Research Article - Journal of Fisheries Research (2018) Volume 2, Issue 2
Impacts of Moringa oleifera leaves and Lannea barteri bark as growth promoting additives and survival rates in the diets of Clarias gariepinus fingerlings.
Suleiman AM*, Orire AM*, Sadiku SOEDepartment of Water Resources, Aquaculture and Fisheries Technology, Federal University of Technology, Minna, Nigeria.
- Corresponding Authors:
- Suleiman AM
Department of Water Resources Aquaculture and Fisheries Technology
Federal University of Technology
Minna, Nigeria
Tel: +234 703 286 7661
E-mail: mgupamiffi@yahoo.com - Orire AM
Department of Water Resources Aquaculture and Fisheries
Technology Federal University of Technology
Minna Nigeria
Tel: +234 703 255 2295
E-mail: abdul.orire@futminna.edu.ng
Accepted date: November 02, 2018
Citation: Suleiman AM, Orire AM, Sadiku SOE. Impacts of Moringa oleifera leaves and Lannea barteri bark as growth promoting additives and survival rates in the diets of Clarias gariepinus fingerlings. J Fish Res. 2018;2(2):6-16.
DOI: 10.35841/fisheries-research.2.2.12-16
Visit for more related articles at Journal of Fisheries ResearchKeywords
Natural Additives, Growth, Clarias gariepinus, Antibiotic.
Introduction
Fish is a source of indispensable amino acids in the diet of human and animals because of its high quality proteins, Omega 3 fatty acids and other nutrients [1]. Demand for fish products is increasing on daily basis while wild stock is rapidly decreasing due to over exploitation and abuse of fisheries rules and regulations [2]. According to Villa-Cruz et al. [3] sustaining fish supplies from captured fisheries will not meet growing demand for fish product globally. Therefore, aquaculture has become an important ventures and fastest growing subsector of agribusiness.
For a sustainable aquaculture, this activity requires high quality feeds which should contain necessary nutrients and complementary feed additives to keep aquatic organisms healthy, fast growth, environmental friendly, and disease resistant [4]. The palatability of diets and feeding rate, the nutritional components of aqua feeds are also of paramount importance to the growth performance of the farmed fish species. Thus, formulation of a diet that meets up with the nutrient requirements of the cultured fish is imperative for the rapid growth rate and survival of aquaculture fish species [5]. Kumar et al. [4] also reported that, feed additives are substances such that they are added in trace amount by providing a mechanism in which dietary deficiencies can be addressed. Additive serves as benefit to the nutrition and the growth of animal concerned. Most of some growth promoting feed additives include hormones, antibiotics, ionospheres, probiotics and some salts [4]. Some of these additives used in feed mill are chemical products especially hormones and antibiotics which may cause unfavourable side effects. The use of Antibiotic Growth Promoters (AGPs) as feed additives in the aquaculture industry has been criticised by government policies and consumers because of possible development of microbial resistance to these products and their potential harmful effects on human health [6].
Attempts to use the natural materials such as medicinal plants could be widely accepted as feed additives to enhance efficiency of feed utilization and animal productive performance [7]. Natural plant products have been reported to promote various activities like anti-stress, growth promotion, appetite stimulation, tonic, immune stimulation and antimicrobial properties in fin fish and shrimps’ larvae culture [8]. Farmers are confronted with the challenge of poor fish growth. This has led to some farmers influencing rapid growth in fish by incorporation of synthetic growth substances into fish feed in a bid to shorten production period which in terms has health implication in consumers [9]. There is need to also mitigate the use of harmful substance in fish production, especially the use of synthetic growth substances with negative health implication to the consumers. This study seeks to investigate the effect Moringa oleifera leaves, stem bark of Lannea barteri and oxytetracycline antibiotic as an additive on the growth performance and survival rates of clariid species (Clarias gariepinus) fingerlings.
