Research Article - Journal of Food Technology and Preservation (2017) Volume 1, Issue 1
Impact of sub-lethal 2(3)-tert-butyl-4 hydroxyanisole (BHA) microcapsules on behavior of Aspergillus flavus in culture medium and peanut seeds.
Daiana Garcia1*, Natalia Soledad Girardi1, Andrea Nesci2, María Alejandra Passone2, Miriam Etcheverry21Doctoral fellow of the National Council of Scientific and Technical Research, Argentina
2Research Career, National Council for Scientific and Technical Research, Argentina
- *Corresponding Author:
- Daiana Garcia
Micro Biology
National Council for Scientific and Technical Research
Argentina
Tel: +54 358-4676113
Fax: +54 358-4676231
E-mail: dgarcia@exa.unrc.edu.ar
Accepted date: December 14, 2016
Citation: Garcia D, Girardi NS, Nesci A, et al. Impact of sub-lethal 2(3)-tert-butyl-4 hydroxyanisole (BHA) microcapsules on behavior of Aspergillus flavus in culture medium and peanut seeds. J Food Technol Pres. 2016;1:11-17
Abstract
The aim of this work was the evaluation of physiological behavior of Aspergillus flavus affected by the application of sub-lethal doses of microencapsulated 2(3)-tert-butyl-4 hydroxyanisole (BHA). For this, peanut meal extract agar (PMEA) and peanut kernels with modified water activity (aw) (0.96 and 0.99) and a sub-lethal dose (0.6 mM) of an BHA formulation was used. Fungal physiological aspects as growth rate, time to growth and AFB1 evolution were modified by the formulation, especially on PMEA at both aw. In general, conidial and vesicle size of the mold only were affected by growth substrate, being higher on sterile seed than on PMEA regardless of aw condition. However, positive correlation (p<0.05) observed in controls between radius and biomass responses was altered by the application of the formulation, mainly under the lowest aw in both substrate evaluated. Finally, presence of encapsulated antioxidant showed significant change in Pearson coefficients respect to the controls for all studied parameters. As conclusion, sub-lethal doses of formulation lead in reduction on growth and toxin accumulation, but conidial and vesicle size were not affected. Results of this work indicate the need to consider both fungal primary and secondary metabolism to determine the effect of food grade antioxidant formulation.
Keywords
Peanut, Aspergillus flavus, Aflatoxin B1, Microcapsules, BHA
Introduction
Peanut (Arachis hypogaea L.) is a very important dried fruit considered as one of the most widely used nuts due to their nutritional properties and taste. Some nutritional characteristics studied for this nut and its oil are the high levels of proteins and minerals with a well-balanced fatty acid and antioxidant profiles [1,2]. Besides, this food is very important for the Argentina economy due to the continuous increase in the yield with a total production of 1.17 million tons in 2015/16 harvest season [3]. Moreover, around 0.44 and 0.68 million tons are exported ranking the first position as peanut exporter since 2012 [4]. Due to the economic importance of peanut, quality and safety are essential for marketing. However, is considered to be a high-risk product for contamination with aflatoxins (AFs) due to frequently contaminated with moulds, particularly Aspergillus flavus and Aspergillus parasiticus, and because of long peanut drying times and occurrence of rainy periods after uprooting [5]. Both aflatoxigenic funguses are between the most important storage moulds founded by many studied in stored peanut [6-11]. Particularly, Passone et al. [12] reported the prevalence of Aspergillus section Flavi aflatoxin producing strains (65 and 75%) on stored peanut in big bags with four different aw levels. Aflatoxin B1 (AFB1) is considered to be a carcinogen type 1 by the International Agency for Research on Cancer [13]. Mycotoxins can be produced in peanut grains in the field, during transport and storage where conditions are suitable for their production becoming a high risk to human and animal health [14]. Mould growth is commonly controlled using synthetic fungicides; however continuous and indiscriminate use of these chemical compounds in foods and feeds, could lead to toxic effects for consumers and to the development of resistances in microorganisms [15]. One alternative to the use of synthetic fungicides which could lead to persistent residues, carcinogenic and toxic effects is the use of harmless substances, like food grade antioxidant as butylated hydroxyanisole (BHA) (phenolic antioxidant). This compound has shown effect against moulds and insect vectors of aflatoxigenic fungi on stored peanuts [16-20]. However, is known that levels of BHA decreased with time when it was applied in peanut food system due to the interaction with physical and biological factors [21] (Passone, et al. 2008c). Girardi et al. [22] applied BHA microencapsulation technology in order to keep food grade antioxidants from the action of environmental factors. Besides, Garcia et al. [23] showed that 20 mm of BHA formulation completely inhibited Aspergillus section Flavi development and AF aflatoxin accumulation; nevertheless lower dose decreased both growth and toxin levels. In this work, our interest focuses on the physiological behavior of Aspergillus flavus affected by the exposure to sublethal BHA formulation. To elucidate this impact the aims of this work were i) to assess growth parameters based on radial growth and biomass dry weight; ii) to study evolution of AFB1 and iii) determinate vesicle and conidial sizes under optical microscope, in presence of sub-lethal inhibitory concentrations of antioxidant formulation, at two different water activities (aw) in peanut meal extract agar (PMEA) and peanut grains.
