- Biomedical Research (2015) Volume 26, Issue 1
Impact of single-dose intravenous paracetamol on lymphocyte DNA damage and oxidative stress in trauma patients.
Ozgur Sogut1*, Leyla Solduk2, Mehmet Tahir Gokdemir3, Halil Kaya3
1Bezmialem Vakif University, Faculty of Medicine, Department of Emergency Medicine, Istanbul, Turkey.
2Diyarbakir Research and Training Hospital, Department of Emergency Medicine, Diyarbakir, Turkey
3Harran University, Faculty of Medicine, Department of Emergency Medicine, Sanliurfa, Turkey.
- Corresponding Author:
- Ozgur Sogut
Department of Emergency Medicine
Faculty of Medicine, Bezmialem Vakif University
Vatan Str, Fatih, 34093
Istanbul, Turkey
Accepted date: July 30 2014
Abstract
We investigated the impact of paracetamol administration on lymphocyte DNA damage and oxidative stress parameters for acute pain management in patients with acute traumarelated pain. Thirty-five adult patients with mild or moderate trauma and 33 eligible healthy volunteers as control subjects were enrolled. Blood samples were obtained from the patients before and at 2 and 12 h after IV administration of paracetamol. Serum lymphocyte DNA damage and total oxidant status (TOS), total antioxidant status (TAS) and oxidative stress index (OSI) were studied as oxidative stress parameters. Serum lymphocyte DNA damage levels were 14.97 ± 12.38 AU (arbitrary units) in Group 1 (before analgesia), 13.94 ± 9.28 AU in Group 2 (2 h after analgesia), and 10.57 ± 8.73 AU in Group 3 (12 h after analgesia). A significant difference was observed among the groups with respect to serum lymphocyte DNA damage (p < 0.01). Mean serum TOS and OSI values were lower in Groups 2 and 3 compared to that in Group 1. Furthermore, mean serum TAS values were higher in Group 3 compared to that in Goup 1. Our data suggest that the treatment of trauma-related acute pain with intravenous paracetamol resulted in decreased DNA damage in human T lymphocytes.
Keywords
Paracetamol (acetaminophen), lymphocyte DNA damage, total oxidant status (T0S), total antioxidant status (TAS), oxidative stress index (OSI)
Introduction
Acute pain is the most common complaint upon admission to the Emergency Department. In a study conducted in France, the proportion of patients who were admitted to the Emergency Department with a complaint of pain was > 80% [1]. Insufficient management of acute pain may lead to thromboembolic and pulmonary complications, prolonged hospitalization, return of the patient to the hospital after discharge for treatment of pain, reduction in quality of life, and development of chronic pain [1,2]. Trauma-related pain is the most frequent cause of acute pain that should be addressed seriously. Acute pain varies according to the severity of trauma and can be classified using a visual analogue scale (VAS). Proper management of pain according to pain severity prevents both deterioration of clinical condition and is important for relieving future chronic pain [3,4]. Paracetamol (acetaminophen) or nonsteroidal antiinflammatory drugs (NSAIDs) are used to manage mild pain, weak opioids are used in addition to paracetamol or NSAIDs, and potent opioids are additionally used for severe pain. Side effects of paracetamol occur less frequently than those of NSAIDs. Therefore, paracetamol is the most commonly prescribed analgesic for treatment of acute pain [2,5].
Paracetamol may be administered via per oral/ per os (PO) or intravenous (IV) routes. The efficacy and safety of IV formulations are obscure compared to placebo or other analgesics [5]. Previous studies have reported that paracetamol reduced oxidative stress by reducing lipid peroxidation and protein oxidation in vitro (in brain and liver cells) and has a potential antioxidant effect [6,7]. Morphine, which is a potent opioid (narcotic) analgesic, causes DNA damage in vitro in human lymphocyte cell cultures [8]. Although paracetamol is known to result in decreased oxidative stress and this impact reduces genotoxicity and DNA damages in vitro [6], existing literature dictates that paracetamol inhibits DNA repair and induces cell death in vivo and in vitro [9]. However no case-control studies have investigated the effect of paracetamol on DNA damage in human T lymphocytes. Therefore, in the present study, we aimed to investigate the effects of paracetamol on human lymphocyte DNA damage and oxidative–anti-oxidative status in patients with mild or moderate trauma and compared with those in controls. As mentioned above, most studies of the safety of paracetamol and morphine were conducted in vitro; in contrast, our study was a clinical trial and used the alkaline comet method, which has high specificity and sensitivity for determining DNA damage.
