventive measures that were part of the bundle were: hand washing before the caregiver's contact with a patient under mechanical ventilation, raising the head of the bed to 30-45°, daily removal of sedation, oral decontamination at Chlorhexidine and the cuf pressure control of the intubation tube. The evaluation of the practices was carried out using a direct observation audit without the knowledge of the care team. In patients suspected of having VAP (CPIS>\6), the diagnosis was made when isolating a pathogen at ≥ 104 CFU/mL in the tracheobronchial aspiration culture.
For any included patient, the following data were collected: patient characteristics at admission (age, sex, comorbidities, reason for admission, reason for mechanical ventilation and the severity score MPM0), data on the evolution of the patient in the service (the length of hospitalization in intensive care unit, the duration of mechanical ventilation and the patient's future), the rate of application of preventive measures and finally the data relating to the VAP (time of occurrence and the microorganisms involved). The primary endpoint was the incidence of VAP and the secondary endpoints were compliance with preventive measures, mortality, incidents or adverse events related to mechanical ventilation.
The data was analyzed using SPSS 23.0 software. Quantitative variables were described by their mean ± standard deviation or their median with their extreme values; qualitative variables by frequency and percentage. Comparisons were made using Pearson's chi-square test or Fisher's exact test, depending on the conditions of application, for qualitative (categorical) variables and student's t-test for quantitative variables. For non-normally distributed numeric variables, the medians were calculated and compared using the non-parametric test.
Results
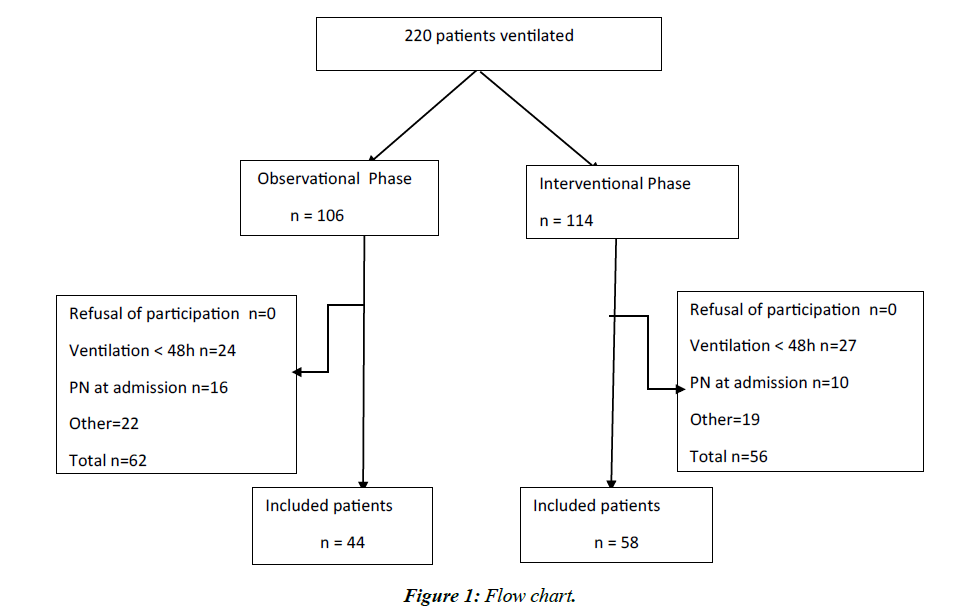
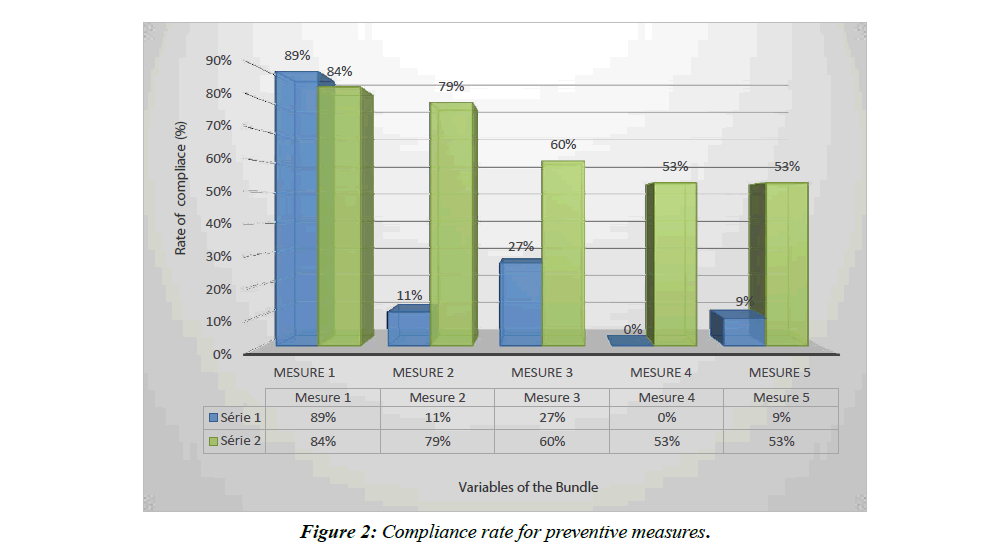
In total, we included 44 patients in the observation period and 58 in the interventional period (Figure 1). The basic characteristics of the patients are shown in Table 1. Figure 2 shows compliance with preventive measures in both groups. The impact of these measures on patient outcomes is presented in Table 2.