Material and Methods
Fungal isolate and preparation of inoculum
A. flavus (RCP08108) a mycotoxigenic isolate was included in this research. The reference in bracket is the code of cultures held in the Microbial Ecology Laboratory Collection, Department of Microbiology and Immunology of the National University of Río Cuarto, Córdoba, Argentina. Isolate was sub-cultured on malt extract agar (MEA) plate and incubated at 25ºC for 7 days to enable significant sporulation. After incubation, a sterile inoculation loop was used to remove the conidia from MEA plates and it was suspended in 5 ml of distilled peptone water solution (0.1%). After homogenization, the suspension was adjusted using a Neubauer counting chamber to achieve final concentration of 1–5×104 spores/ml.
Preparation of antioxidant formulation
An industrial grade antioxidant, 2(3)-tert-butyl-4 hydroxyanisole (BHA) obtained from Eastman Chemical Company was used as core material. Formulation was made by complex coacervation using gelatin (bloom 240) and Arabic gum as core material following the methodology proposed by Girardi et al. [22]. Empty capsules were performed with the same methodology but without the addition of BHA, in order to be used as control by replacing the core material with peanut oil. Sub-lethal dose of antioxidant formulation (0.65 mm) was used in both culture medium and peanut grains.
Culture medium and peanut kernels preparation
Peanut meal extract agar (PMEA) was prepared at 2% (w/v) with a final pH of 6.5. Thirty grams of ground peanut per liter were boiled for 45 min and the resultant mixture filtered through a double layer of muslin. Volume was made up to 1 l and agar?agar at 2% (w/v) was added [24]. Water activity of the basic medium (0.99) was adjusted to 0.96, with known amounts of glycerol [25]. Basic medium was autoclaved at 121°C for 20 min before cooling at 50°C and then poured into 9cm sterile Petri dishes and microcapsules with or without BHA were added to reach 0.6 mm concentration. Water activity of representative samples of each treatment was checked after autoclaving with an AquaLab Water Activity Meter 4TE with an accuracy of ±0.001 (Decagon Devices, Inc.) Peanut kernels were sterilized by autoclaving at 120°C during 20 minutes twice. Water activity of sterile peanut was adjusted by aseptic addition of distilled water to seeds inside sealed containers which were kept at 4°C for 48 hours with periodic hand-shaking during this time. The amount of water necessary to reach the different aw levels was determined by a calibration curve (water activity-ml vs. water to be added/g substrate) previously made [23]. Therefore, aw of seeds was modified at 0.96 and 0.99 by the addition of 200 and 550 μl/g of sterile water, respectively and checked with the Aqua Lab Water Activity Meter 4TE (Decagon Devices, Inc.). Twenty five g of peanut were poured in Petri plates forming a single layer and the formulation with or without antioxidant was added to reach 0.6 mm concentration.
Inoculation and incubation
Petri dishes were inoculated centrally with 2 μl of a 1-5 × 104 spores/ml suspension. Plates with the same aw were enclosed in sealed containers along with beakers containing water glycerol at the same water condition. One hundred PMEA Petri dishes and 90 peanut kernels Petri dishes were inoculated and incubated at 25ºC.
Experimental design
In this work, a series of PMEA (100) and peanut Petri plates (90) with loaded, unloaded and without BHA microcapsules were prepared at 0.96 and 0.99 aw, and inoculated with A. flavus. PMEA and peanut plates contained 30 ml of medium and 25 g of seeds, respectively. During and after incubation period, Petri plates were analyzed (3 replicates per analysis) for determination of colony radius, biomass weight and AF content, conidial and vesicle size.
Growth assessment
Colony radius (mm) and biomass (mg dry weight) were measured at different time periods. Colony radius was daily examined for an overall period of 30 days by measurements at right angles with the aid of a ruler and a binocular magnifier, on PMEA and peanut Petri dishes. Mycelium dry weight was measured as Taniwaki. On culture medium. Colonies were cut from the medium, transferred to a beaker containing distilled water (100 ml approximately), then heated in a streamer for 30 min to melt agar. Mycelium remained intact was collected and transferred to a dried, weighed filter paper and dried at 80ºC for 18 hrs. Then the filter paper was weighed and the dry weight of biomass was calculated by difference.
