Research Article - Biomedical Research (2017) Volume 28, Issue 13
Impact of strengthening clinical management and intervention on prognosis and compliance among patients with heart failure
Mai Chen1#, Li Xue2#, Chunxi Cha1*, Jingxing Liang1 and Pengchao Ma1
1Center of Heart Diseases, the First Agricultural Construction Division Hospital of Xinjiang Production and Construction Corps and Fourth Affiliated Hospital of Shihezi University, Aksu, Xinjiang, PR China
2Department of Cardiology, the Lu'an Second People's Hospital and Affiliated Hospital of West Anhui Health Vocational College, Lu'an, Anhui, PR China
#These authors contributed to the work equally
- *Corresponding Author:
- Chunxi Cha
Center of Heart Diseases
The First Agricultural Construction Division Hospital of Xinjiang Production and Construction Corps and Fourth Affiliated Hospital of Shihezi University
AKsu, XinJiang, PR China
Accepted date: May 12, 2017
Abstract
This study aimed to investigate the impact of strengthening clinical management and intervention on prognosis and treatment compliance among patients with Chronic Heart Failure (CHF). From June 2011 to July 2013, a total of 397 patients with CHF who had been admitted to the Center of Heart Diseases, First Agricultural Construction Division Hospital of Xinjiang Production and Construction Corps and met the diagnostic criteria were randomly divided into 2 groups according to the time of admission and personal preferences: the management-strengthening group (group R, 221 cases) and routine group (group S, 176 cases). All patients were subjected to a standard CHF treatment protocol; group S additionally received Heart Failure (HF) education, outpatient follow-up, and telephonic followup to strengthen the management and intervention. The results of the 2 groups were compared after 12 months. Compared with group R, the mortality and rehospitalization rates of group S were significantly lower, whereas the drug usage and optimal treatment rates were significantly higher; in addition, HFrelated indicators were significantly improved, and costs were significantly reduced (P<0.05). Strengthening clinical management and intervention could significantly decrease rehospitalization and mortality rates, improve prognosis and quality of life, and conserve medical resources.
Keywords
Heart failure, Disease management and intervention, Cardiovascular disease.
Introduction
Heart Failure (HF) is the final stage of a range of organic heart diseases and is defined as a complex clinical syndrome that emerges when cardiac output cannot meet the metabolic needs of the body [1]. Worldwide, the number of patients with HF has reached 22.5 million, and these patients have a 5 y survival rate similar to that of cancer patients, with a rehospitalization rate of approximately 20% within 30 d of discharge [2]; hence, HF has become the leading cause of death in developed countries [3], and is one of the major cardiovascular diseases and public health issues in China, in the 21st century. Many large-scale clinical trials have demonstrated that Angiotensin- Converting Enzyme Inhibitors (ACEIs) and β-receptor blockers could significantly decrease the mortality rate associated with Chronic HF (CHF); however, even with the use of these medications, 10-50% of the patients were rehospitalized within 3-6 months, 40% were rehospitalized or died within 1 y after diagnosis, and 50% died within 4 y [4,5]. Numerous foreign studies have shown that for patients with CHF, disease management and intervention could decrease the rehospitalization and mortality rates, improve prognosis and quality of life, and decrease healthcare costs [6-8].
The management and intervention of HF has become a research focus in health-related fields and thus has received significant attention worldwide. In this study, we aimed to further explore the necessity and effectiveness of management of patients with HF by strengthening clinical management and intervention.
