- Biomedical Research (2010) Volume 21, Issue 4
Impairment of in vitro embryonic development with a corresponding ele-vation of oxidative stress following nicotine treatment in mice: Effect of variation in treatment duration
Kamsani YS, Rajikin MH*, Chatterjee A, Nor Ashikin MNK, Nuraliza ASFaculty of Medicine, Universiti Teknologi Mara, 40450 Shah Alam, Selangor, Malaysia
- *Corresponding Author:
- Mohd. Hamim Rajikin
Faculty of Medicine, Universiti Teknologi MARA
Kompleks Sains & Teknologi
40450 Shah Alam, Selangor Malaysia
Phone: +60 3 5544 2899
Fax: +60 3 5544 2831
E-mail: hamim400@salam.uitm.edu.my
Accepted April 14 2010
Abstract
This study was designed to investigate the effects of nicotine on in vitro embryo development at a variable duration of treatment schedule. A concomitant oxidative stress level was also evaluated. Four- to six-week-old female mice (Mus musculus) were injected (s.c.) with nico-tine (5.0 mg/kg/day) for 7, 14 or 28 consecutive days. Animals were superovulated and co-habitated overnight with fertile male at a ratio 1:1. Forty-eight hours post-coitum, blood samples of the animals were collected for manoldialdehyde (MDA) estimation. On sacrifice, ovaries including the fallopian tubes were excised. Fallopian tubes were flushed and the normal embryos were subjected to in vitro culture. Out of 783 retrieved embryos, only 61% were found normal. Nicotine increased the number of abnormal embryos (61.7 ± 9.3) as compared to controls (40.3 ± 8.1). None of the embryos formed blastocyst following nicotine treatment for 7 days. When the length of treatment was extended for 14 days, embryonic development reached only up to 8-cell stage. However, most of the embryos stopped dividing at 2-cell stage after 28 days of nicotine treatment. Plasma MDA concentration was found to be higher in all the three groups of nicotine-treated experimental mice compared to control groups. Ovarian MDA levels showed a significant difference between the groups of animals treated with nicotine for 14 days and 28 days, but not between two other treated groups, those received nicotine for 7 days and 14 days, respectively. In conclusion, the degree of im-paired development of the preimplanted embryos seems to be directly correlated with the length of nicotine treatment with a corresponding increment of oxidative stress.
Keywords
Nicotine, preimplantation embryo development, malondialdehyde
Introduction
The adverse effects of smoking on reproduction are well documented, yet the current trend of tobacco smoking among the women still remains. Approximately 4% of Malaysian population aged ≥15 years are female smokers (WHO Policy, Strategy Advisory Committee for Tobacco Free Initiative Estimated, 2000). Previous epidemiologi-cal studies from the general population of reproductive age have confirmed that there is a delay in conception [1] and the onset of menopause in female smokers [2]. There-fore, certain components in cigarette smoke may directly or indirectly interfere with embryo development and vi-ability.
Nicotine is present in cigarettes in amounts varying from 0.8 to 1.8 mg per cigarette depending on the brand and size of cigarette [3,4]. As much as 1 mg of nicotine is recorded to be absorbed by smoking a single cigarette [5].
Nicotine is a toxic alkaloid and is quickly absorbed through the respiratory track, mucosa of the mouth and skin [6]. Nicotine also induces oxidative stress through the production of free radicals [7] with an alteration of antioxidant enzymes in various tissues including heart, liver and ovaries as well as blood plasma [7]. This altera-tion of antioxidant enzymes is reflected by an increased level of MDA, an oxidative stress biomarker [8]. It has been reported that various tissues of mice exposed to side-stream cigarette smoke show elevated oxidative DNA damage [9] with a concurrent increase in lipid peroxida-tion and decreased level of antioxidant enzymes [10]. Fur-thermore, increased lipid peroxidation in blood of smok-ers has also been shown to be linked with oxidative dam-age on DNA with carcinogenesis [11]. It seems that peo-ple who smoke or exposed to cigarette smoke are also subjected to nicotine-induced oxidative stress [12]. A number of studies have shown that nicotine delays em-bryo cleavage from 2- to 4-cell stage and results in delayed implantation and prolonged gestation [13,14]. Nico-tine also affects the structure of the oviduct for transport-ing a gamete to the uterus for implantation [15]. Further-more, perturbation of the first and second meiotic divi-sions of the hamster oocyte following nicotine treatment has also been documented [16]. Nicotine, moreover causes a high percentage of pregnancy wastage and re-duces litter size during parturition [14]. Other findings have shown that nicotine delays or decreases LH and prolactin secretion [17].