Subjects and Methods
Study participants
Over a 4-month period (July 2012 through November 2012), 35 consecutive adult patients (mean age, 39.71 ± 20.31 years; range, 18–81 years; 11 females and 24 males) presenting to the emergency department with symptoms of mild or moderate trauma due to various causes (vehicle accident, vehicle-pedestrian accidents, fall from high place, and assault) were enrolled in this prospective clinical study. As the control group, we enrolled 33 age- and sex-matched healthy adults (mean age 37.62 ± 12.23 years, 12 females and 21 males). Patients were provided with basic trauma life support at presentation and advanced trauma life support if required. Written informed consent was obtained directly from patients after vital functions were stabilized. Furthermore, the healthy volunteers were informed about the study protocol, and written consent was obtained from all participants.
Pain severity was determined using a VAS by the same healthcare professional for each patient upon arrival at the emergency department. Subjects who had mild–moderate pain were administered 100 ml (10 mg/mL) paracetamol IV for 20 min. Demographic characteristics of the patients, clinical findings, and pain severity were recorded with the VAS. Subjects were divided into three groups: Group 1 (before analgesia administration), Group 2 (2 h after analgesia administration), and group 3 (12 h after analgesia administration). Inclusion criteria were: adult patients (≥18 years) with traumatic mild or moderate pain according to the VAS, and written informed consent.
This study was conducted in accordance with the 1989 Declaration of Helsinki and was approved by the Ethics Committee of Harran University, Faculty of Medicine.
Exclusion criteria
To investigate the effects of paracetamol on oxidative status and lymphocyte DNA damage, subjects with conditions that may have affected oxidative markers, such as chronic medical disorders (i.e., congestive heart failure, chronic obstructive lung disease, diabetes mellitus, chronic renal failure, hypertension, or malignancy) and those who had coronary artery disease, peripheral vascular disease, or renal dysfunction; subjects using medications such as sedative-hypnotic drugs or stimulatory substances, smokers, subjects with measurable blood alcohol concentrations or who had consumed alcohol prior to the study; those following a special diet, subjects who were pregnant or had elevated human chorionic gonadotropin (hCG) levels detected by a quantitative hCG blood test (β-hCG), patients with severe trauma; and subjects who underwent computed tomography and/or magnetic resonance imaging (MRI) were excluded from the study. None of the subjects was taking drugs known to affect lipid or lipoprotein metabolism. Special care was taken to exclude subjects who were taking anabolic drugs, diuretics, vitamins, or other antioxidants such as vasoactive and beta-blocking agents.
Blood sample collection
Venous blood samples were obtained before analgesia (Group 1) and at 2 (Group 2) and 12 h (Group 3) after analgesia administration, collected into heparinized tubes, and immediately stored on ice at 4°C. The serum was separated by centrifugation at 4,000 rpm for 5 min. Plasma samples were stored at -80°C until analysis.
Assessment of lymphocyte DNA damage by the alkaline comet assay
Lymphocyte isolation for the comet assay was performed by density gradient separation (Histopaque 1077, Sigma- Aldrich, St. Louis, MO, USA). One milliliter of heparinized blood was layered over 1 mL of Histopaque and centrifuged for 35 min at 500 × g and 25°C. The interface band containing lymphocytes was washed with phosphate-buffered saline (PBS) and then collected by 15-min centrifugation at 400 × g. The resulting pellets were resuspended in PBS to obtain 20,000 cells/10 μl. Membrane integrity was assessed by trypan blue exclusion. Endogenous DNA damage in lymphocytes was analyzed by the alkaline comet assay as described by Singh et al. [10] with minor modifications. A fresh lymphocyte cell suspension of 10 μl was mixed with 80 μl of 0.7% low-melting point agarose (Sigma) in PBS at 37°C. Subsequently, 80 μl of this mixture was layered onto slides that had been coated previously with 1.0% hot (60°C) normal-melting point agarose, and the slides were covered with a coverslip at 4°C for at least 5 min to allow the agarose to solidify. After removing the coverslips, the slides were submersed in freshly prepared cold (4°C) lysing solution (2.5 M NaCl, 100 mM EDTA-2Na; 10 mM Tris-HCl, pH 10–10.5; 1% Triton X-100, and 10% DMSO were added just before use) for at least 1 h. The slides were then immersed in freshly prepared alkaline electrophoresis buffer (0.3 M NaOH and 1 mM Na2ETDA, pH 13) at 4°C for unwinding (40 min) and then electrophoresed (25 V/300 mA, 25 min). All steps were conducted under a red light or without direct light to prevent additional DNA damage. After electrophoresis, the slides were stained with ethidium bromide (2 μl/ml in distilled H2O; 70 μl/slide), covered with a coverslip, and vizualized using an epifluorescence microscope (Nikon, Tokyo, Japan) equipped with a rhodamine filter (excitation wavelength 546 nm, barrier filter 580 nm). The images of 100 randomly chosen nuclei (50 cells from each of two replicate slides) were analyzed visually. Each image was classified according to the intensity of the fluorescence in the comet tail, which was rated from 0 (from undamaged) to 4 (maximally damaged) (Figure 1), so that the total score of two replicate slides could be 0–400 arbitrary units (AU). All procedures were performed by the same biochemistry staff, and DNA damage was assessed by a single observer who was unaware of the sample identities.