Aflatoxin B1 analyses from PMEA and peanut seeds
Aflatoxin B1 extraction from PMEA: Three agar plugs (diameter 4 mm) of each colony were removed from center, middle and outer of the colonies at different incubation times and placed in a vial. One ml of methanol was added, and the vials were shaken for 5 s. After 60 min, the extracts were shaken, filtered (Microclar 0.45 μm, MC-NYL-04N), transferred into another vial and stored at 4ºC until the analysis by HPLC instrument (Waters, Mildford, MA, SA).
Aflatoxin B1 extraction from peanut kernels
Determination of AFB1 in peanut kernels was performed according to AOAC´s official method 994.08 with some modifications. Total AFs were extracted at different times from a representative sample (25 g) of ground peanut with 100 ml of acetonitrile: water (84:14 v/v) for 30 min using an orbital shaker, and the supernatants was filtered through Whatman Nº4 filter paper. Then, 5 ml of the extract was applied to a multifunctional cleaned column (R-BIOPHARM Rhone LTD). The filtrate (2 ml) was evaporated to dryness and stored at 4°C until to highperformance liquid chromatography (HPLC) analysis.
Aflatoxin B1 detection and quantification
Aflatoxin B1 was detected and quantified separately by using a HPLC system (Waters 2696 separations module, Waters, Milford, USA). Aflatoxin B1 quantification was performed according to Trucksess. with some modifications. Dry extracts were dissolved in 200 μL of acetonitrile: water (9:1) and derivatized with 700 μl of trifluoroacetic acid:acetic acid:water (20:10:70). One hundred μl of the derivatized solutions were inject in HPLC system (Waters 2696 separation module, Waters, Milford, USA) and chromatographic separations were performed on a stainless steel C18 reverse phase column (150×4.6 mm i.d.,5um particle size, Phenomenex, USA ). Water: methanol: acetonitrile (66.6:16.7:16.7) mixture was used as the mobile phase at a flow rate of 1.5 ml/min. A Waters 2475 module was used for fluorescence detection (λexc 360 nm; λem 440 nm). Quantification was achieved with a software integrator (Empower, Milford, MA, USA). The mycotoxin was quantified on the basis of the HPLC fluorimetric response compared with that of a range of mycotoxins standards. Detection (LOD) and quantification (LOQ) limits of the analytical method were 1.5 ng/g and 4.5 ng/g, respectively.
Conidial and vesicular size evaluation
Size of conidia and vesicles of A. flavus were examined at the final of the assay (20 days) by optical microscopy at 40×magnification (Carl Zeiss, 37081). Diameters of one hundred conidia and twenty vesicles were registered for each treatment. Images were captured and size was estimated by using Motic Images Plus 2.0 (2005) software.
Statistical analysis
Diameters of growing colonies were plotted against time and Baranyi and Roberts model was used to estimate growth rate and time to growth for each condition. Analysis of variance of growth rates, time to growth, conidial and vesicle size and AFB1 accumulation was used in order to assess significant differences due to growth conditions and BHA formulation assayed. Mycotoxins were expressed as ng/g of culture medium or peanut. LSD test was used to establish the differences among mean values of the variables under the different levels of factors at p<0.05. Pearson correlation coefficient was used to evaluate correlations between studied factors.
Results
A. flavus (RCP08108) growth assessment growing on PMEA and peanut seeds treated with BHA formulation
Analysis of variance (ANOVA) was performed to determine the effect of growth substrate (Gs), treatment (T), (aw) and their interactions on fungal growth in culture medium through both colony radius and biomass assays. On the one hand, all factors alone and their interactions affected significantly (p<0.1) both mould growth parameters estimated from colony: radial “growth rate” [μ (mm/d)] and “time to growth” [λ (days)]. Growth rate was highly affected by aw (p<0.1 and F=71.23) followed by Gs (p<0.1 and F=19.56) and T (p<0.1 and F=3.4). While λ was greatly affected by Gs (p<0.1 and F=150.50) and T (p<0.1 F=26.5). On the other hand, both growth parameters obtained from biomass dry weight also were affected by aw and T (aw, p<0.1; F=104.93 and T, p<0.1; F=5.50 for μ and aw, p<0.1; F=12.12 and T, p<0.1; F=119.03 for λ). Table 1 shows the effect of microencapsulated BHA at sub-inhibitory level of 0.6 mm on radial μ (mm/d) and λ (days) values estimated from radial growth and biomass dry weight assays on PMEA and peanut kernel conditioned at different aw (0.99, 0.96). Results showed that on PMEA, for each aw assayed, both growth parameters on radial experiments revealed significant differences between control without capsules (CWC) and BHA formulation (F-BHA) or control with empty microcapsules (CEC), according to LSD test (p<0.05). With this respects, application of microcapsules (with or without antioxidant) modified radial μ and λ in culture media where caused a reduction in μ and an increase in λ in the order of 20 and 50%, respectively, for the both studied water conditions compared with CWC. However, no significant differences (p<0.05) for μ parameter estimated trough biomass assay where found, while λ show a decrease of 90% when mould grew in presence of BHA capsules, regardless aw. As for biomass dry weight, growth studies performed on peanut kernels did not showed significant differences (p<0.05) for μ between treatment and controls samples at the both aw, but λ parameter was significantly increased (48%) by the presence of microcapsules in the substrate.