Materials and Methods
Plants additives preparations
Moringa oleifera leaves procurement: Moringa oleifera leaves were obtained from horticultural garden, Department of Crop Production, Federal University of Technology, Minna. The leaves were taken to Water Resources, Aquaculture and Fisheries Technology (WAFT) Departmental laboratory where they were air dried at room temperature for 5 days, the dry leaves were ground to powdery form [10]. The powdery was further screened with 1 mm mesh size sieve, packed in polythene leather and kept in a deep freezer (4°C).
Lannea barteri stem bark procurement: Lannea barteri stem bark was obtained from Gupa-Miffi via Lapai Local Government Area of Niger State. It was cut into smaller sizes, pounded and sun dried for 2 days, ground into powdery [11]. The ground particle was further reduced with 1 mm mesh size sieve, packed in polythene leather and kept in a deep freezer (4°C).
Methods of extraction of plants additive
The plants extract used for the experiment were Moringa oleifera leaves and bark stem of Lannea bateri. Two methods were used for the extraction of these plants which are ethanol and aqueous extraction.
Ethanol extraction of plants additive: Fifty (50) g of the prepared (Moringa oleifera leaves and Lannea bateri bark) powder were measured and soaked into 500 mL of ethanol (C2H5OH) with 99/100% concentration following the procedures of Nwerze and Nwafor [10] for 72 hr in 1000 mL bottle. The bottles were tightly covered to prevent evaporation of the ethanol. The bottles were thoroughly agitated at intervals and the soaked materials were doubled filtered using muslin cloth. The concentrated liquid collected were then fed to 1 L flask and clipped to a rotary evaporator (RE300) machine rotating on a water bath filled to a capacity with water which was made to boil steadily at 100ᴼC while the solvent was removed. The extracts were poured into 10 mL bottle, exposed to air to ensure complete volatility of the ethanol solvent. The extracts were covered and stored in the laboratory deep freezer at 4°C until used.
Aqueous extraction of plant additive: Fifty (50) g grams of Maringa oleifera leaves and Lannea bateri bark powder were measured and soaked with distilled water of 500 mL volume. The procedure for extraction is as the same as that of ethanol extraction. The aqueous extracts were preserved in the laboratory deep freezer at 4°C until used.
Raw incorporation
The powder prepared from the plants (Moringa oleifera leaves and Lannea bateri bark) were incorporated directly into the diets.
Diet formulation and level of inclusion
Eight diets were formulated for the experiment. Each of the experimental diets was formulated to contain 45% Crude Protein (CP) in consonance with the Commercial Reference Diet (CRD) which was used as control diet. The proximate analysis of the major ingredients as well as formulated diets was determined (Table 1) using AOAC (Association of Official Agricultural Chemists) [12].
| Feed Ingredients (g) |
Diet 1 (CRD) | Dite 2 (NAD) | Diet 3 (MWL) | Diet 4 (MAE) | Diet 5 (MEE) | Diet 6 (LWL) | Diet 7 (LAE) | Diet 8 (LEE) | Diet 9 (ANTB) |
|---|---|---|---|---|---|---|---|---|---|
| FM (72.4% CP) | - | 50.4 | 49.4 | 49.4 | 49.4 | 49.4 | 49.4 | 49.4 | 49.4 |
| MM (14.0%) | - | 44.6 | 43.6 | 43.6 | 43.3 | 43.6 | 43.6 | 43.6 | 43.6 |
| VMP | - | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Oil | - | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Additive | - | - | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Total | - | 100.00 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Compositions(%) | |||||||||
| Moisture | 4.12 | 2.3 | 2.48 | 2.84 | 2.22 | 2.55 | 2.82 | 2.38 | 2.41 |
| Crude Protein | 46.6 | 45.18 | 44.86 | 44.89 | 44.10 | 44.50 | 44.50 | 44.75 | 45.43 |
| Ether Extract | 6.00 | 8.50 | 8.80 | 9.70 | 9.00 | 8.40 | 9.90 | 9.20 | 9.30 |
| Crude Fibre | 2.86 | 2.30 | 2.6 | 2.67 | 2.58 | 2.40 | 2.67 | 2.58 | 2.58 |
| Ash | 17.40 | 14.18 | 18.60 | 17.01 | 15.57 | 17.40 | 16.80 | 13.58 | 14.9 |
| NFE | 23.02 | 27.54 | 22.66 | 22.89 | 26.30 | 24.75 | 23.25 | 27.51 | 25.38 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
CRD:Commercial Reference Diet; NAD:Non Additive Diet; MWL:Moringa Whole Diet; MAE:Moringa Aqueous Extract Diet; MEE:Moringa Ethanol Extract Diet; LWL:Lannea Whole Diet; LAE:Lannea Aqueous Extract Diet; LEE:Lannea Ethanol Extract Diet; ANTB: Antibiotics; FM:Fish meal; VMP:Vitamin and Mineral Premix; CP:Crude Protein.