Measurement of total oxidant status (TOS)
Serum TOS was determined using a novel automated measurement method developed by Erel [11]. Oxidants present in the sample oxidize the ferrous ion-odianisidine complex to ferric ions. The oxidation reaction is enhanced by glycerol, which is abundant in the reaction medium. The ferric ions provide a colored complex with xylenol orange in an acidic medium. Color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. This assay was calibrated with hydrogen peroxide, and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 equiv/L). The assay has excellent precision values of < 2%.
Measurement of total antioxidant status (TAS)
Total antioxidant status in serum was determined using an automated measurement method [12]. In this method, hydroxyl radicals, which are among the most potent of the biological radicals, are produced. In the assay, ferrous ion solution, in Reagent 1, is mixed with hydrogen peroxide, in Reagent 2. The sequentially produced radicals—such as brown-colored dianisidinyl radical cations, produced by the hydroxyl radical—are also potent radicals. The oxidation reactions progress among dianisidyl radicals, and further oxidation reactions develop, increasing color formation. Antioxidants in the sample suppress the oxidation reactions and color formation. Using this assay, the antioxidative effect of the sample against the potent free-radical reactions, which are initiated by the produced hydroxyl radicals, was measured. Results are expressed as milimole (mmol) Trolox equiv/L. The precision of the assay was < 3%.
Calculation of the oxidative stress index (OSI)
The OSI was calculated according to the following formula: OSI (AU) = TOS (μmol H2O2 equiv/L)/TAS (mmol Trolox equiv/L) × 10 – 1.
Statistics
Data analyses were conducted using the SPSS v.11.5 software (SPSS, Inc., Chicago, IL, USA). Numerical data (e.g., oxidative–antioxidative status parameters; TOS, TAS, and OSI levels; and lymphocyte DNA damage) are expressed as means ± standard deviation (SD). Inter-group comparisons (controls vs. patients) were performed using the chi-square and Mann–Whitney U tests. When comparing numerical data (intra-group comparisons) that were normally distributed, identified with the Kolmogorov- Smirnov Z test, the one-way analysis of variance (ANOVA) was used if there were more than two groups (pre-analgesia; Group 1, post-analgesia 2. hour; Group 2 and post-analgesia 12. hour; Group 3). A two-tailed p-value < 0.05 was considered to indicate statistical significance.
Results
Mean VAS scores were 3.42 ± 0.65, 2.11 ± 0.58, and 1.08 ± 0.65 in groups 1, 2, and 3, respectively.