Table 1: Effect of preservatives and pH on Aspergillus niger 13D growth.
| Preservatives | Concentration (%) | pH values | Fungal growth rate* | Maximum mycelial growth¥ |
|---|---|---|---|---|
| Calcium Propionate | 0 | 5.0 | 1.39 ± 0.01a | 8.4 ± 0.1a |
| 5.5 | 1.39 ± 0.01a | 8.5 ± 0.1a | ||
| 6.0 | 1.39 ± 0.01a | 8.5 ± 0.0a | ||
| 0.1 | 5.0 | 0.68 ± 0.04a | 5.9 ± 0.2a | |
| 5.5 | 0.71 ± 0.04a | 5.9 ± 0.2a | ||
| 6.0 | 0.89 ± 0.02b | 7.1 ± 0.3b | ||
| 0.2 | 5.0 | 0.25 ± 0.06a | 0.7 ± 0.1a | |
| 5.5 | 0.35 ± 0.05b | 2.7 ± 0.4b | ||
| 6.0 | 0.42 ± 0.01b | 2.8 ± 0.4b | ||
| 0.3 | 5.0 | 0.00 ± 0.00a | 0.0 ± 0.0a | |
| 5.5 | 0.00 ± 0.00a | 0.0 ± 0.0a | ||
| 6.0 | 0.28 ± 0.06b | 0.8 ± 0.0a | ||
| 0.4 | 5 | 0.00 ± 0.00a | 0.0 ± 0.0a | |
| 5.5 | 0.00 ± 0.00a | 0.0 ± 0.0a | ||
| 6 | 0.00 ± 0.00a | 0.0 ± 0.0a | ||
| Potassium Sorbate | 0.05 | 5.0 | 0.89 ± 0.01a | 8.5 ± 0.1a |
| 5.5 | 1.03 ± 0.15a | 8.5 ± 0.1a | ||
| 6.0 | 0.91 ± 0.09a | 8.5 ± 0.1a | ||
| 0.1 | 5.0 | 0.56 ± 0.04a | 4.5 ± 0.3a | |
| 5.5 | 0.59 ± 0.08a | 5.1 ± 0.5a | ||
| 6.0 | 0.63 ± 0.08a | 5.8 ± 0.0a | ||
| 0.2 | 5.0 | 0.54 ± 0.12a | 3.3 ± 0.7a | |
| 5.5 | 0.45 ± 0.03a | 3.7 ± 0.3a | ||
| 6.0 | 0.50 ± 0.07a | 4.4 ± 0.2a |
*Fungal growth rate: cm of colony days -1.
¥Fungal colony diameter (cm) in WBM agar medium with or without preservatives after 10 days at 30ºC. Variables with the same superscript letter in the same column show no significant differences between them (P<0.05).
Conidial and vesicular size of A. flavus (RCP08108) affected by BHA formulation in culture media (PMEA) and peanut kernels
Effects of single factor as well as their two and three way interactions on conidial and vesicle sizes for both culture media and peanut kernels were determined by ANOVA. Conidial diameter was only affected by Gs (p<0.1 and F=11.3), while vesicle diameter was affected by aw conditions (p<0.1 and F=7.37) and its interaction with Gs (p<0.1 and F=10.37). Conidial and vesicular size of A. flavus (RCP08108) that developed on PMEA and peanut kernels modified at 0.96 and 0.99 aw in presence of microencapsulated BHA, CEC and CWC at the end of the experiment (20 days) are shown in Figure 1A and 1B. No significant differences (p<0.05) between conidial size estimated in treatment (mean=9.4 μm and 11.3 μm for PMEA and peanut kernels, respectively) and control samples (mean=9.4 μm and 11.5 μm for PMEA and peanut kernels, respectively) were found (Figure 1A). However, conidial diameters were significantly higher in peanut kernels than in PMEA according to LSD test (p<0.05) by recording mean sizes of 11.5 and 9.5 μm respectively. Besides, water conditions significant affected (p<0.05) size of the spores on PMEA where differences around of 15% were found between 0.96 aw and 0.99 aw. However, when A. flavus grew on peanut substrate, size of conidia was independent of the kernel water condition. Figure 1B shows that vesicle size was no influenced by the water condition of peanut based agar with a mean diameter of 98 μm. Nevertheless, when A. flavus developed on peanut kernels, size of vesicle was 40% higher at the lower aw (0.96) assayed. No significant differences (p<0.05) between treatment and controls were found, except for the kernels at 0.99aw where fungus developed vesicles 32% higher in presence of F-BHA formulation and CEC compared to CWC.