Primary objective of the present study was to investigate the degree of adverse effect caused by nicotine on in vitro development of mice embryo and a corresponding meas-urement of oxidative stress level when the duration of treatment is extended from 7 to 14 or 28 days
Materials and Methods
Animal Treatment
Forty-eight mice with an average body weight between 25 - 30 gm were housed at 27 °C with 12 h light-dark cycle. Animals were given food pellets and water ad libitum and randomly divided into six groups. Animals of groups 1, 2 and 3 received daily subcutaneous (s.c.) injection of nicotine (5 mg/kg/day) [ICN, USA] for 7, 14 and 28 days, respectively. Animals of groups 4, 5 and 6, which were treated as controls, had 0.9% saline (s.c.) for 7, 14 and 28 consecutive days. On the last day of treatment, animals were superovulated using PMSG (150 IU/kg b.w.) fol-lowed by hCG (75 IU/kg b.w.), mated with the fertile males and sacrificed 48 hours post-coitum.
Sample collection
Blood samples were collected via cardiac puncture. Plasma was separated by centrifugation (2500 rpm, 4°C for 15 minutes) and frozen at -70°C until MDA analysis. Ovaries were freed from the adhering tissues and stored in 1 ml of phosphate buffered saline (PBS) and kept at -70°C until MDA analysis. Fallopian tubes were excised and embryos were flushed under a dissecting microscope (Leica Zoom 2000, Japan) and counted.
Embryo Culture
Normal and abnormal embryos were categorized accord-ing to the criteria set by Ertzeid & Storeng [18]. Only normal embryos were cultured. The embryos were cul-tured in a 35-mm culture dish filled with 100 μl droplets of Whitten’s medium (Sigma Chemical Co., USA) over-laid with mineral oil (Sigma, USA). The cultures were maintained in a humidified atmosphere containing 5% CO2 and 95% air at 37° C for 5 days. Assessment of em-bryonic development was made under the reverse phase (Leica IRB, Japan), and inverted microscope (Olympus 1X81 SF-3, Japan).
Determination of oxidative stress biomarker, MDA in plasma and ovaries
Plasma and ovaries were processed accordingly for evaluation of MDA levels using the theobarbituric acid reactive substances (TBARS) method [19]. The absorb-ance was measured photometrically at 632 nm and the concentrations were expressed as nanomoles MDA per gram protein (nmol/g).
Statistical analysis
Data were analyzed using the SPSS package program (SPSS 16.0, Chicago, IL, USA). A Kolmogorov-Smirnov test was used to test the normality of data distribution. Statistical methods included paired and unpaired two-tailed Student’s t-test with normal distribution. All con-tinuous variables were expressed as mean ± SEM. A p value of <0.05 was considered statistically significant.
