Case Report - Current Pediatric Research (2017) Volume 21, Issue 1
Impact of parasitic infections on nutritional status and micronutrients in Saudi children.
Naglaa M Shalaby1,2, Nehad M Shalaby3, Ashraf O Sayed41Department of Parasitology, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
2Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia.
3Pediatric Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
4Department of pediatrics, Children and Women's University Hospital, Minia University, Egypt.
- *Corresponding Author:
- Nehad MS
Pediatric Department, Faculty of Medicine
Mansoura University, Mansoura, 35516, Egypt.
Tel: 00201093988813
E-mail: amahalawy2002@yahoo.com
Accepted date: November 02, 2016
Abstract
Background: Micronutrients deficiency is a great problem that is augmented by infection and poor nutrition. Copper, zinc, and iron are trace elements needed for human growth. Objective: To investigate the impact of parasitic infections on nutritional status and serum copper, iron, and zinc in western Saudi children. Subjects and methods: A case-control study included 110 parasitic infected children and 90 age and sex matched controls. Anthropometric measures were evaluated using specific Saudi Arabian growth charts. Parasites were detected in stool specimens using standard microscopic methods. Atomic Absorption Spectrophotometer was used for detection of serum zinc, iron, and copper. Data were analyzed statistically using SPSS version 20. Results: Parasitic infected children showed a statistically significant low weight for age, weight for height, and BMI. Serum zinc, iron, and copper were significantly lower in parasitic infected children than control. Serum zinc has the most significant positive correlation with weight for age, weight for height and BMI for age (r=0.6, 0.6, 0.7), respectively, followed by iron. Malnutrition existed in 34.5% of children with parasitic infections with a significant impact on serum zinc. Multiple linear regression models showed a highly negative effect of parasitic infection and a less negative effect to underweight on serum zinc, copper and iron levels. Conclusion: Studied serum micronutrients especially zinc and iron and anthropometric indices were significantly lower in parasitically infected children.
Keywords
Parasites, Zinc, Iron, Copper.
Introduction
In developing countries, there are many health drawbacks of which the micronutrient deficiencies are considered a great problem that is augmented by infectious diseases and poor dieting creating a complex cycle in children, which is difficult to be controlled, especially in preschoolchildren due to their rapid growth rate and needs [1-3]. Inappropriate ingestion of micronutrients, as well as hindering its adequate absorption, which is augmented by illnesses as infections with various parasites eventually lead to a shortage of valuable trace elements [4,5].
For adequate development and growth, children need vital micronutrients, the most common of which are copper, zinc, and iron. They are needed for maintaining a healthy child with intact immunity as they participate in a lot of enzymatic and biological processes [6,7]. WHO has highlighted upon the prevalence of both zinc, and iron in developing countries, and has estimated that the highest incidence is among children [8,9].
Parasitic infections, especially that affecting intestines are widely distributed all over the world and comprise great health concerns, especially for children in poor regions in Latin America, Africa, and Asia [10,11]. The link of poor nutrition and intestinal helminthes infection has been well recognized by many researchers who settled several conclusions regarding age groups at greatest risk and the effect of such infections on growth parameters especially weight and height [12,13]. The intensity and type of parasitic infection contribute to its effect on nutrition [14]. To check how adequate nutrition the children have, one should apply anthropometric indexes which highlight the nutritional status of children; they are beneficial because they are considered a non-invasive, accurate, convenient and simple tool to quantify the degree of under-nutrition or over-nutrition [15].
The targets of this research were to check the influence of parasitic infections on nutritional condition and serum zinc, iron, and copper in preschool and school children in the Kingdom of Saudi Arabia.
Subjects and Methods
Study Area
This research was performed in the western area of Saudi Arabia. The nature of its climate is cool in winter, hot in summer. The annual least and the highest temperature is (20°C) and (48°C), respectively.
Study Design
A case-control study was undertaken over one year from August 2014 to September 2015 after obtaining ethical approval from the Research and Ethic Committee of Faculty of Applied Medical Sciences, King Abdul-Aziz University, Jeddah, Saudi Arabia. Also, an informed consent was taken from the parents of those children joined the study.
