Research Article - Journal of Clinical Oncology and Cancer Research (2020) Volume 3, Issue 1
Impact of smoking on serum osteopontin in patients with hepatitis crelated cirrhosis and Itâ??srole in hepatocarcinogenesis.
Mohammed Mohammed Shamseya1, Sameh Aldesoky Lashen2*, Ayman Mohammed Shamseya2, Marwa Ahmed Madkour1, Magda Abd El-Ghany Megahed3, Thoria Mohammed Dawood3, Osama Ghazy Badr3
1Department of Clinical and Experimental Internal Medicine, Medical Research Institute, University of Alexandria, Alexandria, Egypt
2Department of Internal Medicine, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
3Department of Biochemistry, Medical Research Institute, University of Alexandria, Alexandria, Egypt
- Corresponding Author:
- Sameh Aldesoky Lashen
Department of Internal Medicine
Faculty of Medicine, University of Alexandria
Alexandria, Egypt
Tel: +20 1274117543
E-mail: sameh.lashen@alexmed.edu.eg
Accepted date: July 23, 2020
Citation: Shamseya MM, Lashen SA, Shamseya AM, Madkour MA, Megahed MAE et al. Impact of smoking on serum osteopontin in patients with hepatitis crelated cirrhosis and It’srole in hepatocarcinogenesis. Allied J Clin Oncol Cancer Res 2020;3(1):34-39.
Abstract
Background: Hepatitis C virus (HCV) related liver disease, culminating into hepatocellular carcinoma
(HCC), is a prevalent disease in Egypt. Smoking is linked to multiple health-related problems.
Osteopontin (OPN) is a multifunctional protein, highly expressed in bone. Its overexpression has been
observed in various human tumors. Its role in HCC has generated significant interest.
Aim: To study the effect of heavy smoking on levels of osteopontin and its possible role in HCV related
HCC.
Material and Methods: 180 volunteers were included and divided into 6 equal groups: Group 1:
normal healthy persons. Group 2: hepatitis C-negative smokers. Group 3: HCV-positive cirrhotic nonsmokers.
Group 4: HCV-positive cirrhotic heavy smokers. Group 5: HCV-positive cirrhotic patients
with HCC and non-smokers. Group 6: HCV-positive cirrhotic patients with HCC and heavy smokers.
All subjects were evaluated using as regards HCV-Abs, HBsAg, aminotransferases, serum bilirubin,
serum albumin, serum C-reactive protein, serum iron, serum alpha-fetoprotein, and serum
osteopontin.
Results: The results showed a statistically significant elevation of the mean serum OPN level in group 4
in comparison to both groups 1 and 2, and in group 6 in comparison to both groups 1 and 2. There is
also a statistically significant elevation of the mean serum OPN level in group 2 in comparison to group
1, in group 4 in comparison to both groups 3 and 5, and in group 6 in comparison to both groups 3 and
5.
Conclusion: Smoking increases osteopontin levels in sera of HCV-positive patients compared to its
level in HCV-positive nonsmokers.
Keywords
Hepatitis C, Hepatocellular carcinoma, Smoking, Osteopontin.
Introduction
Hepatitis C virus (HCV) is one of the most important global chronic infections worldwide [1]. The World Health Organization (WHO) estimates that 170 million people are infected with HCV globally and 3–4 million new infections occur annually [2]. Egypt has the highest rate of HCV infection worldwide which is estimated nationally at ~ 14.7 % [3].
Hepatocellular carcinoma (HCC) is a prevalent hepatic disease representing the 6th most common neoplasm and the 3rd common cause of cancer-related deaths all over the world. About 600,000 deaths per year are attributed to HCC [4,5]. The burden of HCC has been increasing in Egypt with a doubling in the incidence rate in the past 10 years [6].
Direct carcinogenicity remains uncertain in HCV induced HCC. Hepatitis C, being a positive, single-stranded RNA virus without a DNA intermediate in its replicative cycle, seems to be less likely to have integration of its nucleic acid sequences into the host genome. However, the observation that the virus possesses an independent role in hepatic carcinogenesis is noteworthy. HCV infection causes chronic inflammation, cell death, proliferation, and cirrhosis of the liver. Thus, HCV related HCC is found almost exclusively in patients with cirrhosis [7].