Table 1: Diets Formulation and Chemical Compositions of Experimental Diets Fed Clarias gariepinus fingerlings for 56 Days.
The ingredients used were maize meal, fish meal, vitamin and mineral premix 3%, vegetable oil and additives extract of Moringa leaves, Lannea bark and Oxytetracycline (Antibiotic) were included at 2% each in the diets (Table 1). The ingredients were measured according to the formulation and were mixed thoroughly to ensure homogeneity. The additives were dissolved into 300 mL of warm water which was added to 1 kg diet that ensure good dough. The dough was fed into a hand pelleting machine. The pelleted diets were oven dried for two days at 60°C. The dried pellets were then broke into small particles to suit the buccal cavity of the fingerlings.
Experimental Procedures
Five hundred and fourty (540) Clarias gariepinus fingerlings of an average weight of 4.60 ± 0.02 g were acclimated for two weeks at Water Resources, Aquaculture and Fisheries (WAFT) Departmental laboratory before they were randomly distributed into 27 tanks of the 50 L recycling plastic tanks of filled with 20 L volume of water each. Each of the tanks was stocked with 20 fish in 3 replicate. Water flow rate was maintained at 1.5 L per min. Water quality parameters were measured weekly and reading were taken throughout the experimental period. Ranges of water quality are shown in Table 2. Temperature and dissolved oxygen was measured using hand held Dissolve Oxygen meter (AZ8403), pH was measured using pocket size pH meter (HI96107), and total conductivity was measured using pen type digital conductivity meter (CT-3030) while total alkalinity was determined chemically in the laboratory following the NIS [13] procedures. Fish were fed 5% of their body weight per day in two equal meals between 10 am and 4 pm for 56 days. The fish were weighed fortnightly and feeding rates were adjusted accordingly. Uneaten feeds faecal matter were siphoned from each tank, oven dried and kept according to each treatment faecal analysis. At the end of the experiment, growth parameters and feed utilization indices were determined using the following formulae;
| Parameters | Ranges NIS, 2007 |
|---|---|
| Temperature (ºC) | 26.0 – 29 |
| pH | 6.9 – 8.0 6.5- 8.5 |
| conductivity (μS/cm) | 150.0 – 220.0 100-500 |
| Total Alkalinity (mg/l) | 80.7 – 95.3 |
| Dissolved Oxygen (mg/l) | 4.0 – 5.7 3-7 mg/l |
NIS= Nigeria Industrial Standard
Table 2: Ranges of Weekly Average Water Quality Parameters Monitored in All Experimental Tanks During56 Days Feeding Trial
Mean Initial Weight (g) = 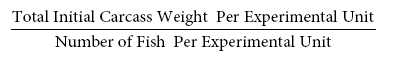
Mean Final Weight (g) = 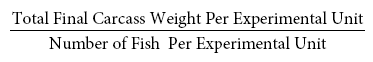
Mean Weight Gain (g) = Mean Final Carcass Weight (g) – Mean Initial Carcass Weight (g) [14]
Specific Growth Rate (%) = 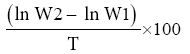
Where; W1 = Fish Initial Weight
W2 = Fish Final Weight
ln = Natural Logarithm
T = Number of Days in the Experiment
Food Conversion Ratio = 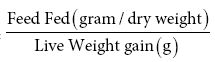
Protein Efficiency Ratio = 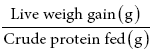
Apparent Net Protein Utilization (%) = 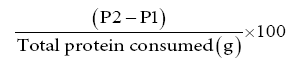
Where; P1 = Protein in Fish Carcass (g) at initial level of the experiment
P2 = Protein in Fish Carcass (g) at the end of the experiment [14]
Survival Rate =  [15]
[15]
Statistical Analysis
Evaluations of growth parameters of the diets were subjected to one-way analysis of variance (ANOVA) using P<0.05 significance level to test for significant difference. The parameters of mean comparison were applied according to Duncan multiple range test [16]. All the statistics were computerised using stat graphics (version 3.0) and Minitab (version 9.2) packages.