Subject Sampling
This research work was done on 110 children infected with different parasites known by their positive stool analysis. Their ages were from 2-15.5 years. The studied groups were 60 males and 50 females. A control group was included with 90 parasite free healthy children 52 were males and 38 were females with matched age and sex. They were enrolled from children, attending different clinics for a regular check-up. The following inclusion and exclusion criteria were applied:
Inclusion criteria
• Children of both sexes.
• Ages were from 2 up to 15 years.
• Underweight or normal weights were only considered.
• No history of vitamins or mineral received by participant children for 6 months preceding the study.
Exclusion criteria
• Overweight, obesity.
• Underweight due to chronic diseases as congenital heart problems, endocrine disorders, tumors.
• Children received mineral-vitamin supplements in the last 6 months. All exclusion criteria were set to rule out any confounders that could affect our results.
Both groups were submitted to the following:
A) Nutritional assessment: The children were nearly naked wearing no shoes while measuring of weight to the nearest 100 gram using a digital electronic scale and of height with a portable anthropometric stadiometer (Seca) to the nearest 0.1 cm. All measurements were undertaken by the same person and recorded as the mean of three consecutive readings. Anthropometric indices were used to estimate the children's nutritional status as follows: The Body Mass Index (BMI) was calculated from a child's weight in kilograms and height in meters (kg/m2) for school-aged children, Weight-for-Height (WFH) for preschool children, Weight-for-Age (WFA) and Height-for-Age (HFA).They were plotted on agesex specific Saudi growth charts [16]. Underweight, stunting, and wasting was defined by values below the 5th centile line for WFA, HFA, and WFH or BMI, respectively.
B) Detection of parasites in stool: Standard steps were used in collecting stool specimens in a clean leak proof stool cups. All data of participating children including name, age, sex serial number, and date of sample collection was recorded obviously on the stool cups. Just after collection, direct wet smear using iodine, saline, and lacto-phenol cotton blue, was performed. Subsequently, formalin-ethyl acetate sedimentation was done in the stool sample and examined by direct wet smear (as previous) and modified Ziehl-Neelsen stain [17]. The parasitological examination was performed by two senior clinical laboratory technicians, independently at the laboratory of Parasitology, King Abdul-Aziz University Hospital. According to their parasitological stool results, parasitically infected children were classified into protozoa and helminthes types.
C) Biochemical estimation: For estimating serum micronutrient levels, zinc, iron and copper, blood specimens were taken by phlebotomists with minimal veno-stasis after overnight fasting to determine the levels of micronutrients. Five milliliters of cubital venous blood samples were collected in sterile tubes and centrifuged for 15 min. Sera were separated and stored in Eppendorfs at −20°C until analysis was done at the Analytical Chemistry Unit, Faculty of Applied Medical Sciences, King Abdul- Aziz University. The concentrations of Zinc, Iron, and Copper of serum samples were determined by Contr AA 700 High-Resolution Continuum Source Atomic Absorption Spectrophotometer [18].
Statistical Analysis
IBM SPSS software package (Statistical Package for Social Sciences, version 20 for Windows) was used to analyze data. Continuous variables were presented as median (interquartile ranges); categorical variables as numbers and percentages. A one-sample Kolmogorov-Smirnov test was used to assess whether the data were normally distributed. The majority of the variables does not follow a normal distribution (P<0.05), they were non parametric variables, thus the results are presented as median (interquartile ranges). Categorical data were analyzed for comparison using chi-square tests or Fisher’s exact test, if chi-squared test is not suitable when the expected values in any of the cells of a contingency table are below 5; Continuous data which not normally distributed were compared using the Mann-Whitney test. Spearman correlation analysis was used to assess the correlation between anthropometric measurements and serum iron, zinc and copper in children with parasitic infections.
A stepwise multiple linear regression analyses were calculated to assess the effects of parasitic infections, BMI for age, weight for age, height for age and age and sex on the serum levels of zinc, iron and copper.
At 5% level of significance, P-value less than 0.05 were considered significant in all analysis. Cut-off value for, iron, copper, zinc, was defined at their serum levels of 60 μg/dl, 75 μg/dl, 75 μg/dl, respectively [19].