Results
Effect of nicotine on in vitro embryo development
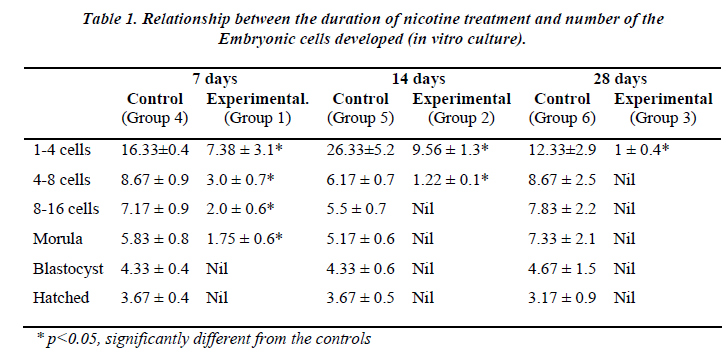
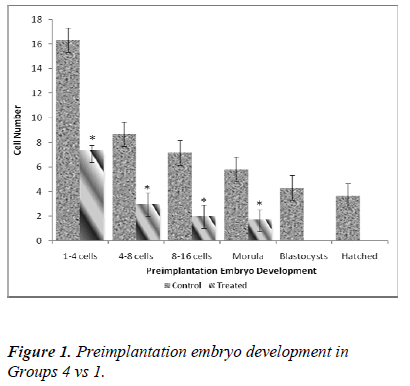
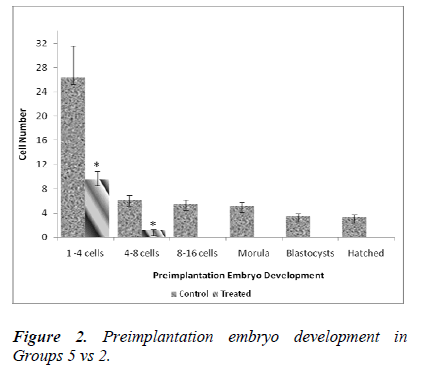
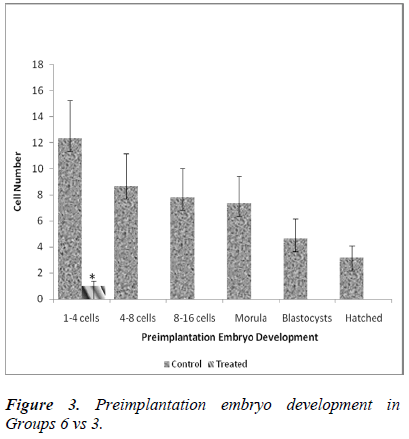
Table 1 shows the effect of nicotine on the rate of in vitro embryo development in nicotine-treated mice. Embryos from the control groups 4, 5 and 6 developed up to a hatched blastocyst stage on day 5. Following 7 days of nicotine treatment (Group 1), the embryos developed until the stage of morula (23.7%) but none of the embryos hatched. Embryos grew up to 8-cell stage only (12.7%) following 14 days of nicotine treatment (Group 2), while in the 28-day nicotine-treated group (Group 3), embryo development ceased at 2-cell stage (100%).
Figure 1 shows significant differences in the developmental stage of 1-4, 4-8, 8-16 cells and morula following 7 days nicotine treatment (Group 1). In the control group (Group 4), the number of 1-4 cells retrieval was 16.33 ± 0.4 whereas in the treated group (Group 1), the value was significantly lower (7.38 ± 3.1; 60.2%). This decreasing pattern also applies to 4-8 cells (8.67 ± 0.9 [control] vs 3.0 ± 0.7 [treated]), 8-16 cells (7.17 ± 0.9 [control] vs 2.0 ± 0.6 [treated]) as well as morula stage (5.83 ± 0.8 [control] vs 1.75 ± 0.6 [treated]). No blastocysts was detected on day 5 in the treated group (Group 1).
Figure 2 shows significant differences in the developmental stage between 1-4 cells and 4-8 cells in 14- day treatment group (Group 2). In control group (Group 5), the number of 1-4 cells retrieval was 26.33 ± 5.2 whereas in the treated group (Group 2), the value was found to be significantly lower (9.56 ± 1.3; 56.2%). A similar decreasing pattern was also applied to 4-8 cells (6.17 ± 0.7 [control] vs 1.22 ± 0.1 [treated]). No further in vitro embryonic development was evident from day 3 onwards in the treated group (Group 2).