Materials and Methods
Study subjects
From June 2011 to July 2013, a total of 397 patients with CHF who were admitted to the Center of Heart Diseases, Fourth Affiliated Hospital of Shihezi University and First Agricultural Construction Division Hospital of Xinjiang Production and Construction Corps and met the diagnostic criteria were enrolled in this study. The study population included 243 men and 154 women (mean age, 63.34 ± 13.85 y). The cases included 47 with New York heart Association (NYHA) grade II, 224 with NYHA grade III, and 126 with NYHA grade IV HF. Heart disease types included coronary heart disease (n=195), hypertensive heart disease (n=76), rheumatic heart disease (n=49), dilated cardiomyopathy (n=37), and other types of cardiomyopathy (n=40). The inclusion criteria were 1) the presence of organic heart disease, including coronary heart disease, hypertension, or cardiomyopathy, after surgery for valvular heart disease; and 2) a NYHA classification grades IIIV. The exclusion criteria were the presence of 1) simple diastolic heart failure; 2) congenital heart disease; 3) severe hypertrophic obstructive cardiomyopathy or restrictive cardiomyopathy; 4) pericardial diseases, pericardial tamponade, or constrictive pericarditis; 5) severe kidney diseases, with a creatinine clearance rate<0.6 ml/s (<36 ml/ min) and serum creatinine levels>265 μmol/L (≥ 3 mg/dl); 6) abnormal laboratory results for liver function and elevated serum glutamic-pyruvic transaminase (SGPT; Alanine Transaminase, ALT) and serum glutamic oxaloacetic transaminase (SGOT; Aspartate Aminotransferase, AST) levels (>4-fold increase over normal values); 7) unstable brain lesions; 8) cognitive impairment, dementia, or severe mental illness; 9) severe physical disabilities and inability to cooperate in the follow-up; and 10) inability or unwillingness to sign the informed consent form. This study was conducted with approval from the Ethics Committee of the Fourth Affiliated Hospital of Shihezi University and First Agricultural Construction Division Hospital of Xinjiang Production and Construction Corps. Written informed consent was obtained from all participants.
Clinical management and intervention
All patients underwent a physical examination, electrocardiogram (ECG), cardiac color ultrasonography, and full chest radiography on the day of hospitalization, as well as urine, blood, thyroid function, and blood biochemistry examinations, dynamic ECG, and N-terminal pro b-type natriuretic peptide (NT-proBNP) testing on the second day. A patient’s HF status was diagnosed based on clinical symptoms and auxiliary examination findings, with reference to the Framingham diagnostic criteria. According to the order of admission and individual preferences, patients were randomly divided into group S (176 cases) and group R (221 cases). All patients were subjected to a standard treatment protocol, including the administration of digitalis preparations, diuretics, and vasodilators, as well as an ACEI/angiotensin II receptor blocker (ARB), β-blocker, and spironolactone. The concentrations of ACEI/ARB and β-blocker were gradually increased to reach the target dose or maximum tolerated dose.
Additionally, some interventions were performed only for Group S. These included outpatient follow-up by a specialist on the day of discharge; the first follow-up aimed to establish a relationship with the patient, and subsequent follow-ups were conducted according to the planned follow-up schedule. A telephonic follow-up was arranged within the first week of discharge by a specialist, and clinical symptoms, including dyspnea, edema, and palpitation, and details of the patient’s (e.g., water intake, food intake and sodium intake) were recorded. Patients whose conditions were stable were followed up telephonically once every 2 w; those with an unstable condition were followed up telephonically once a week to remind them about timely treatment according to the clinical symptoms recorded during follow-up. Outpatient follow-up was arranged at 1, 3, 6, 9, and 12 months after discharge and included a physical examination and necessary auxiliary laboratory examinations. The follow-up duration was 12 months. The follow-up generally included records of the patients' daily living conditions, intake and excretion of liquids, sodium intake, dosages and side effects of medications, and clinical manifestations, along with an exercise tolerance test. Patients and their families were educated about fluid restrictions, diet, and early detection and treatment of HF symptoms. Patients were taught self-management (e.g., monitoring of body weight and symptoms, opportunities and ways to seek help). Optimal treatment and gradually increased the dosage to the specified target dose or maximum tolerated dose.
The above interventions were not performed for group R. After discharge, patients were informed about standard treatment programs, which included the administration of a digitalis preparation, diuretic, and vasodilator, along with an ACEI/ ARB, β-blocker, and spironolactone. The doses of ACEI/ARB and β-blocker were gradually increased to reach the target dose or maximum tolerated dose. Patients were followed up regularly and were allowed to select hospitals and physicians voluntarily, without any restriction. Patients were required to report their follow-up results to the HF clinic; otherwise, the HF clinic would perform a telephonic follow-up and data collection once every 3 months.
Regarding the quality of life assessment, a direct Chinese translation of the "Minnesota Living with Heart Failure Questionnaire" (LiHFe) [9] was used to assess this parameter. The questionnaire comprised 21 simple questions that were related to emotions, physical strength, and economic and social aspects. Each question could be answered on a 6-point scale ranging from 0 (best) to 5 points (worst). All patients were educated about the guidelines before filling out the questionnaire, which was completed within 5-10 min; the score was calculated as the sum of the scores of all questions.