Results
Table 1 show that a hundred and ten children with parasitic infections 93 protozoa and 17 helminthes infections and 90 healthy controls were included in the study. Patients with parasitic infections revealed a statistically significant low Weight-for-Age (WFA), Weight-for-Height (WFH) and Body Mass Index (BMI) p<0.001; based on a cutoff value (<5th percentile). A statistically significant low levels of zinc, iron, and copper, were also identified, compared to healthy control group p<0.01.
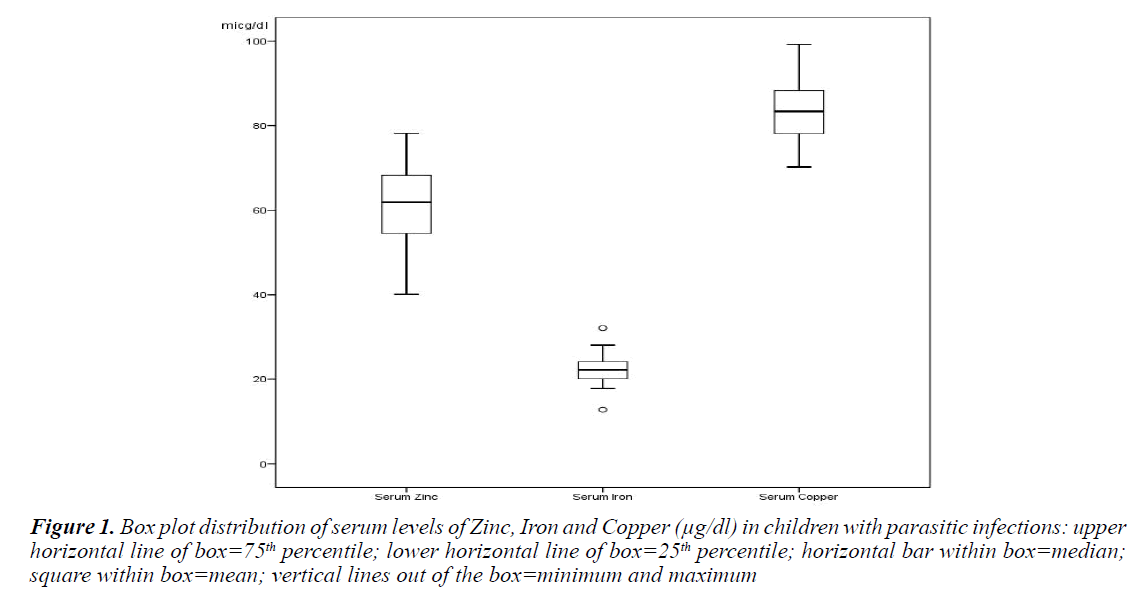
A Box plot distribution illustrated for the median, 25th- 75th interquartile ranges of serum Zinc, Iron, and copper, 62, (54-68); 22 (20-35); 83 (78-88) (μg/dl), respectively, in children with parasitic infections (Figure 1).
Table 2 shows how the parasitic infections were prevalent in the 110 studied children. Five types of a single protozoan infection were identified in 75% of children: Giardia lamblia Table 2 shows how the parasitic infections were prevalent in the 110 studied children. Five types of a single protozoan infection were identified in 75% of children: Giardia lamblia. was the most prevalent protozoa (n=34), followed by Entamoeba hisotolytica (n=21), Cryptosporidium parvum (n=12), Entamoeba coli (n=9), and Isospora (n=4). Two protozoan infection was identified in each patient in 12% of children: Giardia lamblia and Entamoeba hisotolytica (n=7), Giardia lamblia and Cryptosporidium parvum (n=6). Two helminthes were identified in 15% of infected children: Ascaris (n=10) and Hymenolepis nana (n=7).