Figure 3 shows a significant difference in the developmental stage between 1-4 cells obtained on the day of embryo retrieval. In control group (Group 6), the number of 1-4 cells retrieved was 12.33 ± 2.9 whereas in the treated group (Group 3), the value was significantly lower (1 ± 0.4; 8 %). No further embryonic development was observed after day 1 of in vitro culture in the treated group (Group 3).
Nicotine treatment and MDA concentration in plasma and the ovary
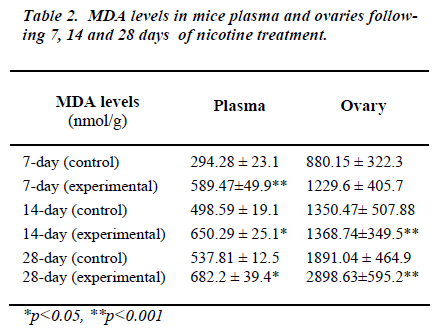
Table 2 shows MDA levels in plasma and the ovary in mice at different treatment schedule with nicotine. In all the nicotine-treated groups, levels of plasma MDA were found to be significantly higher as compared to their re-spective controls.
Although a significant increase in ovarian MDA was re-corded in between the groups treated for 14 and 28 days, yet plasma MDA levels between these two groups did not differ. Although nicotine altered plasma and the ovarian MDA concentrations, yet its effects on the ovarian MDA level was found to be much more pronounced.
Discussion
In 1985 Mitchell and Hammer [20] reported that nicotine of 5.0 mg/kg body weight in rats for 4 consecutive days produced less number of blastocyst as compared to the control. The same dosage of nicotine was used for ultra-structure studies [21,22] and pregnancy at term [15]. We were, therefore tempted to examine and compare whether 5.0 mg/kg/day of nicotine for a period of 7, 14 or 28 days could alter in vitro development of embryo until the stage of blastocyst hatching. Our results of delayed embryonic cleavage from 1- to 4-cell stage on day 2 of pregnancy confirmed the findings of other researchers [14,15,20]. Effects of chronic treatment of nicotine for consecutive 30 days on preimplantation embryo development have been documented [21,23]. Norfilza et al [21] have recorded widened previtelline space and highly densed mitochon-dria in mice embryos following a chronic nicotine (5.0 mg/kg/day) treatment for 30 days. Another study has shown that twice-daily injections of nicotine (5.0 mg/kg/day) from day 1 through day 5 of pregnancy re-duced oviducal blood flow and the rate of embryo cell proliferation [20]. Using nicotine at a dose of 5.0 mg/kg/day for 7, 14 or 28 days, it was found that nicotine for 7 consecutive days prevented embryos to develop be-yond the stage of morula. However, embryos in 14-day treatment group did develop up to 8-cell stage only, while nicotine for 28 consecutive days ceased embryo develop-ment at 2-cell stage.
Our results therefore indicate that prolonged exposure of nicotine has a greater negative impact on in vitro embryo development. It has been reported that embryos in rats developed only up to a 4-cell stage when nicotine treat-ment is continued for 30 days [21]. An identical treatment schedule in mice is found to prevent blastocysts formation on day 4 in culture (Rozzana et al [23]). Nicotine expo-sure (5.0 mg/kg/day) in rat for four consecutive days however, resulted in a less number of blastocyst as com-pared to control [20] and only 33% of pregnancy in rats evidently continued until term [14].
In present study, we retrieved 783 embryos of which only 61% were found normal. However, more than half of the retrieved embryos (60.5%) from the nicotine-treated mice were found to be abnormal. The decline in the number of retrieved oocytes has previously been reported [24,25].