Statistical analysis
All data were imported into an Excel database, and the SPSS 17.0 statistical package (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis. Continuous variables were expressed as means ± standard deviations (͞X ± s) or classification percentages, whereas classification variables were expressed as percentages. Continuous variables of independent samples were analysed using the t-test, whereas classification variables were analysed using the chi-square test with a multivariate logistic regression model to adjust for confounding factors. A P value of <0.05 was considered statistically significant.
Results
Baseline data
The 397 selected patients were randomly divided into 2 groups: group R (221 cases; 136 men, 85 women; mean age, 62.51 ± 14.19 y; 10 cases (4.52%) lost to follow up); and group S (176 cases; 107 men, 69 women; mean age, 63.58 ± 13.63 y; 100% outpatient follow-up rate with each patient followed up at least 5 times). The 2 groups did not differ significantly with respect to baseline characteristics (P>0.05) such as sex, age, NYHA grade, NT-proBNP level, Left Ventricular Ejection Fraction (LVEF), HF type, comorbidities, medication, and rate of target-reaching dose achievement (Table 1).
| Variable | Group R (n=221) | Group S (n=176) | P |
|---|---|---|---|
| Male | 136 (61.5%) | 107 (60.8%) | 0.859 |
| Female | 85 (38.5%) | 69 (39.2%) | 0.762 |
| Age (years old, x̄ ± s) | 62.51 ± 14.19 | 63.58 ± 13.63 | 0.518 |
| Level II | 25 (11.31%) | 22 (12.5%) | 0.671 |
| Level III | 123 (55.7%) | 101 (57.4%) | 0.684 |
| Level IV | 73 (33%) | 53 (30.1%) | 0.464 |
| LVEF (x̄ ± s, %) | 43.62 ± 11.12 | 44.01 ± 13.62 | 0.851 |
| ρ (NT-proBNP)/(ng/L) | 5284.25 ± 5676.62 | 5319.76 ± 5915.81 | 0.932 |
| ACEI (case, %) | 154(69.7%) | 124 (70.5%) | 0.844 |
| β-blocker (case, %) | 170 (76.9%) | 136 (77.3%) | 0.923 |
| ARB (cases, %) | 13 (6.16%) | 11 (6.25%) | 0.858 |
| ACEI plus β-blocker (case, %) | 86 (38.9%) | 69 (39.2%) | 0.945 |
| Aldosterone antagonist (cases, %) | 133 (60.2%) | 105 (59.7%) | 0.901 |
| Diuretics (cases, %) | 163 (73.8%) | 131 (74.4%) | 0.857 |
| Digoxin (cases, %) | 68 (30.8%) | 55 (31.3%) | 0.904 |
| ACEI target-reaching dosage (cases, %) | 11 (4.98%) | 9 (5.11%) | 0.942 |
| β-blocker target-reaching dosage (cases, %) | 4 (1.81%) | 4 (2.27%) | 0.707 |
| Coronary heart disease | 109 (49.3%) | 86 (48.9%) | 0.915 |
| Hypertension heart disease | 43 (19.5%) | 33 (18.8%) | 0.834 |
| Rheumatic heart disease | 26 (11.8%) | 23 (13.1%) | 0.647 |
| Dilated cardiomyopathy | 20 (9.05%) | 17 (9.66%) | 0.808 |
| Renal dysfunction | 22 (9.95%) | 18 (10.2%) | 0.916 |
Table 1: Comparison of baseline characteristics between the two groups.