| Variable | Parasitic infections (n=110) |
Healthy Controls (n=90) |
      p |
|---|---|---|---|
| Age, years | 9 (5.5-12.6) | 8 (5-12) | 0.5 |
| Gender Male Female |
60 (55.5%) 50 (45.5%) |
52 (58%) 38 (42%) |
0.7a |
| Weight,kg | 24 (17.2-37.9) | 26 (18.8-39.1) | 0.25 |
| Height, cm | 126(108-146) | 126 (104-144) | 0.9 |
| BMI for age | 15.8 (13.9-18) | 17.9 (16.3-19.9) | 0.1 |
| BMI centile | 25(2.9-50) | 50 (50-50) | 0.001* |
| Weight for age | 10 (2.9-25) | 50 (25-50) | 0.001* |
| Height for age | 25 (10-50) | 25 (25-50) | 0.1 |
| Weight for height | 50 (8.8-50) | 75 (50-90) | 0.01* |
| Serum Zinc, µmol | 62 (54-68) | 78 (73-83) | 0.001* |
| Serum Iron, µmol | 22 (20-35) | 33 (30-35) | 0.001* |
| Serum Copper, µmol | 83 (78-88) | 89 (83-93) | 0.001* |
| Parasites Protozoa Helminthes |
93 (85%) 17 (15%) |
-- -- |
-- -- |
Notes: Continuous variables are presented as median
(interquartile ranges); Categorical variables are numbers
with percentages
Abbreviations: BMI, Body Mass Index. aChi-square test;
others are Mann-Whitney Test
*P is significant
Table 1. Comparison between parasitic infections and controls participating in the study.
Figure 1. Box plot distribution of serum levels of Zinc, Iron and Copper (µg/dl) in children with parasitic infections: upper horizontal line of box=75th percentile; lower horizontal line of box=25th percentile; horizontal bar within box=median; square within box=mean; vertical lines out of the box=minimum and maximum
No statistically significant difference was noted between protozoa and helminthes infections, concerning anthropometric measurements and micronutrients in serum (zinc, Iron, and copper) (Table 3).
| Parasite species | No | % |
|---|---|---|
| Giardia lamblia | 34 | 31 |
| Entamoeba hisotolytica | 21 | 19 |
| Cryptosporidium parvum | 12 | 11 |
| Entamoeba coli | 9 | 8.2 |
| Isospora | 4 | 3.5 |
| Giardia lamblia & Cryptosporidium parvum | 6 | 5.5 |
| Giardia lamblia & Entamoeba hisotolytica | 7 | 6.4 |
| Ascaris | 10 | 9 |
| Hymenolepis nana | 7 | 6.4 |
Table 2. Prevalence and type of protozoa and helminthes infections in studying children (n=110)
| Variable | Protozoa (n=93) |
Helminthes (n=17) |
p |
|---|---|---|---|
| Weight for age = 5th centile <5th centile (underweight) |
51 (69%) 42(31%) |
13 (94%) 4 (6%) |
X2 =2.8a 0.07 |
| Height for age = 5th centile <5th centile (stunted) |
80 (86%) 13 (14%) |
16 (94%) 1 (6%) |
X2 =0.7 0.30b |
| Weight for height = 5th centile <5th centile (wasted) |
68 (77%) 25 (23%) |
15 (88%) 2 (12%) |
X2=1.8 0.15b |
| BMI centile = 5th centile <5th centile (wasted) |
59 (69%) 34 (31%) |
14 (94%) 3 (6%) |
X2=1.1 0.23a |
| Serum zinc Low Normal |
52 (56%) 41 (44%) |
11 (65%) 6 (35%) |
X2=0.45 0.35a |
| Serum Iron Low Normal |
88 (95%) 5 (5%) |
15 (88%) 2 (12%) |
X2=0.08 0.6 b |
| Serum Copper Low Normal |
4 (4%) 89 (96%) |
1 (6%) 16 (94%) |
X2=0.09 0.58b |
aChi-square test; bFisher?s test.