Smoking causes a variety of adverse effects on organs that have no direct contact with the smoke itself such as the liver [8]. Smoking yields chemical substances with cytotoxic potentials [9]. These chemicals created by smoking induce oxidative stress associated with lipid peroxidation leading to activation of stellate cells and the development of fibrosis [10]. Smoking increases the production of pro-inflammatory cytokines such as interleukin (IL) 1 and 6 and tumor necrosis factor-α (TNF-α) involved in liver cell injury [11]. It has been reported that smoking increases fibrosis score and histological activity index in chronic hepatitis C (CHC) patients [8].
Osteopontin (OPN), a glycoprotein was first identified in 1986 in osteoblasts, is a multifunctional protein, highly expressed in bone [12]. The putative functions of OPN include bone mineralization, regulation of immune cell function, inhibition of calcification, control of tumor cell phenotype, and cell activation [13]. OPN overexpression has been observed in various human tumors, including carcinomas of the stomach, lung, breast, colon, prostate, and pancreas [14]. The mechanism by which OPN enhances tumor development and particularly metastasis is still poorly understood [15]. The expression level of OPN in tumor tissues or in the blood of cancer patients has been positively correlated with worse prognosis in many cancer types [16]. The role of OPN in HCC has also generated significant interest, especially with regard to its roles as a prognostic factor [17]. In a previous gene expression profiling study, OPN has been identified as one of the leading genes associated with the metastasis of HCC [18].
The Aims of the Study
The aim is to study the effect of heavy smoking on levels of OPN and its possible role in hepatocarcinogenesis in patients with hepatitis C.
Materials and Methods
Our study was a case-control study. We recruited the study subjects from Internal medicine department (Hepatology Unit) at the Medical Research Institute, Alexandria University. We included 180 volunteers. They were divided into six equal groups (30 volunteer/ group) as followings: Group-1: normal healthy persons with no history of liver diseases and no history of smoking, group-2: normal healthy persons with no history of liver diseases and with current history of heavy smoking, group-3: patients with HCV related cirrhosis with no history of smoking, group-4: patients with HCV related cirrhosis with current history of heavy smoking, group-5: patients with HCV related HCC with no history of smoking, and group-6: patients with HCV related HCC with current history of heavy smoking.
Quantification of smoking was done using the smoking index (SI) defined as the number of cigarettes smoked per day multiplied by the number of years of smoking and heavy smokers are defined as having SI ≥ 301 [19].
Exclusion criteria included decompensated cirrhosis (Child- Pugh class B or C), hepatitis B infection, other causes of chronic liver disease, diabetes mellitus, obesity, hypertension, cardiac diseases, rheumatic diseases, immunologic diseases or cancers (other than HCC).
All subjects included in the study were subjected to full history taking, the clinical evaluation including anthropometric measures with the calculation of body mass index (BMI). Laboratory investigations including viral hepatitis markers, complete blood count (CBC), serum transaminases, liver function tests, C-reactive protein (CRP), serum iron, serum alpha-fetoprotein (AFP) were done. Plasma levels of osteopontin were measured using human osteopontin immunoassay Quantikine® ELISA kit (Catalog #DOST00, RandD SystemsInc®, USA and Canada) following manufacturer instructions. Imaging studies including abdominal ultrasound (US) and triphasic computed tomography of the abdomen (CT) were done to diagnose HCC.
Statistical Analysis
The results of this study were tabulated and statistically analyzed using ANOVA, paired-t-test and Chi-square test as appropriate. Results were considered to be significant at p ≤ 0.05. Duncan's method was used to find the significance between every two groups as follows: the same small letters indicate that there was no significant difference between these groups, while the different letters indicate that there was a significant difference between these groups.
Results
The clinic laboratory parameters and their corresponding statistical comparisons are shown in Table 1.