Nicotine-induced pregnancy complications such as spon-taneous abortion, low birth weight and delayed concep-tion are also evident [22,23]. Nicotine-related formation of reactive oxygen species (ROS) such as superoxide an-ion (O2¯), hydrogen peroxide (H2O2) and free radical (OH¯) [26,27] causes pro-oxidants to outnumber antioxi-dants [7], and results in oxidative stress. Free radicals are found in ovarian lutein cells [28], follicular fluid [29], and around the blastocyst [21]. It has been demonstrated that oxidative stress induces mitochondrial and cellular DNA damage [30,31] and promotes in vitro ageing [32]. In-creased concentration of plasma and ovarian MDA fol-lowing nicotine treatment for 7, 14 or 28 days is a consis-tent proof of the generation of oxidative stress in our ex-perimental animals. Moreover, decrease in ovarian perfu-sion by nicotine [23] could possibly cause a significant increase in MDA concentration in the ovarian tissue par-ticularly following 14 or 28 days of nicotine treatment. Increased MDA levels in blood/serum of smokers [11] and an increase in cotinine concentration in follicular mi-croenvironment are also evident [33].
In conclusion, the degree of nicotine-induced in vitro em-bryo dysgenesis seems to be correlated with the duration of nicotine exposure. Moreover, corresponding increase in plasma and ovarian levels of MDA, an indicator of oxida-tive stress also suggests that the impaired embryogenesis is possibly caused by nicotine-related generation of free radicals.
Acknowledgement
This work was funded by the Fundamental Research Grant Scheme (FRGS) No 600-IRDC/ST/FRGS.5/3/1343 to Professor Dr Mohd. Hamim Rajikin.
References
- Zenzes MT. Cigarette smoking as a cause of delay in conception. Reprod Med Rev 1995; 4: 189-205.
- Midgette AS, Baron J. Cigarette smoking and the risk of natural menopause. Epidemiology 1990; 1: 474-480.
- Benowitz NL, Kuyt F, Jacob P III, Jones RT. Cotinine disposition and effects. Clin. Pharmacol Ther 1983; 34:604-611.
- Rosa M, Pacifici R, Altieri I. et. al. How the steady- state cotinine concentration in cigarette smokers is di- rectly related to nicotine intake. Pharmacol Epidemiol Drug Util 1992; 52: 324-329.
- Barbieri RL, McShane M, Ryan KJ. Constituents of cigarette smoke inhibit human granulosa cells aroma- tase. Fertil Steril 1986; 46: 232-236.
- Gandini L, Lombardo F, Lenzi A et. al. The in-vitro effects of nicotine and cotinine on sperm motility. Hum Reprod 1997; 12: 727-733.
- Sies H. Strategies of antioxidant defence. Eur J Bio- chem 1993; 215: 213-219.
- Spiteller G. Review: on the chemistry of oxidative stress. J. Lipid Mediat 1993; 7: 199-221.
- Howard DJ, Briggs LA, Pristos CA. Oxidative DNA Damage in Mouse Heart, Liver, and Lung Tissue Due to Acute Side-Stream Tobacco Smoke Exposure. Arch of Biochem and Biophys 1998; 352 : 293–297.
- Helen A, Krishnakumar K, Vijayammal PL, Augusti KT. Antioxidant effect of onion oil (Allium cepa. Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicol Lett 2000; 116: 61-68.
- Kalra J, Chaudhary AK, Prasad K. Increased produc- tion of oxygen free radicals in cigarette smokers. Int J Exp Pathol 1991; 72: 1-7.
- Suleyman H, Gumustekin K, Taysi S, Keles S, Oztasan N, Aktas O, Altinkaynak K, Timur H, Akcay F, Akar S, Dane S, Gul M. Beneficial effects of Hippophae rham- noides L. on nicotine induced oxidative stress in rat blood compared with vitamin E. Biol Pharma Bull 2002; 2(59): 1133-1136.
- Yoshinaga K, Rice C, Krenn J, Pilot R L. Effects of nicotine on early pregnancy in the rat. Biol. Reprod 1979; 20: 294-303.