Table 3. Comparison of protozoa and helminthes infections regarding some anthropometric measurements, serum Zinc, Iron, and Copper (n=110).
| Anthropometric index | Serum Iron | Serum Zinc | Serum copper |
| Weight-for-age | 0.4 (p<0.001)* | 0.6 (p<0.001)* | 0.2 (p<0.03)* |
| Height-for-age | 0.3 (p<0.05)* | 0.1 (p>0.5) | 0.08 (p>0.5) |
| Weight-for-height | 0.3 (p<0.003)* | 0.6 (p<0.001)* | 0.1 (p> 0.3) |
| BMI for age | 0.3 (p<0.007)* | 0.7 (p<0.0001)* | 0.1 (p> 0. 4) |
*P is significant
Table 4. Correlations between anthropometric measurements and serum iron, zinc and copper in children with parasitic infections (n=110).
| Malnourished (n=38) |
Nourished (n=72) |
p | |
|---|---|---|---|
| Serum Zinc Low Normal |
32 (84%) 6 (16%) |
15 (21%) 57 (79%) |
0.001* X2=40.8 |
| Serum Iron Low Normal |
36 (95%) 2(5%) |
69 (96%) 3 (4%) |
0.60 X2=0.07 |
| Serum Copper Low Normal |
3 (8%) 35 (92%) |
1 (1%) 71 (99%) |
0.40 X2=0.49 |
*P is significant
Table 5. Serum micronutrients in infected children according to their nutritional status (n=110) .
Correlations between serum micronutrients and anthropometric measurements in children with parasitic infections are shown in Table 4, serum zinc has the most significantly positive correlation with weight for age, weight for height and BMI for age (r=0.6, 0.6, 0.7; respectively, p<0.001). While serum iron, has a less significant positive correlation with weight for age, weight for height and BMI for age (r=0.3) for all anthropometric measures (p<0.003). Serum copper has also a weak but significant positive correlation shown only with weight for age, otherwise, no significant correlations with other anthropometric indices.
The most parameters affecting serum zinc negatively in stepwise multiple linear regression model were parasitic infection and underweight (B1=-12.95 and B2=-0.18, with a p<0.001 and 0.01, respectively), while BMI for age had a significant positive effect on serum zinc level (B3=2.18, p<0.001), with an R2 of the model of 0.63.Also, parasitic infection showed a large negative effect (B1=-3.64, p<0.01) and a small negative effect of underweight (B2=-0.18, p<0.03) on serum copper level. In serum copper, parasitic infection had a negative effect (B1=-3.65, p<0.001), a weak negative effect to underweight, had on serum copper (B2=-0.18, p<0.03) with R2 of 0.19.Similarly, parasitic infection and underweight, had a negative effect on iron serum level, (B1=-8.9, and B2=-0.13, with a p<0.001 and 0.003, respectively).
Table 5 shows that 34.5% of children with parasitic infections had malnutrition and there was a statistically significant difference of serum zinc between the malnourished and nourished children with parasitic infections (p<0.001). However, in the two studied groups, they were not statistically different as regard the serum iron and copper.
Discussion
Trace elements deficiencies and poor nutrition dramatically hinder adequate human health and socio-economic development. Both developed and developing countries are interested in the burden of trace elements deficiency disorders, the highest prevalence is found in Sub-Saharan Africa and South Asia [20].
In the present study, zinc, iron and copper serum levels were significantly lower in parasitic patients compared to controls. The path physiology is not clearly understood; however, micronutrients deficiencies may be linked to malabsorption due to mucous affection. Patients with Giardiasis, in particular, may have intestinal lesions caused by Giardia trophozoites may impair intestinal zinc absorption to a great extent. Additionally, infection by various parasites affects obviously the level of serum zinc due to its shifting to the liver [21]. Besides, the intestinal parasites use carbohydrates, lipids, minerals, vitamin and other food sources of the host in order to gain essential energy of the life cycle [22].
The results of the present study are comparable with those reported recently by Arbabi et al. [23] they found that serum levels of trace elements such as magnesium, zinc, and copper were reduced with infection by Giardia lamblia and in enterobiasis. These findings were supported by many other researches which clarified the poor absorption of several micronutrients caused by intestinal parasites [24,25]. Some studies not only showed that patients with parasitic infections had micronutrients deficiencies, but also their treatment with anti-parasitic medications had improved their serum levels [26,27]. For example, Olivares et al. [28] reported that serum copper, zinc, and magnesium deficiencies have significantly improved three months after treatment of patients with Enterobius vermicularis and Giardia lamblia infections. Similarly, another study from Mexico reported that eradication of Giardia lamblia led to a marvelous increase in the mean serum zinc levels after treatment for six months in Mexican schoolchildren [29]. In the present study, serum iron was significantly lower in patients with parasitic infections than children of the healthy group; the observed results are similar to earlier researches which stated that there was a deficient status of iron in the sera of parasite infected children [30,31].