Results
Growth parameters and nutrients utilization of experimental fish (C. gariepinus) fed supplemented plants additives of three different extraction methods (whole, aqueous and ethanol) in their diets are shown in Table 3 with significant differences (P<0.05). The survival rate of the experimental fish was between 90% - 98%. There was improvement on the growth performance of the fish that were fed the plant additives even though their performance were significantly lower (P<0.05) than those of control diet (Commercial Reference Diet (CRD)), Non-Additive Diet (NAD) and Oxytetracycline (Anti-biotic -ANTB). However, among the diets containing plant additives, the best growth response was achieved in Lannea Plant Whole (LWL) based diet (Table 3). There was no significant difference (P>0.05) for Specific Growth Rate (SGR) of the fish in the group of CRD, NAD and ANTB as the values were 2.46% ± 0.21, 2.48% ± 0.17 and 2.23% ± 0.23 respectively. There was no significant difference (P>0.05) in all values recorded for Feed Conversion Ratio (FCR) while CRD, NAD and ANTB were not significant different (P>0.05) from each other in their Protein Efficiency Ratio (PER) values (2.02% ± 0.18, 2.06% ± 0.14 and 2.00% ± 0.21). Similarly, there were no significance difference (P>0.05) in all the diets containing plant additives which are MWL (1.42 ± 0.09), MAE (1.42 ± 0.13), MEE, (1.60 ± 0.13), LWL (1.73 ± 0.05), LAE (1.48 ± 0.07) and LEE (1.60 ± 0.09) in Protein Efficiency Ratio. The Apparent Net Protein Utilization (ANPU) was highest in LEE (98.33 ± 2.89) based diet while the lowest value was recorded in ANTB (5.57 ± 1.92) based diet (P<0.05).
| Growth Parameters | Diet 1 (CRD) | Diet 2 (NAD) | Diet 3 (MWL) | Diet 4 (MAE) | Diet 5 (MEE) | Diet 6 (LWL) | Diet 7 (LAE) | Diet 8 (LEE) | Diet 9 (ANTB) |
|---|---|---|---|---|---|---|---|---|---|
| MIW (g) | 4.61a ± 0.12 |
4.59a ± 0.032 |
4.63a ± 0.08 |
4.63a ± 0.04 |
4.62a ± 0.02 |
4.55a ± 0.06 |
4.60a ± 0.08 |
4.57a ± 0.06 |
4.55a ± 0.02 |
| MFW (g) | 18.37a ±2.32 | 18.48a ± 1.63 |
11.33c ± 0.54 | 11.46c ± 0.99 | 13.06b ± 1.34 |
13.63b ± 0.64 |
11.85c ± 0.66 |
12.87bc ± 0.84 |
15.99ab ± 2.11 |
| MWG (g) | 13.76a ± 2.27 |
13.87a ± 1.67 |
6.70cd ± 0.62 |
6.83cd ±0.96 | 8.45bc ± 1.32 |
9.08b ± 0.58 |
7.26c ± 0.60 |
8.30bc ± 0.79 |
11.42ab ±2.09 |
| SGR (%)/day | 2.46a ± 0.21 |
2.48a ±0.17 | 1.60b ± 0.12 |
1.61b ± 0.15 |
1.85b ± 0.19 |
1.96b ± 0.06 |
1.69b ± 0.70 |
1.85b ± 0.10 |
2.23a ± 0.23 |
| FCR | 1.10a ± 0.10 |
1.08a ± 0.07 |
1.57a ± 0.10 |
1.57a ± 0.10 |
1.39a ± 0.11 |
1.29a ± 0.04 |
1.51a ± 0.07 |
1.39a ± 0.08 |
1.12a ± 0.11 |
| PER | 2.04a ± 0.18 |
2.06a ± 0.14 |
1.42ab ± 0.09 |
1.42ab ± 0.13 | 1.60ab ± 0.13 |
1.73ab ± 0.05 |
1.48ab ±0.07 | 1.60ab ± 0.09 |
2.00a ± 0.21 |
| ANPU (%) | 91.40ab ± 5.53 |
79.06bc ± 6.64 | 34.81cd ± 1.71 | 66.45c ± 6.05 | 82.18b ± 9.18 |
31.72de ± 1.41 | 33.19d ± 1.82 |
93.28a ± 3.94 |
5.57e ± 1.92 |
| Survival (%) | 95.00ab ± 0.00 |
93.33b ± 2.89 |
98.33a ± 2.89 | 90.00c ± 8.66 | 95.00ab ± 0.00 | 91.67bc ± 5.77 | 98.33a ± 2.89 |
98.33a ± 2.89 |
93.33b ± 7.64 |