Comparison of death and rehospitalization rates
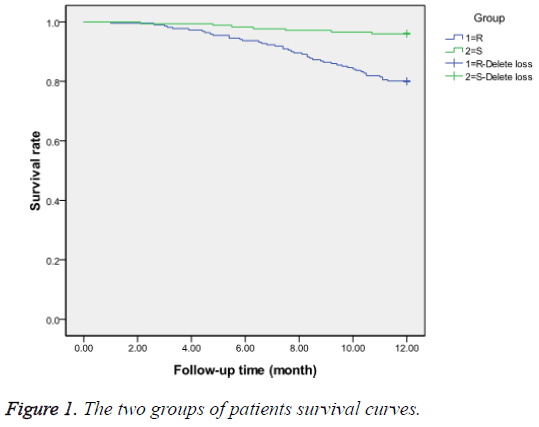
During the follow-up period, group R experienced 44 (19.9%) deaths, including 14 (6.3%) cases of sudden death, 28 (12.7%) deaths due to HF deterioration, and 2 (0.9%) deaths from other causes, whereas group S experienced only 7 (3.98%) deaths, including 1 (0.57%) sudden death, 5 (2.84%) deaths due to worsening HF, and 1 (0.57%) death from other causes. In group R, 154 (69.7%) patients were rehospitalized, including 138 (62.4%) for worsening HF, 12 (5.4%) for other cardiovascular diseases, and 4 (1.8%) for other reasons. In group S, 72 (40.9%) patients were rehospitalized, including 61 (34.7%) for worsening HF, 9 (5.1%) for other cardiovascular diseases, and 2 (1.1%) for other reasons. A Kaplan-Meier survival curve analysis is presented in Figure 1.
Cardiac function-related parameters
A skewed difference was observed in the plasma NT-proBNP levels of the 2 groups, which were then subjected to a logarithmic analysis. During follow-up, the mean Left Ventricular End Systolic Dimension (LVESD), Left Ventricular End Diastolic Dimension (LVEDD), LVEF (%), left ventricular Fractional Shortening (FS) (%), and logNT-proBNP values were significantly improved in group S relative to the baseline values and those of group R (P<0.05); however no significant difference was observed in the left atrial diameter (LAD; P>0.05). The mean LVEF (%), FS (%), and logNT-proBNP values in group R improved significantly relative to the baseline values (P<0.05), whereas no significant differences were observed in cardiac function-related parameters, such as LAD, LVEDD, and LVESD (P>0.05). The improvements in cardiac function-related parameters were significantly better in group S than in group R (P<0.05; Table 2).
| Related parameters | Group R (n=167) | Group S (n=169) | ||
|---|---|---|---|---|
| Before management and intervention | 12 months after management and intervention | Before management and intervention | 12 months after management and intervention | |
| LAD | 46.32 ± 9.69 | 43.65 ± 11.20 | 46.94 ± 8.73 | 44.23 ± 10.65 |
| LVESD | 49.54 ± 12.19 | 47.96 ± 13.90 | 50.23 ± 11.55 | 44.10 ± 11.82ab |
| LVEDD | 61.92 ± 11.25 | 59.69 ± 12.22 | 61.18 ± 10.49 | 56.17 ± 10.81ab |
| LVEF (%) | 43.62 ± 11.12 | 44.35 ± 12.07a | 44.01 ± 13.62 | 48.20 ± 13.93ab |
| FS (%) | 22.27 ± 7.83 | 23.32 ± 8.25a | 22.46 ± 7.67 | 25.27 ± 7.95ab |
| Lg (NT-proBNP) | 3.56 ± 0.36 | 3.54 ± 0.33a | 3.54 ± 0.38 | 3.47 ± 0.29ab |
Table 2: Comparison of cardiac function-related parameters between the two groups.
Comparison of quality of life
With respect to the scores obtained on the LiHFe questionnaire [9], the physical score, mood score, and total quality of life score were significantly better among patients in group S than those at baseline and among patients in group R (P<0.05); however, no significant differences in symptom and social restriction scores were observed in either group before the intervention and during follow-up (P>0.05) (Table 3).
| Item of LiHFe | Group R (n=167) | Group S (n=169) | ||
|---|---|---|---|---|
| Before management and intervention | 12 months after management and intervention | Before management and intervention | 12 months after management and intervention | |
| Physical score | 16.23 ± 4.65 | 15.63 ± 4.61 | 16.95 ± 4.51 | 13.91 ± 4.53ab |
| Mood score | 9.08 ± 4.39 | 7.35 ± 4.46 | 9.49 ± 3.91 | 6.44 ± 3.18ab |
| Symptom score | 7.90 ± 2.28 | 7.29 ± 2.60 | 8.02 ± 2.78 | 7.41 ± 3.07 |
| Social restriction score | 7.96 ± 2.45 | 7.38 ± 2.69 | 8.19 ± 2.76 | 7.45 ± 3.04 |
| Total score | 41.51 ± 7.40 | 37.42 ± 7.52 | 42.53 ± 8.25 | 35.06 ± 8.71ab |
Table 3: Comparison of quality of life between the two groups.