In contrast to the results of the present study, some other studies reported a controversial association between parasitic infections and micronutrients. Results of a study from Turkey revealed that children with giardiasis had increased serum levels of copper, whilst zinc and iron levels were low [26]. Similar findings were reported by other researchers [32-35]. Such controversial results of several studies could be explained by the inability of the body to store zinc causing a decrease in its level to a great extent. Conversely, the storage of copper is mainly in its binding form to ceruloplasmin which is considered one of the important acute phase reactant that increases in various infections explaining why serum level of copper increases during such conditions. In the present study, low serum copper could be explained also that many children are underweight or stunted and have inadequate ingesting of foods with high bioavailability of copper such as meat, poultry, and fish that is evidently found with poor nutrition.
In this study, serum iron levels were remarkably lower (p<0.0001) in children having parasitic infections than the control group. The outcomes of the current study were also similar to those reported by other studies [26,32].
In the current research, 35% of parasites infected children were malnourished (wasted and or stunted) versus 65% nourished. It was demonstrated that serum zinc was the only micronutrient significantly lower in malnourished than the nourished ones (p<0.001). Correlation analysis between serum micronutrients and the anthropometric measurements shown positive significant correlations of serum iron and zinc with most of anthropometric measurements (weight-for-age, weight- for-height and BMI-for-age). On the contrary, serum copper did not have a significant correlation with all anthropometric measurements, except with weight for age (r=0.2). Many researchers observed the same as well [1,36]. Furthermore, multiple linear regression model shown a highly negative effect of parasitic infection (B1=-12.95, B1=-3.64, -8.9, respectively) and a less negative effect to underweight (B2=-0.18, -0.13) on serum zinc, copper and iron levels.
Some reasons may be responsible for the low iron deficiency in this study, such as a combination of reduced intake, deficient absorption. It cannot be ignored that the parasitic infection has a systemic effect and parasites utilize iron, which is essential for their growth and multiplication [37]. Iron deficiency anemia is estimated by WHO at approximately half of children whose ages ranged from 4 to 15 years old in developing countries [38]. As mentioned before, zinc cannot be stored in the body so its serum level could be easily dropped, especially in susceptible pre-schoolchildren because of their higher growth demands. The zinc deficiency prevalence was estimated by WHO. It was 31%, ranging from 4 to 73% across various areas all over the world [39]. In the present study, anthropometric measurements indicated that 84% of children with malnutrition who are short, and or wasted have a significant lower zinc concentration versus 21% in normally nourished children. Many reporters defined that short stature and decreased height-for-age was mainly affected by deficient levels of serum zinc as it is considered one crucial factor in the metabolism of nucleic acids and hence the protein synthesis, consequently impeding the whole process of growth in children [4,40].
One of the striking observations of the present study is no significant difference of serum iron and copper between the malnourished and normally nourished groups in children with parasitic infections. The explanations for this result could be related to other factors, not investigated, such as heavy parasitic infections, small number of study children or some dietetic factors, for example, decreased bioavailability of most minerals and increased levels of phytate in the food due to the consumption of a diet based mainly on plants [41].
The present study is not without limitations; first, the dietary intake of the children have not been monitored, nor calculated, since the ingestion of low bioavailability food of most of the micronutrients may contribute to deficiencies of serum zinc and iron especially in children living in low socioeconomic status, this occurs because of inhibition of absorption by phytates, polyphenols and oxalates, which are found in diets mainly based on plants [41]. The main inhibitor is phytate; it can bind divalent minerals such as Zn2+ and Fe2+, thus hindering their body absorption [42]. Another limitation is the study did not involve developmental milestones assessment, other studies, recently, have focused on the relationship between intestinal parasitic infection, malnutrition, and child development. Investigators from Jamaica demonstrated improved academic performance in children who had been dewormed with anthelmintic drugs [43,44].