BHA formulation effects on trend of AFB1 accumulation by A. flavus (RCP08108)
ANOVA test of the effect of Gs, aw and T and their interactions showed that accumulation of AFB1 at the final of incubation period (20 days) was statistically affected by Gs (p=0.00 and F=134.2), aw (p=0.00 and F=52.2), T (p=0.00 and F=28.9) and their two and three way interactions [(Gs*aw; p=0.00 and F=24.8); (Gs*T; p=0.00 and F=12.6); (aw*T; p=0.00 and F=14.01) and (Gs*aw*T; p=0.01 and F=7.04)]. For the two aw assayed, AFB1 levels detected in controls were significantly higher respect to BHA treatment according to LSD test (p<0.05), regardless of substrate evaluated. On the one hand, when AFB1 accumulation on PMEA-CWC was evaluated, levels of this metabolite sharply increased in the order of 6437.6 and 3150.5 μg/g at 0.96 and 0.99 aw, respectively, after 14 days of incubation, being statistically higher (p<0.05) (50%) at the lowest water stress condition (0.96) (Figure 1B). A. flavus strain growing in presence of CEC produces a pick of AFB1 at 11 and 14 days of incubation at 0.96 and 0.99 aw respectively, being the levels lower (1.7 to 2.5 times) than in CWC during all incubation period, regardless of substrate aw. Similar trend of toxin accumulation was found when fungus grew in culture medium that with the addition of microcapsules, showing levels of 2105.2 μg/g at 0.96 aw and 1491.9 μg/g at 0.99 aw at the end of the assay. On the other hand, levels of AFB1 on peanut kernels in CWC showed a gradual increase of the toxin until 11 days where a decline occurred for both aw. Final levels detected were 6361.2 and 7180.6 μg/g at 0.96 and 0.99aw, respectively. For CEC and F-BHA, toxin evolution was dependent of aw condition. In the case of CEC, at 0.96 aw, AFB1 accumulation was increased with time except at 11 days where a decreased of 60% was observed compared with 7 days. At this aw condition, mean level estimated was14286.5 μg/g at 20 days of incubation. Although at 0.99 aw a production pick of 9084.1 μg/g was observed at 7 days and levels registered at the end of the experiment (20 days) were 3 times lower (5311.2 μg/g) compared with the lowest water condition (0.96). Finally, production pattern of the toxin in presence of F-BHA at 0.96 aw, showed a sharp increase at 7 days of incubation and this level was maintained around of 11122.5 μg/g until 20 days where a decline of the toxin accumulation was found (6935.8 μg/g). However, at the highest water condition (0.99) AFB1 was increasing with time showing a production pick at 7 days (8227.1 μg/g) with a mean final level of 6259.5 μg/g.
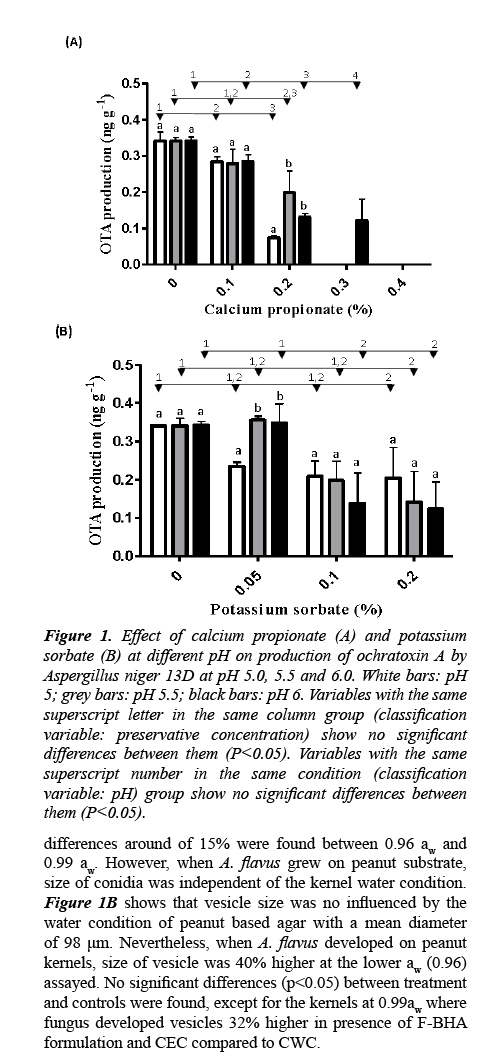
Figure 1: Effect of calcium propionate (A) and potassium sorbate (B) at different pH on production of ochratoxin A by Aspergillus niger 13D at pH 5.0, 5.5 and 6.0. White bars: pH 5; grey bars: pH 5.5; black bars: pH 6. Variables with the same superscript letter in the same column group (classification variable: preservative concentration) show no significant differences between them (P<0.05). Variables with the same superscript number in the same condition (classification variable: pH) group show no significant differences between them (P<0.05).differences around of 15% were found between 0.96 aw and 0.99 aw. However, when A. flavus grew on peanut substrate, size of conidia was independent of the kernel water condition.