Table 1. Basic characteristics of patients.
| Before intervention (n=44) |
After intervention (n=58) |
p | |
|---|---|---|---|
| Demography | |||
| Age/years. Mean ± SD | 52 ± 19.3 | 45.7 ± 19.6 | 0.110 |
| Median (min-max) | 56 (17-86) | 42.5 (16-83) | |
| Sex Male (%) | 25 (56.8) | 29 (50.0) | 0.494 |
| MPMo Score (%) | |||
| Mean ± SD | 42.4 ± 33.3 | 29.2 ± 31.5 | 0.490 |
| Mean ± SD | 26.8 (1.9-98.3) | 13.8 (0.9-99.4) | |
| Co- morbidity n (%) | |||
| HTA | 18 (40.9) | 20 (34.5) | 0.506 |
| Diabetes | 6 (13.6) | 6 (10.3) | 0.609 |
| Other | 9 (20.5) | 11 (19.0) | 0.851 |
| Admissions n (%) | |||
| Medical | 23 (52.3) | 35 (60.3) | 0.415 |
| Surgical | 12 (27.3) | 9 (15.5) | 0.146 |
| Traumatic | 9 (20.4) | 14 (24.1) | 0.659 |
| Main Diagnosis at admission n (%) | |||
| Stroke | 15 (34.1) | 12 (20.7) | 0.129 |
| Severe injuries | 10 (22.7) | 14 (24.1) | 0.868 |
| Brain tumors | 7 (15.9) | 4 (6.9) | 0.146 |
| Sepsis | 9 (20.5) | 12 (20.7) | 0.977 |
| Metabolic disorders | 2 (4.5) | 0 (0.0) | 0.101 |
| Other | 8 (18.2) | 20 (34.5) | 0.077 |
| Indications of mechanical ventilation n (%) | |||
| Cardio vascular | 6 (13.6) | 8 (13.8) | 0.982 |
| Neurological | 36 (81.8) | 39 (67.2) | 0.098 |
| Respiratory | 3 (6.8) | 14 (24.1) | 0.020 |
Table 2. Impact of the preventive measures introduced.
| Indicator | Pre-Intervention Phase | Post-Intervention Phase | p |
|---|---|---|---|
| VAP: n (%) | 11 (25) | 10 (17.2) | 0.337 |
| Density of incidence /1000 day of MV | 33.74 | 18.05 | 0.222 |
| MV duration : Mean ± SD | 7.4±7.4 | 7.0 ± 6.6 | 0.77 |
| Median [extremes] | 4.5 [2-41] | 4 [2-30] | 0.929 |
| Length of stay : Mean ± SD | 9.4 ± 9.8 | 9.5 ± 8.9 | NA |
| Median [extremes] | 5 [2-41] | 6.5 [2-43] | 0.919 |
| Mortality (%) | 88.6 | 86 | 0.716 |
In the observational phase, 11 out of 44 patients presented a VAP for 326 days of ventilation, an incidence density of 33.74 VAP for 1000 days of mechanical ventilation. In contrast, in the interventional phase, 10 out of 58 patients presented with VAP for 554 days of ventilation, an incidence density of 18.05 VAP for 1000 days of invasive ventilation. The causative organisms are detailed in Table 3.