Comparison of medication usage and proportion of patients achieving the target-reaching dosage
At baseline, there was no statistical difference between the 2 groups in terms of medication usage and the proportion of patients using a target-reaching dosage (P>0.05). During follow-up, the rates of ACEI, β-blocker, ARB, diuretic, and ACEI plus β-blocker usage were significantly higher in group S than in group R (P<0.05), and the percentage of patients using the target-reaching dosages of ACEI and β-blocker was also dramatically higher in group S than in group R; there was no significant difference in the proportion of digoxin use between the 2 groups (P>0.05; Table 4).
| Variable | Group R (n=167) | Group S (n=169) | P |
|---|---|---|---|
| ACEI (cases, %) | 118 (70.66%) | 140 (82.84%) | 0.024 |
| β-blocker (cases, %) | 129 (77.25%) | 155 (91.71%) | 0.001 |
| ARB (cases, %) | 11 (6.59%) | 28 (16.57%) | 0.02 |
| ACEI plus β-blocker (cases, %) | 66 (39.52%) | 110 (65.09%) | 0 |
| Aldosterone antagonist (cases, %) | 110 (65.87%) | 138 (81.66%) | 0.005 |
| Diuretics (cases, %) | 129 (77.25%) | 154 (91.12%) | 0.004 |
| Digoxin (cases, %) | 56 (33.53%) | 59 (34.91%) | 0.954 |
| ACEI target-reaching dosage (cases, %) | 9 (5.39%) | 29 (17.16%) | 0.003 |
| β-blocker target-reaching dosage (cases, %) | 4 (2.40%) | 35 (20.71%) | 0 |
Table 4: Comparison of medication usage and proportion of target-reaching dosage between the two groups.
Medical costs
Medical cost statistics were obtained by consulting the clinical and hospitalization records of patients with HF, as well as reviewing the records of telephonic follow-ups of outpatients with HF. In group S, which had higher outpatient service expenditures and lower hospitalization expenditures, the total cost was lower than that of group R (P<0.05; Table 5).
| Variable | Group R (n=167) | Group S (n=169) | P |
|---|---|---|---|
| Outpatient cost | 894.25 ± 428.94 | 1914.87 ± 214.36 | 0 |
| Hospital cost | 13134.45 ± 8234.35 | 8538.15 ± 9756.78 | 0.039 |
| Total cost | 14026.68 ± 8203.65 | 10453.18 ± 10543.67 | 0.044 |
Table 5: Comparison of medical expenses (Chinese Yuan (RMB)) between the two groups.
Main factors related to rehospitalization and death
A logistic multivariate regression analysis was conducted, using rehospitalization and death as dependent variables and the risk factors (e.g., exercise therapy, sex, age, LVEF, NTproBNP, diabetes, ventricular arrhythmia, chronic lung disease, stroke, creatinine, hemoglobin, and fasting blood sugar) as independent variables. The results revealed that a stronger outpatient follow-up and low NT-proBNP level were protective against rehospitalization and death, whereas ventricular arrhythmias, renal dysfunction, and chronic lung disease promoted these negative outcomes; these results are shown in Table 6.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Regular outpatient follow-up | 0.309 | 0.149~0.602 | 0.011 |
| NT-proBNP | 0.997 | 0.997~1.000 | 0.032 |
| Ventricular arrhythmias | 7.798 | 2.350~25.653 | 0.014 |
| Renal dysfunction | 4.401 | 1.010~17.213 | 0.029 |
| Chronic lung disease | 7.502 | 2.176~25.512 | 0.001 |
Table 6: Predictors of rehospitalizations and deaths based on Multiple factors analysis.