| Mean ± SD | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | P value |
|---|---|---|---|---|---|---|---|
| Age (years) | 42.73 ± 4.15 | 42.2 ± 2.39 | 41.16 ± 8.6 | 42.76 ± 7.65 | 46.51 ± 7.6 | 46.32 ± 8.1 | 0.851 |
| Gender (M/F) | 13/2 | 36800 | 16/9 | 25/0 | 18/7 | 25/0 | <0.001 |
| BMI (kg/m2) | 24.1 ± 2.4 | 24.4 ± 3 | 23.9 ± 3.8 | 24.3 ± 4.1 | 23.3 ± 3.2 | 23.1 ± 3.1 | 0.587 |
| Spleen (cm) | 11.13 ± 1.36b | 11.3 ± 1.34b | 13.8 ± 1.08a | 14.2 ± 1.12a | 14.8 ± 2.11a | 14.9 ± 2.13a | <0.001 |
| Platelets x103 (cells/µl) | 192 ± 40a | 190 ± 36a | 127 ± 52b | 124 ± 59b | 88 ± 41 c | 92 ± 47c | 0.035 |
| ALT (U/L) | 20.07 ± 8.96b | 25.3 ± 9.5b | 30.84 ± 10.1a | 38.79 ± 10.2a | 31 ± 14.2a | 30.2 ± 12.3a | 0.033 |
| AST (U/L) | 18.53 ± 8.11b | 19.8 ± 5.25b | 29.88 ± 9.19a | 31.4 ± 12.8a | 31 ± 11.7a | 31.1 ± 11.2a | 0.015 |
| ALP (U/L) | 68.38 ± 18.79c | 66.8 ± 18.34c | 72.48 ± 27.9b | 75.92 ± 22.89b | 120.2 ± 31.2a | 122 ± 29.21a | 0.023 |
| γ-GT (U/L) | 21.87 ± 9.3c | 20.5 ± 7.35c | 47 ± 13.3b | 54.88 ± 14b | 67.2 ± 21.53a | 69.3 ± 22.8a | 0.002 |
| Albumin (g/dl) | 4.31 ± 0.64a | 4.38 ± 0.66a | 4.02 ± 0.61a | 3.9 ± 0.48a | 2.7 ± 0.38b | 2.62 ± 0.29b | 0.032 |
| Bilirubin | 0.85 ± 0.09c | 0.82 ± 0.08c | 1.8 ± 0.34b | 1.6 ± 0.28b | 4.1 ± 2.08 a | 4.12 ± 2.03a | <0.001 |
| INR | 1.25 ± 0.17c | 1.2 ± 0.28c | 1.44 ± 0.46b | 1.45 ± 0.24b | 1.92 ± 0.38a | 1.93 ± 0.31a | 0.037 |
| Iron (µg/dL) | 96.27 ± 25.18c | 96.5 ± 43.06c | 118.8 ± 28.1b | 130.04 ± 35.9a | 134.2 ± 39.4a | 138.4 ± 41.6a | 0.026 |
| AFP (ng/ml) | 0.46 ± 0.35c | 0.55 ± 0.4c | 2.74 ± 2.37b | 3.45 ± 2.3b | 687 ± 119.3a | 698 ± 123.4a | < 0.001 |
Table 1: Clinico-laboratory data with their statistical comparisons among the study groups.
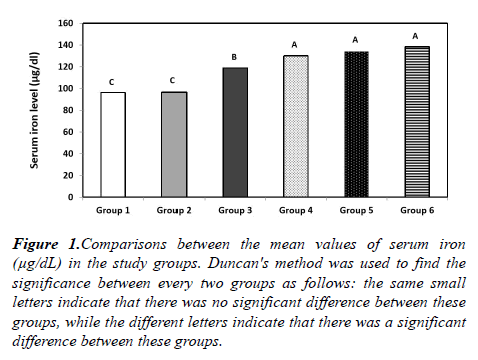
Regarding serum iron, the mean serum iron level was (96.27 ± 25.18 μg/dl) in group 1, (96.5 ± 43.06 μg/dl) in group 2, (118.8 ± 28.02 μg/dl) in group 3, (130.04 ± 35.98 μg/dl) in group 4, (134.21 ± 39.38 μg/dl) in group 5 and (138.43 ± 41.57 μg/dl) in group 6 showing statistically significant difference among the 6 groups (P=0.026). These results also showing a statistically significant elevation of serum iron in each of the groups 4-6 in comparison to each of the groups 1-3. Also, there is a statistically significant elevation of serum iron in group 3 in comparison to either group 1 or 2. While there is no statistically significant difference between groups 4, 5, and 6, or between groups 1 and 2 (Figure 1).
Figure 1: Comparisons between the mean values of serum iron (μg/dL) in the study groups. Duncan's method was used to find the significance between every two groups as follows: the same small letters indicate that there was no significant difference between these groups, while the different letters indicate that there was a significant difference between these groups.