Figure 1B: shows that vesicle size was no influenced by the water condition of peanut based agar with a mean diameter of 98 μm. Nevertheless, when A. flavus developed on peanut kernels, size of vesicle was 40% higher at the lower aw (0.96) assayed. No significant differences (p<0.05) between treatment and controls were found, except for the kernels at 0.99aw where fungus developed vesicles 32% higher in presence of F-BHA formulation and CEC compared to CWC.
Correlation among studied parameters of A. flavus (RCP08108) for BHA formulation and controls
Pearson correlation coefficients of the experiments were performed on PMEA and peanut kernels for AFB1 accumulation, colony radius and biomass dry weight through experimental time to evaluate toxin evolution together growth (Table 2). Respect to PMEA assay, for both radius (mm) and biomass dry weight (mg) a significant positive correlation (p<0.05) was found only for CWC at 0.96 aw. However, for the highest water condition (0.99) all treatment presented positive correlation except for mycelial weight under CEC treatment. Regarding experiments performed on peanut, only significant positive correlation (p<0.05) between colony radius and AFB1 accumulation was found for CWC at both aw.
Table 2. Percentage of undissociated organic acid related to the pHa.
| Undissociated weak organic acid (%) | ||||
|---|---|---|---|---|
| Weak organic acid | ph | Ph 5.0 | Ph 5.5 | Ph 6.0 |
| Propionic Acid | 4.88 | 44.93 | 26.13 | 6.05 |
| Sorbic Acid | 4.76 | 40.79 | 21.99 | 3.19 |
On the other hand, Pearson correlation coefficients between final levels of AFB1, conidial and vesicle size recorded on PMEA and peanut kernels were estimated for treatment and controls. No correlations were found in controls (CEC and CWC) for the fungal parameters studied. However, BHA formulation showed a significant positive correlation (p<0.05) between AFB1 versus conidial size. No significant correlations were found between AFB1 versus vesicle size and conidial versus vesicle size.
Discussion
Antifungal and Antiaflatoxigenic effect of free and microencapsulated BHA against A. flavus and aflatoxin accumulation has been demonstrated in previous works [16- 20,23], In the present article we were focused to assess the physiological behavior of A. flavus (RCP08108) in presence of a sub-lethal dose (0.65 mm) microencapsulated food grade antioxidant. For this, the mould was exposed to BHA formulation on culture medium and peanut kernels conditioned at two different aw. With this respect, our results showed that growth parameters of A. flavus obtained in this research were affected by all studied factors (aw, Gs and T) except λ which was not influenced by aw. For both PMEA and peanut kernels, growth of the mould decreased by the presence of empty microcapsules and antioxidant formulation with similar data of growth between them. This is agreeing with results founded by Garcia et al. [23]. Which conclude that wall material of microcapsules impart specific properties of the coating increasing antifungal effect. Our data showed a reduction of μ and increase in λ in presence of microencapsulated BHA being growth higher on peanut kernels than on culture medium. This reduction in mould growth due to the addition of a low dose of microencapsulated BHA is agree with Passone. which studied the effect of sub-lethal doses of a mix of BHA:BHT (1+1 mm and 5+5 mm) against A. parasíticus and A. flavus Besides, there are many works that studied the effect of aw on A. flavus under different culture media and grains [14,24] Most of these works found that low levels of aw produce a decrease of growth mould and that growth parameters were different between culture medium and kernels. For this, it is necessary to evaluate Eco physiological behavior of aflatoxigenic strains in food system where exist other factors such as kernel components: fat, carbohydrate, protein, salt and pH which could influence in the effectiveness of the antimicrobial potency Klich and Pitt taxonomy described A. flavus conidial size between 3-6μm in Czapek Yeast extract agar (CYA). However this fungal feature in our work was around 50 and 63% higher in PMEA and peanut kernels respectively, compared with the above reference. Besides, conidial size was not affected by the presence of microcapsules but values were different among growth substrates with the highest diameters in kernels. With respect to vesicle, formulation only affected its size when A. flavus grew in peanut kernels conditioned at the highest aw (0.99), where values obtained for controls were similar to those reported in Klich and Pitt. Meanwhile, vesicle sizes of A. flavus estimated in presence of F-BHA were 50% lower than in this