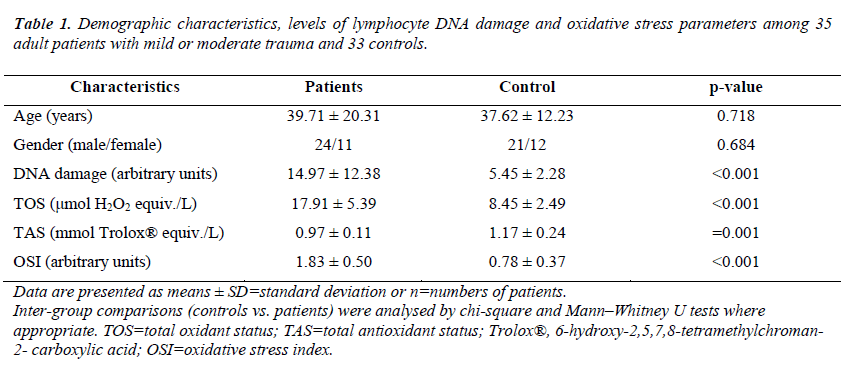
A significant difference was observed between pre-analgesia (Group 1) and post-analgesia (Group 2) in terms of mean VAS scores (95% confidence interval [CI], 1.11–1.51; p = 0.000). Additionally, a significant difference was found between the mean VAS score of Groups 2 (2.11 ± 0.58) and 3 (1.08 ± 0.65) (95% CI, 0.78–1.27; p = 0.035). A significant difference was found between the mean VAS score of Groups 1 (3.42 ± 0.65) and 3 (1.08 ± 0.65) (95% CI. 2.10– 2.57; p = 0.006). ). No significant differences were observed between patients with mild or moderate trauma and the controls with respect to age or gender (p = 0.718 and p = 0.684, respectively). The mean serum lymphocyte DNA damage value was significantly higher in patients with mild or moderate trauma compared with those in the controls (p < 0.001). Likewise, serum TOS and OSI levels were significantly higher (both comparisons, p < 0.001), whereas plasma TAS levels were significantly lower in patients with mild or moderate trauma compared with those in the controls (p = 0.001). The demographic characteristics, levels of lymphocyte DNA damage and oxidative stress parameters among 35 adult patients with mild or moderate trauma and 33 controls are shown in Table 1.
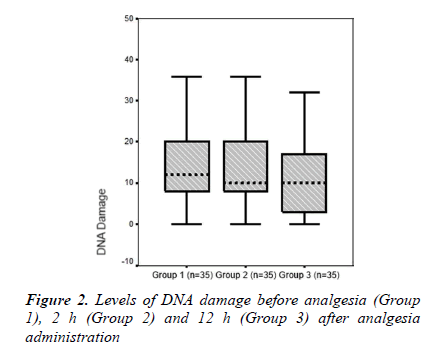
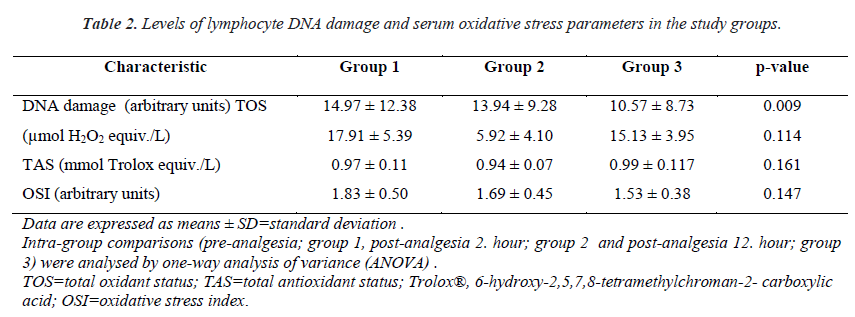
The mean serum lymphocyte DNA damage value (AU) was higher in Group 1 than in Group 2 (14.97 ± 12.38 and 13.94 ± 9.28, respectively). Group 3, which comprised patients with 12 h after analgesia administration, had the lowest mean lymphocyte DNA damage value (10.57 ± 8.73). A significant difference was found between groups in terms of serum lymphocyte DNA damage (p = 0.009, Table 2 and Figure. 2).
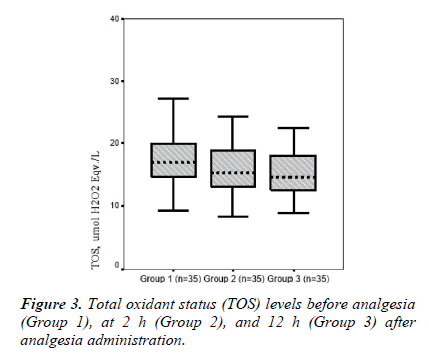
Mean TOS values were 17.91 ± 5.40, 15.92 ± 4.10, and 15.13 ± 3.95 in Groups 1, 2, and 3, respectively. Mean serum TOS levels tended to be lower after analgesia administration in groups 2 and 3 compared to those in Group 1, whereas there was no significant difference in TOS levels between groups (p = 0.114; Table 2 and Figure 3).