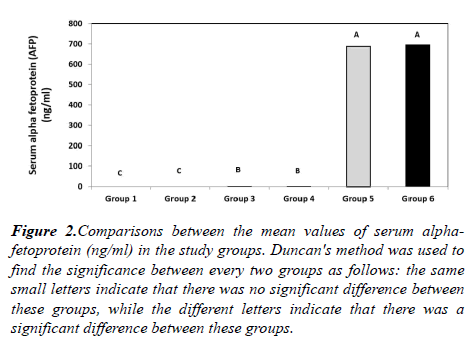
The mean serum AFP level was (0.46 ± 0.35 ng/ml) in group 1, (0.55 ± 0.4 ng/ml) in group 2, (2.74 ± 2.37 ng/ml) in group 3, (3.45 ± 2.3 ng/ml) in group 4, (687 ± 119.32 ng/ml) in group 5 and (698 ± 123.41 ng/ml) in group 6 showing a highly statistically significant difference among the six groups (p<0.001). These results also showing a statistically significant elevation of the mean AFP level in group 3 in comparison to both groups 1 (p=0.003) and 2 (p=0.018). In addition, there is a highly statistically significant elevation of the mean serum AFP level in group 4 in comparison to both groups 1 and 2, in group 5 in comparison to groups 1-4 and in group 6 in comparison to groups 1-4 (p=0.001). Meanwhile, no statistically significant difference was found neither between groups 1 and 2, between groups 3 and 4, nor between groups 5 and 6 (Figure 2).
Figure 2: Comparisons between the mean values of serum alphafetoprotein (ng/ml) in the study groups. Duncan's method was used to find the significance between every two groups as follows: the same small letters indicate that there was no significant difference between these groups, while the different letters indicate that there was a significant difference between these groups.
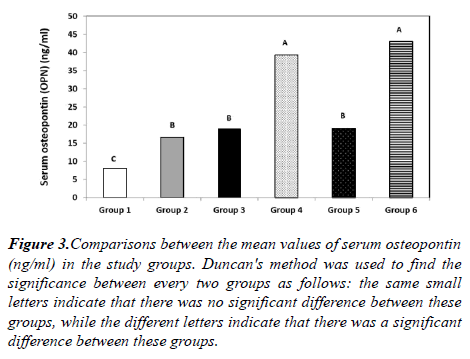
The mean serum OPN level was (8.07 ± 3.04 ng/ml) in group 1, (16.65 ± 4.47 ng/ml) in group 2, (18.92 ± 5.6 ng/ml) in group 3, (39.3 ± 6.84 ng/ml) in group 4, (19.11 ± 6.01 ng/ml) in group 5 and (43.1 ± 7.54 ng/ml) in group 6 showing a highly statistically significant difference among the 6 groups (p<0.001). These results also show a highly statistically significant elevation of the mean serum OPN level in group 3 in comparison to group 1, in group 4 in comparison to both groups 1 and 2, in group 5 in comparison to group 1 and in group 6 in comparison to both groups 1 and 2 (p<0.001). There is also a statistically significant elevation of the mean serum OPN level in group 2 in comparison to group 1 (p=0.004), in group 4 in comparison to both groups 3 and 5 (p=0.005), and in group 6 in comparison to both groups 3 and 5. No statistically significant difference, as regards serum OPN level, was found between groups 2, 3 and 5, or between groups 4 and 6 (Figure 3).
Figure 3: Comparisons between the mean values of serum osteopontin (ng/ml) in the study groups. Duncan's method was used to find the significance between every two groups as follows: the same small letters indicate that there was no significant difference between these groups, while the different letters indicate that there was a significant difference between these groups.
Discussion
The prevalence of HCC associated with hepatitis C is high in Egypt [20]. The pathogenesis of HCC is multifactorial, highly associated with chronic viral hepatitis, alcohol consumption, hepatic toxins, and genetic modification, such as hemochromatosis or lack of alpha 1-antitrypsin [21]. Smoking causes a variety of adverse effects on the liver. [8] Osteopontin (OPN) is a multifunctional glycoprotein, which has a close relation to various types of tumors including HCC [14,18].
The current study results revealed that hepatitis C-positive subjects groups have highly statistically significantly elevated serum alpha-fetoprotein levels than those of hepatitis Cnegative subjects groups, but no significant difference was found neither between hepatitis C-positive cirrhotic smokers and hepatitis C-positive cirrhotic non-smokers, between HCC smokers and HCC non-smokers, nor between hepatitis Cnegative smokers and hepatitis C negative nonsmokers regarding serum AFP levels.