Table 3. Isolated bacteria.
| Microorganism | 1st Phase n=11 % |
2nd Phase n=10 % |
|---|---|---|
| % | % | |
| Staphylococcus aureus | 9 | 0 |
| Streptococcus pneumoniae | 9 | 20 |
| Enterobacter cloacae | 36 | 40 |
| Klebsiella pneumoniae | 0 | 20 |
| Escherichia coli | 18 | 10 |
| Pseudomonas aeruginosa | 18 | 10 |
| Acinetobacter baunanii | 9 | 0 |
As for the secondary endpoints, there was no difference in terms of mechanical ventilation duration, length of stay in intensive care unit, or all-cause resuscitation mortality.
Discussion
With the introduction of our bundle, team compliance during the interventional phase improved compared to the observational phase, and the VAP rate decreased from 33.7 to 18.05 per 1000 days of artificial ventilation between the two periods even do this difference was not significant. This result is consistent with the literature. Indeed, several publications show the same effect of VAP prevention with a nearly 50% decrease in the incidence of VAP by implementing a "ventilator-bundle" [6,8- 12]. However, the lack of a statistically significant difference in our study is due to lack of power.
The incidence density (DI) of VAP observed in the observational phase of this study was very high: 33.7 VAP/1000 days of VM. In the literature, it varies according to the type of service and the type of patient. Recent studies find figures around 15 to 20 PAVM per 1000 days of ventilation in multi-purpose resuscitation services like ours [6,8-11]. In addition to the fact that our cohort was predominantly composed of cerebrolesioned patients, a population otherwise associated with an increased risk of VAP [13], it is probably the absence of a local protocol for the prevention of VAP that explains this high density of incidence as demonstrated by a French survey [14].
In our study, the compliance with the preventive measures in the interventional phase, although significantly improved, was not perfect with a compliance to all measures of 32.75%.
This partial compliance is in fact equivalent to a non-compliance. In fact, the teams that set up a prevention bundle reported a drastic reduction in the incidence of VAP when all the measures of the bundle were respected concomitantly, according to an "all or nothing" strategy. Thus, the impact of the "ventilator bundle" is maximum if the compliance with the "bundle" and with all its components, is at least 95% according to IHI [5]. This partly explains why, although the VAP rate between the two phases of our study decreased significantly, the difference was not statistically significant.
However, we do not have to be ashamed of our results since, in the literature, compliance with recommended preventive measures against VAP was short (12 months) unlike other teams. In the study by Rello et al. in 2013, for example, the second " is often insufficient [15,16]. In addition to the reasons given in the literature to explain this low compliance, especially practical difficulties and a lack of resources [17], in our department, this is also explained by the duration of the study which interventional" phase extended over 22 months. Even more in the study of Bouadma et al. in 2010, where it lasted 30 months. We believe that over time, adherence to the measures implemented in our service will continue to improve.
The preventive measures that we incorporated in our bundle were chosen among the measures recommended in the literature taking into account their cost and ease of implementation. Thus sophisticated measures such as subglottic aspiration, although popular with other teams, were not part of our bundle. In fact, the 5 measures we had chosen were already known to practitioners but were not subject to special attention and training. Our job was to draft a protocol and train all medical and paramedical personnel to apply these measures.
Despite the reduced incidence of VAP, our bundle had no impact on the morbidity associated with this complication. The mean duration of mechanical ventilation and length of stay in the two groups in our study were not statistically different. This could be explained by a lack of power, as our sample is small compared to studies that have reported a positive impact on ventilation time or length of stay in ICU [12,18,19]. Anyway, the heterogeneity of the bundles used in the literature, the absence of randomized study and the possibility of many biases reproached to all these studies, do not make it possible at the moment to know the real impact of the ventilator bundle on the future of resuscitation patients [20,21].
Our study also did not show a significant impact on the mortality of mechanically ventilated patients despite the reduced incidence of VAP. This is consistent with the literature. Indeed, the majority of studies showing the effectiveness of the bundles have not allowed to show an associated decrease in mortality. This could be explained by the low mortality attributable to VAP [2] and the need to include a very large number of patients to be able to demonstrate this impact. Moreover, the very high proportion of comatose patients in our series, a population whose prognosis is very dark in our context, could partly explain this lack of efficacy, as evidenced by the very high mortality rates in the two groups of our study. In comparison with the vast international survey on the prognosis of patients under mechanical ventilation, mortality in intensive care was much lower (31%) [22].