absence. At the lowest water condition (0.96 aw), diameters of vesicle were similar between treatments but doubled their size at the highest aw condition. As our knowledge, there are no researches which evaluated the effect of chemical agent on conidial and vesicular magnitude of A. flavus. However, it is important remark that, conidial cell born from conidiophore being responsible to the propagation of the fungus to new habitats giving rise to new individuals. Besides, sporulation processes is commonly associated with toxic metabolites production. Regarding AFB1 accumulation in this work level of this toxin detected at the end of the experiment were between 1-6.4 times higher in peanut kernels than in PMEA coinciding with μ and conidial diameters increases. May be, this is because AFB1 is related to secondary metabolism which is commonly associated with sporulation processes in molds. Thus toxin is secreted by growing colonies together conidia development. Besides, samples treated with F-BHA presented the lowest levels of the toxin for both growth substrate and aw. However, previous studies suggest that subinhibitory antioxidant levels carried out to aflatoxin production stimulation. In particular, Passone. founded high levels of the toxin accumulation and an induction in aflD gene expression involved in aflatoxin biosynthesis of A. flavus in PMEA when a sub-lethal dose of BHA and butylated hydroxytoluene mixture (1+1 mM) was used. On the other hand, toxin evolution for CWC was different from CEC and F-BHA regardless of substrate and water availability. For CWC, toxin accumulation by A. flavus increased parallel with the expansion of the colony radius regardless of growth substrate according to the significant positive correlation evidenced by Pearson analysis. A. flavus (RCP08108) behavior registered in CWC was also observed by Garcia et al. [25] in maize and maize agar medium (MAM) for AFB1 and AFB2, where toxin accumulation increased together with growth of the isolate. However, for CEC and F-BHA treatments, toxin levels tended to decrease over time. Therefore, a lack of significant correlation was observed by the presence of both types of microcapsules (full or empty). Besides, a not clear trend of Pearson coefficients for AFB1 accumulation versus biomass dry weight was observed. However, Mellon and Garcia reported that AFB1 accumulation parallel increased with fungal biomass in liquid based maize, medium agar maize (MAM) and maize kernels. By contrast, Shih and Marth, observed that aflatoxin production generally increase during the logarithmic and deceleration phases of fungal growth, suggesting that toxin is either a metabolite produced by growing hyphae or is converted biosynthetically from some other compounds by growing hyphae. It may expect that production of mycotoxins, as secondary metabolites, to follow a curve paralleling that of growth but slightly delayed, however regulation of secondary metabolism is poorly understood and the relationship between the rates of primary and secondary metabolism is not clear. Finally, correlations among growth features of A. flavus showed differences when Pearson correlations were evaluated for controls and F-BHA. No correlations were found for the both evaluated controls between AFB1, conidial and vesicle size. However, a significant positive correlation between AFB1 and conidial size was found for the F-BHA treatment. Nevertheless, no correlation was observed when all fungal parameters and treatment were integrated for Pearson analysis. As conclusions, presence of F-BHA sub-inhibitory effect could lead in changes in growth parameters as μ, and λ, also altering A. flavus toxin evolution with time by modifying final concentrations, regardless of substrate. Results of this work indicate the need to consider both fungal primary and secondary metabolism to determine the effect of food grade antioxidant formulation allowing us to predict that there are no risks in the estimation of aflatoxins in those areas of the storage system where F-BHA could not be distributed homogeneously.
Acknowledgement
This work was carried out by grants from
National Agency for Scientific and Technological Promotion (ANPCYT), FONCYT-PICT 1507/14. SECYT-UNRC. 2015- 2018.
References
- Alam S, Shah H, Afzal M, et al. Influence of calcium propionate water activity and storage time on mold incidence and aflatoxins production in broiler starter feed. Animal Feed Science and Technology. 2014;188:137-44.
- Alcano Md, Jahn R, Scherer C, et al. Susceptibility of Aspergillus spp. to acetic and sorbic acids based on pH and effect of sub-inhibitory doses of sorbic acid on ochratoxin A production. Food Research International. 2016;81:25-30.