Data are presented as means ± SD=standard deviation or n=numbers of patients. Inter-group comparisons (controls vs. patients) were analysed by chi-square and Mann–Whitney U tests where appropriate. TOS=total oxidant status; TAS=total antioxidant status; Trolox®, 6-hydroxy-2,5,7,8-tetramethylchroman- 2- carboxylic acid; OSI=oxidative stress index.
Table 1. Demographic characteristics, levels of lymphocyte DNA damage and oxidative stress parameters among 35 adult patients with mild or moderate trauma and 33 controls.
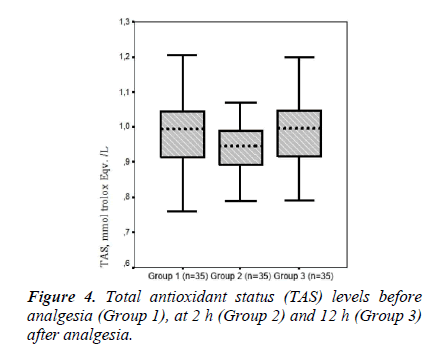
The mean serum TAS values were 0.97 ± 0.11, 0.94 ± 0.07, and 0.99 ± 0.11 in Groups 1, 2 and 3, respectively. Mean serum TAS level tended to be higher in Group 3 compared to that in Group 1, whereas there was no.
Discussion
This is the first in vivo clinical trial of the effects of paracetamol, one of the most commonly used drugs for managing acute pain in adult patients subjected to mild or moderate trauma, on DNA damage and serum oxidative stress parameters in human T lymphocytes. In addition, we used the alkaline comet method, which has high specificity and sensitivity for determining DNA damage.
Pain is the most common cause of emergency room admission, and such pain is usually acute and most commonly caused by trauma [1,13]. Management of acute pain in trauma patients is an essential part of management both for patients and in emergency medicine practice because failure to control pain increases mortality and morbidity. Analgesia must be effective, fast, safe, and appropriate for the patient’s clinical condition. Both deterioration of the current clinical condition and potential chronic pain can be prevented with proper pain management [3].
Paracetamol is a commonly used analgesic for managing acute pain. It may be administered via PO or IV routes and has proven analgesic and antipyretic effects [5,14]. In addition, reasons for preferring paracetamol include no side effects such as gastric mucosal irritation, nausea, vomiting, sedation, confusion, hemorrhage and thrombocytopenia, and no significant difference in terms of efficacy compared to other NSAIDs or opioids [15-18]. The analgesic effect of acetaminophen begins within 10 min, peaks within 1 h, and lasts 4-6 h when administered at 15 mg/kg [14- 15].
The efficacy of PO paracetamol has been demonstrated in many studies, and fewer side effects occur compared to other analgesics [5,14,16]. Several previous studies have shown that IV paracetamol not only effectively manages patients’ pain but can also help reduce the adverse reactions of opioid use, therefore it seems to be clinically efficacious and safe [17-19]. There are fewer side effects from IV paracetamol use than other analgesics and, therefore, it was preferred in the present study. We administered a single dose of 100 ml (10 mg/ml) paracetamol via the IV route for 20 min in cases who had mild or moderate pain by the VAS. While the mean VAS was 3.42 ± 0.65 before analgesia, it was 2.11 ± 0.58 2 h after analgesia was administered. A significant difference was found between the preanalgesia and 2 h group (p = 0.000). An approximate 40% reduction in pain severity was observed at 2 h after administering parenteral paracetamol.
A significant difference was detected between the mean VAS value before analgesia (3.42 ± 0.65) and that 12 h after analgesia (1.08 ± 0.65) (p = 0.006). Thus, pain severity decreased by ~70% 12 h after analgesia provision. The efficacy and safety of IV paracetamol are controversial compared to placebo and other analgesics [5]. According to the our results, a single dose of 1 gram parenteral paracetamol was safe and effective for rapid and effective elimination of mild-moderate pain in trauma patients.