Conclusion
Serum micronutrients: zinc, iron and copper and anthropometric indices were significantly lower in children with parasitic infections than controlled children group. Multiple regression analysis showed that parasitic infection and underweight had a negative effect on all micronutrients studied. However, they were no significant differences of micronutrients between protozoa and helminthes infections. Serum zinc andiron correlated positively with most of the anthropometric indices; however, serum copper only had a weak positive correlation with weight for age. Further large studies are recommended to clarify more the relations between parasitic infections, micronutrients, development and cognition in children.
Acknowledgement
Many thanks to laboratory technicians for their valuable help in carrying out this work.
References
- Amare B, Moges B, Fantahun B, et al. Micronutrient levels and nutritional status of school children living in Northwest Ethiopia. Nutr J 2012; 11: 108.
- Coop RL, Holmes PH. Nutrition and parasite interaction. Int J Parasitol 1996; 26: 951-962.
- Walravens PA, Krebs NF, Hambidge KM. Linear growth of low income preschool children receiving zinc supplement. Am J Clin Nutr 1983; 38: 195-201.
- Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr 2003; 133:1485S-1489S.
- Gibson SR. Principles of Nutritional Assessment. 2nd ed., Oxford University Press: New York 2005.
- Hotz C, Lowe NM, Araya M, Brown KH. Assessment of the trace element status of individuals and populations: The example of zinc and copper. J Nutr 2003; 133: 1563S-1568S.
- Failla ML. Trace elements and host defense: recent advances and continuing challenges. J Nutr 2003;133: 1443S-1447S.
- Caulfield LE, Black RE. Zinc deficiency. In comparative quantification of health risks. WHO, World Health Organization. Geneva 2004.
- WHO/UNICEF/UNU. Iron deficiency anemia assessment, prevention, and control: A guide for program managers. World Health Organization, Geneva 2001.
- Wegayehu T, Tsalla T, Seifu B, et al. Prevalence of intestinal parasitic infections among highland and lowland dwellers in Gamo area, South Ethiopia. BMC Public Health 2013; 13: 151.
- Zarebavani M, Dargahi D, Einollahi N, et al. Serum levels of zinc, copper, vitamin B12, folate and immunoglobulins in individuals with giardiasis. Iran J Public Health 2012; 41: 47-53.
- Egger RJ, Hofhuis EH, Bloem MW, et al. Association between intestinal parasitoses and nutritional status in 3-8 year old children in northeast Thailand. Trop Geogr Med 1990; 42: 312-323.
- Thein-Hlaing, Thane-Toe, Than-Saw, et al. A controlled chemotherapeutic intervention trial on the relationship between Ascaris lumbricoides infection and malnutrition in children. Trans R Soc Trop Med Hyg 1991; 85: 523-528.
- Walker SP, Robinson RD, Powell CA, et al. Stunting, intestinal parasitism and the home environment. Trans R Soc Trop Med Hyg 1992; 86: 331-332.
- http://www.fao.org/docrep/005/y4249e/y4249e0b.htm
- Mohammad IE, Abduallah AA, Abduallah SA, et al. The 2005 growth charts for Saudi children and adolescents (No. AR-20-63). King Abdul-Aziz City for Science and Technology, Riyadh, KSA 2009.
- Garcia LS. Diagnostic medical parasitology. 5th ed. Washington, DC: ASM Press 2007.
- Nuttall KL, Gordon WH, Ash KO. Inductively coupled plasma mass spectrometry for trace element analysis in the clinical laboratory. Ann Clin Lab Sci 1995; 25: 264-271.
- Sauberlich HE. Laboratory tests for the assessment of nutritional status. 2nd ed. Boca Raton, London: CRC press 1999.
- Black RE, Victora CG, Walker SP, et al. Maternal and child under nutrition and overweight in low-income and middle-income countries. Lancet 2013; 382: 427-451.
- Singh A, Smoak BL, Patterson KY, et al. Biochemical indices of selected trace minerals in men: Effect of stress. Am J Clin Nutr. 1991; 53: 126-131.