- Mokhtar N, Rajikin M, Zakaria Z. Role of tocotrienol- rich palm vitamin E on pregnancy and preimplantation embryos in nicotine-treated rats. Biomed Res 2008; 19:181-184.
- Talbot P, Riveles K. Smoking and reproduction: the oviduct as a target of cigarette smoke. Reprod Biol En- docrinol 2005; 3: 52.
- Racowsky C, Hendricks RC, Baldwin KV. Direct ef-fects of nicotine on the meiotic maturation of hamster oocytes. Reprod Toxicol 1989; 3:13-21.
- Blake CA, Scaramuzzi RJ, Norman RL, Kanematsu S Sawyer CH.Effect of nicotine on the proestrous ovula- tory surge of LH in the rat. Endocrinology 1972; 91:1253-1258.
- Ertzeid G, Storeng R. Adverse effects of gonadotrophin treatment on pre- and postimplantation development in mice. J Reprod Fert 1992; 96: 649-655.
- Ledwozyw A, Michalak J, Stepien A, Kadziolla A. The relationship between plasma triglycerides, cholesterol, total lipids and lipid peroxidation product during hu-man arteriosclerosis. Clinical Chemica Acta 1986; 155: 275-284.
- Mitchell JA, Hammer RE. Effects of nicotine on ovi- ductal blood flow and embryo development in the rat. J Reprod Fertil 1985; 74: 71-76.
- Mokhtar N, Rajikin M, Latif ES, Mar MR, Top AGM. Deleterious effects of nicotine on the ultrastructure of oocytes: Role of γ-tocotrienol. Med Sci Monit 2009; 15: 378-383.
- Tarin JJ, Vendrell FJ, Ten J et al. The oxidizing agent butyl hydroxyperoxidase induces disturbances in spin- dle organization, c-meiosis and aneuploidy in mouse oocytes. Mol Hum Reprod 1996; 2: 895-901.
- Rozzana MS, Zaiton Z, Rajikin MH, Fadzilah S, Zanariyah A. Supplementation with alpha lipoic acid improves the in vitro development of embryos in nico- tine-treated mice. Biomed Res 2005; 16: 28-32.
- Zenzes MT, Reed TE, Casper RF. Effect of age and cigarette smoking in the maturation of human oocytes. Hum Reprod 1997; 9: 101-109.
- Joesbury KA, Edirisinghe WR, Phillips MR, Yovich JL. Evidence that male smoking affects the likelihood of a pregnancy following IVF treatment: application of the modified cumulative embryo score. Hum Reprod 1998; 13: 1506-1513.
- Pryor WP, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate and peroxinitrite. Ann NY Acad Sci 1993; 686: 13-28.
- Church DF, Pryor WA. Free radical chemistry of ciga- rette smoke and its toxicological implications. Environ Health Perspec 1990; 87: 9741-9745.
- Zenzes MT, Puy L, Bielecki R. Immunodetection of cotinine in granulose-lutein cells of women in IVF ex- posed to cigarette smoke. Fertil Steril 1997; 68: 76-82.
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocr 2005; 3: 1-21
- Ames BN, Motchnik PA, Fraga CG et al. Antioxidant prevention of birth defects and cancer. In Mattison DR, Olshan A (eds). Male mediated Developmental Toxi- cology. Plenum Press, New York USA, pp 243-259.
- Ballinger SW, Bouder TG, Davis GS et al. Mitochon- drial genome damage associated with cigarette smok- ing. Cancer Res 1996; 56: 5692-5697.
- Tarin JJ. Aetiology of age-associated aneuploidy: a mechanism based on the ‘free radical theory of ageing’. Hum Reprod 1995; 10: 1563-1565.
- Paszkowski T, Clarke RN, Hornstein MD. Smoking induces oxidative stress inside the graafian follicle. Hum Reprod 2002; 17: 921-925.