These results come in accordance to a study [22]. They studied the clinical significance of plasma OPN level as a biomarker of HCC. They found a statistically significant elevated plasma AFP levels in hepatitis C patients than in healthy subjects. Another study [23], investigated biochemical parameters in relation to serum AFP and leptin levels in Iraqi patients with chronic liver diseases. Their study indicated that serum AFP levels were statistically significantly elevated in HBV and HCV patients than its levels in control subjects (p<0.05), in addition, there was no significant difference between HBV and HCV patients (p>0.05).
The current study also revealed that HCV-positive cirrhotic smokers were found to have statistically significantly elevated levels of serum osteopontin in comparison to its levels in HCV-positive cirrhotic non-smokers, as do smoker patients with liver cirrhosis and HCC who have statistically significantly elevated levels of serum OPN in comparison to its levels in non-smoker patients with liver cirrhosis and HCC. The present study results also revealed that the mean serum OPN level in hepatitis C-negative smokers is statistically significantly elevated in comparison to its levels in hepatitis C-negative nonsmokers (healthy control) (p<0.005).
These findings are supported and studied the mechanism of HCV-induced osteopontin and its role in epithelial to mesenchymal transition of the hepatocyte [24]. They suggested that HCV infection induces OPN via altered Ca2+ homeostasis in the endoplasmic reticulum (ER) and elevation of reactive oxygen species (ROS) in the mitochondria. Moreover, it is measured osteopontin levels at different stages of hepatic fibrosis and inflammation in HCV-infected subjects [25]. They found that plasma OPN level is correlated with the severity of liver fibrosis and inflammation, suggesting OPN could be used as a biomarker to evaluate the severity of liver damage in HCV subjects.
Furthermore it has studied the role of serum osteopontin as a diagnostic biomarker for early HCC [26]. They found that the mean serum OPN level in HCC patients was not significantly different from HCV patients while both were significantly higher than the control group ( p<0.001). The previously mentioned study [22] found a significant higher osteopontin level in HCV patients than healthy subjects (p<0.05).
Osteopontin overexpression has been observed in various human tumors [14]. OPN enhances migration of many different cell types through its interaction with integrins [27] and receptors of the CD44 family to deliver signals to cells to promote cell adhesion, chemotaxis, and extracellular matrix degradation [28]. The ability of cancers to metastasize is strongly associated with migration, so this aspect of OPN function is likely important in metastasis. OPN was shown to up-regulate hyaluronic acid synthase, which may contribute to the survival of cells in the absence of adhesion, another key feature of metastatic cells [29].
OPN antibodies can suppress pulmonary metastasis of HCC cells in a nude mouse model, suggesting that it plays a significant role in the metastatic potential of HCC [24]. A previous study involving the silencing OPN mRNA expression in HCC cell lines suggested it may also have proliferative effects [30].
Philips et al. [31] studied the role of osteopontin in hepatocarcinogenesis. Their study demonstrated that OPN, as an extracellular matrix protein, binds to αvβ integrins and CD44 family of receptors to propagate cellular signals on extracellular matrix (ECM) degradation, angiogenesis, and tumor cell apoptosis. The mechanisms by which OPN promotes tumor cell survival are still unclear [32].
In a study it is aimed at studying the impact of down-regulation of osteopontin on the growth of HCC, they concluded that OPN may facilitate tumorigenesis and metastasis through prevention of tumor cells from apoptosis [33]. They also stated that enhanced sensitivity of HCC cells to chemotherapeutic drugs through blockade of nuclear factor kappa Beta activation by OPN silencing through RNA interference-mediated depletion of osteopontin may have a great potential in the development of therapeutic regimens for cancer treatment.
Conclusion
Smoking increases OPN levels in sera of HCV-positive patients compared to its level in HCV-positive nonsmokers. Increased osteopontin production may play an important role in the progression of liver fibrosis and cirrhosis through activation of several inflammatory pathways in those patients and may also be associated with an increased probability of HCC. We suggest that targeting the osteopontin mediated carcinogenic pathways may represent a promising way in prevention and control of HCC. Also, patients with chronic hepatitis C should be informed that smoking cigarettes could worsen their liver disease.
References
- Marinho RT, Barreira DP. Hepatitis C, stigma and cure. World J Gastroenterol. 2013; 19(40):6703-6709.
- MohdHanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology. 2013; 57(4):1333-1342.
- Miller FD, Abu-Raddad LJ. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci. 2010; 107(33):14757-14762.