Discussion
CHF, the last stage of a range of cardiovascular diseases, is a complex clinical syndrome. Despite great progress in the diagnosis and treatment of CHF in recent years, 40% of patients are rehospitalized or die within 1 y of diagnosis, and approximately 50% of patients die within 5 y [1]. To improve the treatment efficacy and survival rate and decrease the rehospitalization rate and healthcare costs, the European Society of Cardiology (ESC) and American College of Cardiology/American Heart Association (ACC/AHA) have recommended strengthening the monitoring of patients undergoing treatment for CHF [2].
A study by Kaldara et al. [5] regarding clinical management and intervention for patients with CHF revealed a significantly higher LVEF and significantly improved NYHA grade in the disease management and intervention group, compared to the conventional treatment group (P<0.01). A large number of foreign studies [10-12] found that clinical management and interventions for patients with HF could significantly decrease the incidence of cardiovascular events and improve patients’ quality of life. Ducharme et al. [4] followed up 230 HF cases and showed that clinical management and intervention could decrease the incidence of cardiovascular events (HF-related rehospitalization and emergency medication) and improve patients’ quality of life. Hebert et al. [7] performed a 12-month telephonic follow-up; after an investigation using the LiHFe survey, the quality of life was found to be significantly higher in the management group than in the control group, in agreement with the results of our study.
Akosah et al. [6] reported that the rate of ACEI application among patients with CHF was 34-35% after discharge but only 38% after 1 y of outpatient treatment. After strengthening outpatient management, this rate increased to 84%, and the annual mortality and hospitalization rates decreased from 42% to 21%. The results of a study by Leung et al. [8] showed that the percentage of patients who reached the guidelinerecommended target ACEI/ARB and β-blocker doses was significantly higher in the management group than in the conventional treatment group (P<0.05). A few foreign studies [13-15] have demonstrated that among patients with HF, the percentage of patients achieving the target ACEI/ARB and β- blocker doses recommended by guidelines was higher among the group with management than among the group without management, although this factor did not significantly decrease the hospitalization and death rates, in contrast to the findings of this study. This discrepancy might be attributable to short-term patient follow-up, poor compliance of patients in the absence of regular outpatient follow-up, and the lack of multicenter and large-scale clinical research.
Ho et al. [16] reported significant correlations of medication compliance with the cardiovascular event-related rehospitalization rate, all-cause mortality, and cardiovascular death among patients with HF. A large number of foreign studies have shown that post-discharge CHF management could significantly improve drug utilization and decrease the rate of CHF-combined endpoint events [17-22]. Numerous studies have also shown that among patients with CHF, treatment compliance was significantly higher in the management and intervention group than in the control group (P<0.05), consistent with the results of our study.
Berg et al. [23] telephonically followed up 533 patients with CHF over a 1-year period; the results showed that the HFrelated rehospitalization rate and emergency admission rate of the follow-up group were 44% and 22% lower, respectively than those of the control group, and follow-up was associated with a total medical care cost reduction of $1792. A metaanalysis conducted in Europe indicated that management and intervention could decrease the rates of HF aggravation or other cardiovascular event-related rehospitalization by 30% and the rates of rehospitalization and death-combined endpoint events by 18% [24]. Akosah et al. [6] followed up patients with HF for 12 months after discharge and found that the rehospitalization rate and death-combined endpoint event rate decreased by 20% within 3 months, and by 22% at 1 year. In the present study, although group S had significantly higher outpatient expenditures, this group had reductions in both the rehospitalization rate and hospitalization expenditures, resulting in a lower overall medical cost compared to that of group R, in agreement with the results of foreign studies.
In summary, in addition to significantly reducing the rates of rehospitalization and mortality and improving patients’ prognoses and quality of life, the strengthening of clinical management and intervention might also conserve medical resources within the social healthcare system.
Acknowledgements
This work was funded by Science and Technology Research Projects of Xinjiang Production and Construction Corps (No. 2011BA048).
Conflict of Interest
All authors have no conflict of interest regarding this paper.
References
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medine (ESICM). Eur Heart J 2008; 29: 2388-2442.
- Hunt SA, American College of Cardiology, American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Task Force on Practice Guideline (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005; 46: 1-82.
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, ODonnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics-2009 update: are port from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119: 21-181.
- Ducharme A, Doyon O, White M, Rouleau JL, Brophy JM. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ 2005; 173: 40-45.
- Kaldara E, Sanoudou D, Adamopoulos S, Nanas JN. Outpatient management of chronic heart failure. Expert Opin Pharmacother 2015; 16: 17-41.