Average values on the same row carrying similar superscripts are not significantly different from each other (P>0.05). MIW:Mean Initial Weight; MFW:Mean Final Weight; MWG:Mean Weight Gain; SGR:Specific Growth Ratio; FCR:Food Conversion Ratio; PER:Protein Efficiency Ratio; ANPU: Apparent Net Protein Utilization.
Table 3: Growth parameters and Nutrient Utilization of Experimental Diets Fed Clarias gariepinus fingerlings for 56 Days
Discussions
Findings show that, the experimental fishes accepted all the diets. However, the Specific Growth Rate (SGR) values obtained from the group of fish fed with Commercial Reference Diet (CRD), Non Additive Diet (NAD) and Anti-biotic (ANTB) were better than those fed with plant additive diets (2.23 ± 0.23). The poor growth performance observed from plant additive diets may be as result of the crude plant additives that were incorporated into the diets without the deactivation of antinutritive factors such as saponins, tannins and phytic acid that may hinder fish digestion [17] might be the cause. This were in disagreement with the findings of Dada [18] who reported that, Oreochromis niloticus fingerlings fed on diet supplemented by probiotics exhibited greater growth than those fed with control diets. The Feed Conversion Ratio (FCR) for all the diets additives and non-additive diets were in conformity with that of the values obtained by Nina [19]; Ovie and Adejayan [20]; Dada [18]. The latter reported that, FCR values ranging between 1.10 and 1.23 for Clarias gariepinus are not significant different (P>0.05) in the various growth parameters even though they were fed with varying levels of garden snails Limicolaria spp. The high survival rate recorded could be as a result of used plants and constant monitoring of water quality as the highest survival rates were observed from fish of plant additives based diets. This was also an indication that, the used plants were not toxic to the fish health as it was in line with the confirmation of Adewumi [21] who used Moringa oleifera leaf meal as protein supplement in C. gariepinus diet. The mean water quality parameter measured during the experiment were within the range of NIS [13] standard and non-toxic phyto-nutrients content of additives as the highest survival rate was recorded in the group of fishes that were fed plant additives.
Conclusion
The results from the experiment indicate that the plants used for the experiments were not toxic to fish health. This is an encouragement to the use plant of medicinal values as an additive in aquaculture diets. The fishes of Lannea Whole (LWL) based diet and Lannea Ethanol Extract (LEE) diets expressed better growth compared with Moringa based diets.
Recommendation
Further experiments will be required in subjecting the additives to various inclusion levels in order to establish the optimal inclusion level of plant additive in the fish diet. Achieving this will boost fish growth performance using plant additive within a limited time and further discourage fish farmers from using antibiotic and synthetic fish growth promoters which have been established to negatively impact residual effects on the consumers.
References
- Shim SM, Ferruzi MG, Kim YC, et al. Impact of Phytochemical-rich foods on Bioaccessbility of Mercury from Fish. Food Chem. 2009;112:46-50.