- Chiang JK, Koo M, Kuo TB, et al. Association between cardiovascular autonomic functions and time to death in patients with terminal hepatocellular carcinoma. J Pain Symptom Manag. 2010; 39(4):673-679.
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. Journal of the National Cancer Institute. 2008; 100(10):698-711.
- Lehman EM, Wilson ML. Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: A systematic review and meta-analysis. Int J Cancer. 2009; 124(3):690-697.
- Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998; 75(3):347-354.
- Pessione F, Ramond MJ, Njapoum C, et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology. 2001; 34(1):121-125.
- Yuen ST, Gogo Jr AR, Luk IS, et al. The effect of nicotine and its interaction with carbon tetrachloride in the rat liver. Pharmacotoxicol. 1995; 77(3):225-230.
- Husain K, Scott BR, Reddy SK, et al. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol. 2001; 25(2):89-97.
- Moszczynski P, Zabinski Z, Moszczynski Jr P, et al. Immunological findings in cigarette smokers. Toxicollett. 2001; 118(3):121-127.
- Gursoy G, Acar Y, Alagoz S. Osteopontin: A multifunctional molecule. J Med Sci. 2010; 1:55-60.
- Mazzali M, Kipari T, Ophascharoensuk V, et al. Osteopontin—a molecule for all seasons. Q J Med. 2002; 95(1):3-13.
- Anborgh PH, Mutrie JC, Tuck AB, et al. Role of the metastasis‐promoting protein osteopontin in the tumour microenvironment. J Cell Mol Med. 2010; 14(8):2037-2044.
- Chakraborty G, Jain S, Behera R, et al. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr Mol. 2006; 6(8):819-30.
- Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004; 90(10):1877-1881.
- Kim J, Ki SS, Lee SD, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006; 101(9):2051-9.
- Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus–positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003; 9(4):416-23.
- Singh N, Aggarwal AN, Gupta D, et al. Quantified smoking status and non-small cell lung cancer stage at presentation: analysis of a North Indian cohort and a systematic review of literature. J Thorac Dis. 2012; 4(5):474.
- Waked IA, Saleh SM, Moustafa MS, et al. High prevalence of hepatitis C in Egyptian patients with chronic liver disease. Gut. 1995; 37(1):105-107.
- Muntane J, Gonzalez R, Ranchal I, et al. Mechanisms of liver cell injury. Rev EspEnferm Dig. 2007; 99(7):405.
- Salem M, Atti SA, El Raziky M, et al. Clinical significance of plasma osteopontin level as a biomarker of hepatocellular carcinoma. Gastroenterol Res. 2013; 6(5):191.
- Zainal IG, Safaa AA, Obead WK. Biochemical parametersin relation to serum alpha fetoproteincand leptin levels in Iraqi pateints with chronic liver diseases. Int J Life SciFarma Res. 2013; 3(1):16-22.
- Iqbal J, McRae S, Banaudha K, et al. Mechanism of hepatitis C virus (HCV)-induced osteopontin and its role in epithelial to mesenchymal transition of hepatocytes. J Biol Chem. 2013; 288(52):36994-7009.
- Huang W, Zhu G, Huang M, et al. Plasma osteopontin concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected subjects. ClinChim Acta. 2010; 411(9-10):675-678.
- Abdel-Hamid M, Ellakwa DE, Omar NN. Role of serum osteopontin level as a diagnostic biomarker for early hepatocelular carcinoma. Int J Cancer Res. 2014; 10(1):37-45.
- Todoric J, Loffler M, Huber J, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n− 3 polyunsaturated fatty acids. Diabetologia. 2006; 49(9):2109-2119.
- McAllister SS, Gifford AM, Greiner AL, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008; 133(6):994-1005.
- Cook AC, Chambers AF, Turley EA, et al. Osteopontin induction of hyaluronan synthase 2 expression promotes breast cancer malignancy. J Biol Chem. 2006; 281(34):24381-24389.
- Sun BS, Dong QZ, Ye QH, et al. Lentiviral‐mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008; 48(6):1834-1842.
- Phillips RJ, Helbig KJ, Van der Hoek KH, et al. Osteopontin increases hepatocellular carcinoma cell growth in a CD44 dependant manner. World J Gastroentero: WJG. 2012 Jul;18(26):3389-3399
- Khan SA, Lopez‐Chua CA, Zhang J, et al. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J Cell Biochem. 2002; 85(4):728-736.
- Zhao J, Dong L, Lu B, et al. Down-regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology. 2008; 135(3):956-968.