- Hesham MS, Edariah AB, Norhayati M. Intestinal parasitic infections and micronutrient deficiency: a review. Med J Malaysia 2004; 59: 284-293.
- Arbabi M, Esmaili N, Parastouei K, et al. Levels of zinc, copper, magnesium elements and vitamin B12, in sera of schoolchildren with Giardiasis and Entrobiosis in Kashan, Iran. Zahedan.J Res Med Sci 2015; 15: 29-32.
- Abou Shady O, El Raziky MS, Zaki MM, Mohamed RK. Impact of Giardia lamblia on growth, serum levels of zinc, copper and iron in Egyptian children. Biol Trace Elem Res. 2011; 140: 1-6.
- Culha G, Sangün MK. Serum levels of zinc, copper, iron, cobalt, magnesium and selenium elements in children diagnosed with Giardia intestinalis and Enterobiosis vermicularis in Hatay, Turkey. Biol Trace Elem Res 2007; 118: 21-6.
- Ertan P, Yereli K, Kurt O, et al. Serological levels of zinc, copper and iron elements among Giardia lamblia infected children in Turkey. Pediatr Int 2002; 44: 286-288.
- Karakas Z, Demirel N, Tarakcioglu M, Mete N. Serum zinc and copper levels in southeastern Turkish children with giardiasis or amebiasis. Biol Trace Elem Res 2001; 84: 11-18.
- Olivares JL, Fernández R, Fleta J, et al. Serum mineral levels in children with intestinal parasitic infection. Dig Dis 2003; 21: 258-261.
- Quihui L, Morales GG, Méndez RO, et al. Could giardiasis be a risk factor for low zinc status in school children from northwestern Mexico? A cross-sectional study with longitudinal follow-up. BMC Public Health 2010; 10: 85.
- Stoltzfus RJ, Chwaya HM, Tielsch JM, et al. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. Am J Clin Nutr. 1997; 65: 153-159.
- Stoltzfus RJ, Dreyfuss ML, Chwaya HM, et al. Hookworm control as a strategy to prevent iron deficiency. Nutr Rev 1997; 55: 223-232.
- Demirci M, Delibas N, Altuntas I, et al. Serum iron, zinc and copper levels and lipid peroxidation in children with chronic giardiasis. J Health Popul Nutr 2003; 21: 72-75.
- Tanyuksel M, SayalA, Aydin A. Trace element levels in some parasitic disease. Acta Parasitol Turcica 1995; 19: 315-321.
- Kilic E, Yazar S, Saraymen R. Serum zinc and magnesium levels in patients with blastocystosis. Biol Trace Elem Res. 2004; 98: 21-26.
- El Gohari Y, Galal SH, Boulos LM. Trace element levels in some parasitic diseases. J Egypt SocParasitol 1984; 14: 179-87.
- Alemnji GA, Thomas KD, Durosinmi MA, et al. Haematogram and serum iron status of malnourished Nigerian children. East Afr Med J 1995; 72: 605-608.
- World Health Organization: Management of severe malnutrition. A manual for physicians and other senior health workers. Geneva 1999. 4.
- UNICEF/UNU/WHO. Iron deficiency anemia: Assessment, prevention and control. 2001.
- Caulfield LE, Black RE. Zinc deficiency. In Comparative Quantification of Health Risks. WHO, World Health Organization. Geneva 2004.
- Prasad AS. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr 2009; 28: 257-265.
- Lazarte CE, Soto A, Alvarez L, et al. Nutritional status of children with intestinal parasites from a tropical area of Bolivia, emphasis on zinc and iron status. Food Nutr Sci 2015; 6: 399-411.
- Lonnerdal B. Phytic acid-trace element (Zn, Cu, Mn) interactions. Int J Food Sci Tech 2002; 37: 749-758.
- Nokes C, Cooper ES, Robinson BA, et al. Geohelminth infection and academic assessment in Jamaican children. Trans R Soc Trop Med Hyg 1991; 85: 272-273.
- Oberhelman RA, Guerrero ES, Fernandez ML. Correlations between intestinal parasitosis, physical growth, and psychomotor development among infants and children from rural Nicaragua. Am J Trop Med Hyg 1998; 58: 470-475.