The originality of this study is the fact that it is the first to implement and validate the international recommendations on prevention of VAP in our community. Most studies in this field come from the West and do not take into account our realities on the ground. However, the results of our study must be interpreted taking into account the methodological weaknesses inherent in this type of study. Like all before/after studies, confusion cannot be ruled out. Nevertheless, the initial characteristics of our patients were similar overall in both groups, thus ensuring their comparability.
Conclusion
The results of our study confirm that the implementation of a prevention protocol type «bundle» effectively reduces the incidence of VAP. On the other hand, our study failed to show a significant impact on the morbidity and mortality of patients placed under mechanical ventilation in our department.
Conflict of Interest
We have no conflict of interest to report regarding this study.
References
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis Occurrence in Acutely Ill Patients Investigators: Sepsis in European Intensive Care Units: Results of the SOAP study. Crit Care Med. 2006;34(2):344-53.
- Timsit JF, Zahar JR, Chevron S. Attributable mortality of ventilator-associated pneumonia. Curr Opin Crit Care. 2011;17(5):464-71.
- American thoracic society; infectious diseases society of America. Guidelines for ventilator-associated, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388.
- Zilberberg MD, Shorr AF, Kollef MH. Implementing quality improvements in the intensive care unit: ventilator bundle as an example. Crit Care Med. 2009;37(1):305-9.
- http://www.ihi.org/ihi/programs/campaign.
- Zack JE, Garrison T, Trovillion E, et al. Effect of an education program aimed at reducing the occurrence of ventilator-associated pneumonia. Crit Care Med. 2002;30(11):2407-12.
- Luna CM, Blanzaco D, Niederman MS, et al. Resolution of ventilator-associated pneumonia: Prospective evaluation of the clinical pulmonary infection as an early clinical predictor of outcome. Crit Care Med. 2003;31(3):676-82.
- Eom JS, MS Lee, Chun HK, et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: a multicenter study. Am J Infect Control. 2014;42(1):34-7.
- Berenholtz SM, JC Pham, Thompson DA, et al. Collaborative cohort study of an intervention to reduce ventilator-associated pneumonia in the intensive care unit. Infect Control Hosp Epidemiol. 2011;32(4):305-14.
- Bouadma L, Deslandes E, Lolom I, et al. Long-Term Impact of a Multifaceted Prevention Program on Ventilator-Associated Pneumonia in a Medical Intensive Care Unit. Clin Infect Dis. 2010;51(10):1115-22.
- Morris AC, Hay AW, DG Swann, et al. Reducing ventilator-associated pneumonia in intensive care: the impact of implementing a care bundle. Crit Care Med. 2011;39(10):2218-24.
- Rello J, Afonso E, Lisboa T, et al. A care bundle approach for prevention of ventilator-associated pneumonia. Clin Microbiol Infect. 2013;19(4):363-9.
- Croce MA, Brasel KJ, Coimbra R, et al. National Trauma Institute prospective evaluation of the ventilator bundle trauma patients: does it really work? J Trauma Acute Care Surg. 2013;74(2):354-60.
- Fischer MO, Garreau N, Jarno P, et al. Multicenter survey on ventilator-associated pneumonia prevention in intensive care. Ann Fr Anesth Reanim. 2013;32(12):833-7.
- Cason CL, Tyner T, Saunders S, et al. Nurses' implementation of guidelines for ventilator-associated pneumonia from the Centers for Disease Control and Prevention. Am J Crit Care. 2007;16(1):28-36.
- E. Bertholet. Prevention of Mechanically Ventilated Pneumonia (VAP): Results of the SRLF 2008 Investigation. Resuscitation. 2010;19(4):366-73.
- Julie E, Mangino, Paula Peyrani, et al. Development and Implementation of a Performance Improvement Project in Adult Intensive Care Units: An Overview of the Improving Medicine through Pathway Assessment of Critical Therapy in Hospital-Acquired Pneumonia (IMPACT-HAP) study. Critical Care. 2011;15(1):R38.
- Game FL, Jeffcoate WJ. Dressing and Diabetic Foot Ulcers. Plast Reconstr Surg. 2016:138(3S):158S-64S.
- Bloos F, Muller S, Harz A, et al. Effects of staff training on the care of mechanically ventilated patients: a prospective cohort study. Br J Anaesth. 2009;103(2):232-7.
- Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;Suppl 2:S133-54.
- Klompas M. Prevention of ventilator-associated pneumonia. Expert Rev Anti Infect Ther. 2010;8(7):791-800.
- Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345-55.