- Akosah KO, Schaper AM, Havlik P, Barnhart S, Devine S. Improving care for patients with chronic heart failure in the community: the importance of a disease management program. Chest 2002; 122: 906-912.
- Hebert PL, Sisk JE, Wang JJ, Tuzzio L, Casabianca JM, Chassin MR, Horowitz C, McLaughlin MA. Cost-effectiveness of nurse-led disease management for heart failure in an ethnically diverse urban community. Ann Intern Med 2008; 149: 540-548.
- Leung AW, Chan CY, Yan BP, Yu CM, Lam YY, Lee VW. Management of heart failure with preserved ejection fraction in a local public hospital in Hong Kong. BMC Cardiovasc Disord 2015; 15: 12.
- Rector TS, Kubo SH, Cohn JN. Patients self-assessment of their congestive heart failure part 2: content, reliability and validity of a new measure, the Minnesota Living with heart failure questionnaire. Heart Fail 1987; 3: 198-209.
- Kato N, Kinugawa K, Seki S, Shiga T, Hatano M, Yao A, Hirata Y, Kazuma K, Nagai R. Quality of life as an independent predictor for cardiac events and death in patients with heart failure. Cicr J 2011; 75: 1661-1669.
- Fujita B, Lauten A, Goebel B, Franz M, Fritzenwanger M, Ferrari M, Figulla HR, Kuethe F, Jung C. Impact of diabetes mellitus on quality of life in patients with congestive heart failure. Qual Life Res 2012; 21: 1171-1176.
- Rector TS. Overview of the Minnesota living with heart failure questionnaire. retrieved from http://www.license.umn.edu/products/Minnesota-Living-With- Heart-Failue-Questionnaire Z94019, 2012.
- Correia J, Silva FF, Roque C, Vieira H, Providencia LA. Impact of a specialized outpatient heart failure follow-up program on hospitalization frequency and functional status of patients with advanced heart failure. Rev Port Cardiol 2007; 26: 335-343.
- Desai AS. Home monitoring heart failure care does not improve patient outcomes: looking beyond telephone-based disease management. Circulation 2012; 125: 828-836.
- Writing Committee Members, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: e240-e327.
- Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. Medication nonadherence is associated with a Broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008; 155: 772-779.
- Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol 2008; 31: 153-158.
- European Heart Rhythm Association, European Society of Cardiology, Heart Rhythm Society, Heart Failure Society of America, American Society of Echocardiography, American Heart Association, European Association of Echocardiography, Heart Failure Association, Daubert JC, Saxon L, Adamson PB, Auricchio A, Berger RD, Beshai JF, Breithard O, Brignole M, Cleland J, Delurgio DB, Dickstein K, Exner DV, Gold M, Grimm RA, Hayes DL, Israel C, Leclercq C, Linde C, Lindenfeld J, Merkely B, Mont L, Murgatroyd F, Prinzen F, Saba SF, Shinbane JS, Singh J, Tang AS, Vardas PE, Wilkoff BL, Zamorano JL. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Heart Rhythm 2012; 9: 1524-1576.
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803-869.
- Zannad F, Gattis Stough W, Rossignol P, Bauersachs J, McMurray JJ, Swedberg K, Struthers AD, Voors AA, Ruilope LM, Bakris GL, OConnor CM, Gheorghiade M, Mentz RJ, Cohen-Solal A, Maggioni AP, Beygui F, Filippatos GS, Massy ZA, Pathak A, Piña IL, Sabbah HN, Sica DA, Tavazzi L, Pitt B. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J 2012; 33: 2782-2795.
- Chatterjee S, Biondi-Zoccai G, Abbate A, Dascenzo F, Castagno D, Van Tassell B, Mukherjee D, Lichstein E. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ 2013; 346: 55.
- Wikstrand J, Wedel H, Castagno D, McMurray JJ. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med 2014; 275: 134-143.
- Berg GD, Wadhwa S, Johnson AE. A matched-cohort study of health services utilization and financial outcomes for a heart failure disease-management program in elderly patients. J Am Geriatr Soc 2004; 52: 1655-1661.
- Gonseth J, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. The effectiveness of disease management programme in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J 2004; 25: 1570-1595.