- FAO. Food and Agriculture Organization of the United Nations. FAO year book: Fisheries and Aquaculture Statistics. 2016;105.
- Villa-Cruiz V, Davilla J, Viana MT, et al. Effect of broccoli (Brassica oleracea) and its phytochemical suforaphane on balanced diets on the detoxification of enzyme levels of Tilapia (Oreochromis niloticus) exposed to carcinogenic and mutagenic pollutant. Chemospher.2009;74:1145-51.
- Naga PKB, Mahaboobi S, Akhileshlesh T. Effect of Feed Additives on Growth Performance of Fish. J Fishscicom.2016;10(3):84-87.
- Ajiboye OO, Yakubu AF, Adams TE. A Perspective on the Ingestion and Nutritional Effects of Feed Additives in Farmed Fish Species. World J. Fish & Marine Sci. 2012;4(1):87-101.
- Lim SJ, Jang E, Lee SH, et al. Antibiotic Resistance in Bacteria Isolated from Freshwater Aquacultures and Predictions of the Persistence and Toxicity of Antimicrobial in Aquatic Environment. J Environ Sci Health B. 2013;48:495-504.
- Adekunle AD. Effect of herbal growth promoter feed additive in fishmeal on the performance of Nile Tilapia (Oreochromis niloticus (L)). Egypt Acad J Biolog Sci. 2012;4(1):111-7.
- Citarasu T. Herbal biomedicines: A new opportunity to aquaculture industry. Aquacult Int. 2010;18:403-4.
- Baruah K P, Norouzitallab D, Debnath AK, et al. Organic acids as non-antibiotic nutraceuticals in fish and prawn feed. Aquacul Health Int. 2008;12:4-6
- Nwerze NO, Nwafor FI. Phytochemical, Proximate and Mineral Composition of Leaf Extracts of Moringa oleifera Lam. From Nsukka, South-Eastern Nigeria. IOSR J Pharm Biol Sci. 2014;91-103.
- Koné WM, Soro D, Dro B, et al. Chemical Composition, Antioxidant, Antimicrobial and Acetylcholinesterase Inhibitory Properties of Lannea Barteri (Anacardiaceae). Aust J Basic Appl Sci. 2011;5(10):1516-23.
- AOAC. Official Method of Analysis 16th Edition, Association of Official Analytical Chemists,Washington D.C. U.S.A. 2010.
- Nigerian Industrial Standard. NIS 554. Nigerian Standard for Drinking Water Quality. Approved by Standard Organisation of Nigeria Governing Council. 2007.
- De Silva SS, Anderson TA. Fish Nutrition in Aquaculture. Chapman and Hall. Melbourne. 1995.
- Bbole L, Mumba C, Mupenda N, et al. Analysis of growth performance and haematological parameters of Oreochromis niloticus fed on a varying diet of Moringa oleifera Lam. leaf meal as an additive protein source. International Journal of Fisheries and Aquaculture. 2016;8(11):105-11
- Duncan DB. Multiple Range and Multiple F test. Biometrics. 1955;11:1-42.
- Tiamiyu, Lateef, Okomoda, et al. Growth Performance of Clarias gariepinus Fed Varying Levels of Sorghum bicolor Waste Meal. International Journal of Aquaculture. 2016;6(20):1-7
- Dada AA. Effect of Herbal Growth Promoter Feed Additive in Fish Meal on the Performance of Nile Tilapia (Oreochromis niloticus). Egypt. Acad. J. Biolog. Sci. 2012;4(1):111-7.
- Nina C. Feather Meal A Useful Addition to Fish Feed. Journal of Animal Nutrition. (feed additives). 2015.
- Ovie SO, Adejayan AB. Effects of Supplementing Fish Meal with Garden Snail (Limicolaria Spp.) in Clarias gariepinus Diets. Sciencepub. 2010;2:58-62.
- Adewumi AA. Moringa oleifera (Lam) as a Protein Supplement in Clarias gariepinus Diet. Advances in Research.2014;2(11):580-9.