- Arroyo M, Aldred D, Magan N. Environmental factors and weak organic acid interactions have differential effects on control of growth and ochratoxin A production by Penicillium verrucosum isolates in bread. International Journal of Food Microbiology. 2005;98:223-31.
- Bento J, Pena A, Lino C, et al. Determination of ochratoxin A content in wheat bread samples collected from the Algarve and Bragança regions Portugal: Winter 2007. Microchemical Journal. 2009;91:165-69.
- Duarte S, Bento J, Pena A, et al. Ochratoxin A exposure assessment of the inhabitants of Lisbon during winter 2007/2008 through bread and urine analysis. Food Additives and Contaminants. 2009;26:1411-20.
- Felizardo RJ, Câmara NO. Hepatocellular carcinoma and food contamination: Aflatoxins and ochratoxin A as great prompter. World Journal of Gastroenterology: WJG. 2013;19:3723.
- Gerez CL, Dallagnol A, Ponsone L, et al. Ochratoxin A production by Aspergillus niger: effect of water activity and a biopreserver formulated with Lactobacillus plantarum CRL 778. Food Control. 2014;45:115-19.
- Hussein HS, Brasel JM. Toxicity metabolism and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101-34.
- Mar?n S, Guynot M, Neira P, et al. Risk assessment of the use of sub-optimal levels of weak-acid preservatives in the control of mould growth on bakery products. International Journal of Food Microbiology. 2002;79:203-11.
- Meeting JFWECOFA, Organization WH and Safety IPOC. Safety evaluation of certain mycotoxins in food: Food & Agriculture Org. 2001.
- Membré JM, Kubaczka M, Chèné C. Growth rate and growth–no-growth interface of Penicillium brevicompactum as functions of pH and preservative acids. Food Microbiology. 2001;18:531-38.
- Milani J, Heidari S. Stability of Ochratoxin a During Bread Making Process. Journal of Food Safety. 2016.
- Mitchell D, Parra R, Aldred D, et al. Water and temperature relations of growth and ochratoxin A production by Aspergillus carbonarius strains from grapes in Europe and Israel. Journal of Applied Microbiology. 2004;97:439-45.
- Paíga P, Morais S, Oliva-Teles T, et al. Determination of ochratoxin A in bread: evaluation of microwave-assisted extraction using an orthogonal composite design coupled with response surface methodology. Food and Bioprocess Technology. 2013;6:2466-477.
- Patterson M, Damoglou A. The effect of water activity and pH on the production of mycotoxins by fungi growing on a bread analogue. Letters in Applied Microbiology. 1986;3:123-25.
- Perši N, Pleadin J, Kova?evi? D, et al. Ochratoxin A in raw materials and cooked meat products made from OTA-treated pigs. Meat Science. 2014;96:203-10.
- Ricke S. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Science. 2003;82:632-39.
- Samson RA, Hoekstra ES, Frisvad JC, et al. Introduction to food-and airborne fungi: Centraalbureau voor Schimmelcultures (CBS). 2004.
- Schmidt-Heydt M, Baxter E, Geisen R, et al. Physiological relationship between food preservatives environmental factors ochratoxin and otapksPV gene expression by Penicillium verrucosum. International Journal of Food Microbiology. 2007;119:277-83.
- Stratford M, Nebe-von-Caron G, Steels H, et al. Weak-acid preservatives: pH and proton movements in the yeast Saccharomyces cerevisiae. International Journal of Food Microbiology. 2013;161:164-71.
- Stratford M, Plumridge A, Nebe-von-Caron G, et al. Inhibition of spoilage mould conidia by acetic acid and sorbic acid involves different modes of action requiring modification of the classical weak-acid theory. International Journal of Food Microbiology. 2009;136:37-43.
- Tong CH, Draughon FA. Inhibition by antimicrobial food additives of ochratoxin A production by Aspergillus sulphureus and Penicillium viridicatum. Applied and Environmental Microbiology. 1985;49:1407-411.
- Torino M, Taranto M, Sesma F, et al. Heterofermentative pattern and exopolysaccharide production by Lactobacillus helveticus ATCC 15807 in response to environmental pH. Journal of Applied Microbiology. 2001;91:846-852.
- Woo CSJ, El-Nezami H. Maternal-Fetal Cancer Risk Assessment of Ochratoxin A during Pregnancy. Toxins. 2016;8:87.
- Yang Y, Bastos M, Chen KY. Effects of osmotic stress and growth stage on cellular pH and polyphosphate metabolism in Neurospora crassa as studied by 31 P nuclear magnetic resonance spectroscopy. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1993;1179:141-47.