Oxidative stress can be defined as an increase in oxidants and/or a decrease in antioxidant capacity [20]. Reactive oxygen species (ROS) play an important role in the pathogenesis of several neurodegenerative processes, including cell death, motor neuron diseases, and axonal injury [18,21,22]. ROS include very transient substances such as hydroxyl radicals, superoxide anions, hydrogen peroxide, and nitric oxide, which lead to lipid peroxidation and oxidation of DNA and proteins. When ROS generation increases to an extent that overcomes the cellular antioxidants, the result is oxidative stress [23].
Oxidative stress-induced acute inflammatory responses play an important role in acute pain arising from trauma [24]. Recent clinical data in trauma patients indicate that oxidative stress parameters increase proportionally to severity of trauma during the early period; however, antioxidants decrease [24,25]. Oldham et al. [25] measured antioxidant levels on days 1, 2, 3, 4, 6, and 8 after classifying patients according to trauma severity score. They reported that antioxidants decreased in 9.9% of mild trauma patients and 34.3% of severe trauma patients, and that this reduction continued for 7 days.
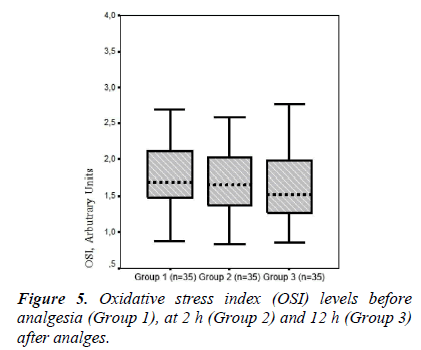
In the present study, TOS and OSI levels declined in Groups 2 and 3 (TOS, 15.92 ± 4.10 and 15.13 ± 3.95; respectively) (OSI, 1.70 ± 0.45 and 1.53 ± 0.38, respectively) compared to those in the pre-analgesia group (Group 1, 17.91 ± 5.40 and 1.83 ± 0.51, respectively). Armagan et al. [7] showed that paracetamol decreases oxidative stress by reducing lipid peroxidation and protein oxidation in brain cells in vitro. Consistent with that study, TOS and OSI values as an oxidative stress parameters tended to decline together with the reduction in pain after paracetamol administration. However, a significant difference was not found between groups in terms of TOS and OSI levels. Garrido et al. [6] reported that paracetamol has a potential antioxidant effect in hepatic cells in vitro. In our study, serum TAS levels tended to increase at 12 h after analgesia (0.99 ± 0.11, Group 3) compared to those pre-analgesia (0.97 ± 0.11, Group 1).
Morphine affects the immune system and causes DNA damage through its effect on the K opioid receptor in CD3+ T cells in vitro. Morphine-induced DNA damage has been reported to arise based on K-opioid receptor activity, which causes immune suppression through p53-mediated signal transduction [8]. However, no clinical studies have investigated the effect of paracetamol on DNA damage in T lymphocytes in vivo. In the present study, the mean DNA damage values (AU) declined significantly in Groups 2 and 3 (13.94 ± 9.28 and 10.57 ± 8.73, respectively) compared to that in Group 1 (14.97 ± 12.38 AU; group 1).
A significant difference was detected between the groups in terms of DNA damage (p = 0.009). The degree of DNA damage was high in trauma patients but decreased gradually after analgesia administration at 2 and 12 h. TOS and OSI values as oxidative stress parameters, and lymphocyte DNA damage decreased significantly after treatment of trauma-related acute pain with paracetamol. The lymphocyte DNA damage that develops in trauma patients may be mediated by oxidative mechanisms. The present study is unique in that it is the first conducted in vivo to show that paracetamol decreased oxidative stress and lymphocyte DNA damage in patients with trauma. Specifically, a single dose of 1 gram intravenous paracetamol administration for pain management following trauma may lead to reduced posttaumatic complications and, ultimately, reduced morbidity and health care costs.
Conclusion
Our preliminary study showed that acute pain developing in patients subjected to trauma led to oxidative stress, which resulted in oxidative DNA damage in lymphocytes. However, TOS and OSI as oxidative stress parameters and lymphocyte DNA damage decreased significantly following intravenous administration of paracetamol. DNA damage in lymphocytes of trauma patients might be explained by oxidative damage. On the basis of our data, we suggest that a single dose of 1-gram intravenous paracetamol administration might be regarded as a clinically efficacious and safe analgesia for pain management in patients following trauma. Future controlled clinical trials conducted with larger samples are needed to support and extend these data.
The Trial Registration No:
B.30.2.HRÜ.0.20.05.00.050.01.04-208
Conflict of Interest Statement
The authors had no conflicts of interest to declare in relation to this article.
Footnote:
The study protocol was carried out in accordance with the International Committee of Medical Journal Editors (ICMJE) and it was approved by the Ethical Committee of Harran University, Faculty of Medicine, Turkey
References
- Milojevic KG, Cantineau JP, Ruiz R, Coudert B, Bataille S, Boutot F, et al. Can severe acute pain escape visual analog scale screening in the ED Am J Emerg Med 2004; 22: 238-241.
- Karcioglu O. Management of pain and practice of analgesia in the emergency setting. Türk Aile Hek Derg 2010; 14: 53-63.
- Cohen SP, Christo PJ, Moroz L. Pain management in trauma patients. Am J Phys Med Rehabil 2004; 83: 142-161.
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983; 17: 45-56.
- McNicol ED, Tzortzopoulou A, Cepeda MS, Francia MB, Farhat T, Schumann R. Single-dose intravenous paracetamol or propacetamol for prevention or treatment of postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2011; 106: 764-775.
- Garrido A, Arancibia C, Campos R, Valenzuela A. Acetaminophen does not induce oxidative stress in isolated rat hepatocytes: its probable antioxidant effect is potentiated by the flavonoid silybin. Pharmacol Toxicol 1991; 69: 9-12.
- Armagan G, Kanit L, Yalcin A. Effects of nonsteroidal antiinflammatory drugs on D-serineinduced oxidative stress in vitro. Drug Chem Toxicol 2012; 35: 393-398.
- Tsujikawa H, Shoda T, Mizota T, Fukuda K. Morphine induces DNA damage and P53 activation in CD3+ T cells. Biochim Biophys Acta 2009; 1790: 793-799.
- Hongslo JK, Holme JA. DNA damages caused by paracetamol. Tidsskr Nor Laegeforen 1994; 114: 1204-1206.
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988; 175: 184-191.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005; 38: 1103-1111.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004; 37: 112-119.
- Berthier F, Potel G, Leconte P, Touze MD, Baron D. Comparative study of methods of measuring acute pain intensity in an ED. Am J Emerg Med 1998; 16: 132-136.
- Hall LG, Oyen LJ, Murray MJ. Analgesic agents. Pharmacology and application in critical care. Crit Care Clin 2001; 17: 899-923, viii.
- Zed PJ, Krenzelok EP. Treatment of acetaminophen overdose. Am J Health Syst Pharm 1999; 56: 1081- 91; quiz 1091-3.
- Gotzsche PC. Non-steroidal anti-inflammatory drugs: the pendulum swings. BMJ 2000; 320: 1058- 1061.
- Craig M, Jeavons R, Probert J, Benger J. Randomised comparison of intravenous paracetamol and intravenous morphine for acute traumatic limb pain in the emergency department. Emerg Med J 2012; 29: 37-39.
- Bektas F, Eken C, Karadeniz O, Goksu E, Cubuk M, Cete Y. Intravenous paracetamol or morphine for the treatment of renal colic: a randomized, placebocontrolled trial. Ann Emerg Med 2009; 54: 568-574.
- Ko MJ, Lee JH, Cheong SH, Shin CM, Kim YJ, Choe YK, et al. Comparison of the effects of acetaminophen to ketorolac when added to lidocaine for intravenous regional anesthesia. Korean Journal Anesthesiol 2010; 58: 357-361.
- Toklu HZ, Hakan T, Biber N, Solakoglu S, Ogünc AV, Sener G. The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radical Research 2009; 43: 658-667.
- Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol 1998; 18: 667-682.
- Lemineur T, Deby-Dupont G, Preiser JC. Biomarkers of oxidative stress in critically ill patients: what should be measured, when and how Curr Opin Clin Nutr Metab 2006; 9: 704-710.
- Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000; 153: 83-104.
- Rael LT, Bar-Or R, Aumann RM, Slone DS, Mains CW, Bar-Or D. Oxidation-reduction potential and paraoxonase-arylesterase activity in trauma patients. Biochem Biophys Res Commun 2007; 361: 561-565.
- Oldham KM, Wise SR, Chen L, Stacewicz- Sapuntzakis M, Burns J, Bowen PE. A longitudinal evaluation of oxidative stres in trauma patiens. J Parenter Enteral Nutr 2002; 26: 189-197.