- Biomedical Research (2012) Volume 23, Issue 1
Immunoreactive Detection of Glial Fibrillary Acidic Protein (GFAP) from the Brain of Bombyx mori
Jae-Sun Choi1* and Cheolin Park2
1Division of Life Science, College of Life Science and Biotechnology, Korea University
2Department of Biomedical Laboratory Science, Daegu Health College, 702-722 KOREA
- Corresponding Author:
- Cheolin PARK
Department of Biomedical Laboratory Science
Daegu Health College, San 7
Taejeon1-dong, Buk-ku
Daegu 702-722 KOREA
Phone: +82-53-320-4508, Fax:+53-320-1450
e-mail: cheolinpark@dhc.ac.kr
Accepted date: September 29 2011
Abstract
Glial fibrillary acidic protein (GFAP) is the primary intermediate filament protein as a marker for the identification of astrocytes in the central nervous system in vertebrates. This present study was performed to identify GFAP-immunoreactive neurons in invertebrate (moth) by the light microscopy immunohistochemistry and immunoblotting. Our results indicated the presence of GFAP-like positive cell processes and cell bodies in the developing stages. The amount of GFAP immunoreactivity was maximized in the 3rd instar larva but diminished it, approaching to 5th instar. This changed amount of GFAP was also confirmed by immunoblotting with developing stages. GFAP immunoreactivity was also observed in both axons within nervi corpora cardiaci (NCC) I +II and corpora allata (NCA). We suggest that this glial filament protein may be conserved in the evolution of the invertebrate nervous systems and that it may be used as a label for some types of glial cells and as neurotransmitter of neuromodulator in the moth.
Keywords
GFAP, Bombyx mori, Immunocytochemistry, Brain, Neurotransmitter
Introduction
Glial cells regulate physiological and biochemical processes in the nervous system [1] and are implicated in the guidance of neuron outgrowth during development [2].
Glial fibrillary acidic protein (GFAP) is an intermediate filament (IF) protein and is expressed in the central nervous system in astrocyte cells. It is involved in many cellular functioning processes, such as cell structure and movement, cell communication, and the functioning of the blood brain barrier. It was first isolated from human multiple sclerosis plaques [3] and it has been biochemically characterized by the use of polypeptide analysis and immunogenic determinations [4].
To identify the sites of GFAP production and and its release is obviously necessary for investigating the regulatory mechanism for GFAP secretion. Localization of the source of GFAP in the brain has been studied by employing a variety of methods such as the observation of histological changes in the neurosecretory cells (NSCs). GFAP-like immunoreactivity has been shown in enteric glia [5], Schwann cells of unmyelinated peripheral nerve fibers [6,7], lens epithelium [8], adult brain of lizards [9], and in the visual system of the crab [10]. Filaments of morphological structure similar to the intermediate glial filament have been reported in the glial cells of many mammals, birds, reptiles, fishes [11], and in some invertebrates such as crab [10] and zebra fish [12] and snail [13]. Primary role of GFAP in vertebrate is confined in astrocytes, as a cytoskeleton stabilizer but also found in several invertebrates [14,15,16,17].
Paula et al.[13] confirmed that molecular weight of GFAP in the snail, Megalobulimus abbreviatus, particularly in cerebral ganglia and subesophageal mass, by immunohistochemistry and immunoblotting was about 55kDa. In this paper, we firstly describe and confirm the appearance of GFAP-immunoreactive cells in the embryonic brain of Bombyx mori and its immunoreactive distribution at developing stages as well as its molecular weight by immunblotting.
Materials and methods
Animals
Cold-treated eggs of Bombyx mori were hatched about 10 days after incubation at 27-28°C with relative humidity of 60-70%. Larvae were reared on an artificial diet (mostly mulberry leaves) under a long-day photoperiod regimen (17 hrs light-7 hrs dark).
Wholemount immunocytochemistry
Wholemount immunocytochemistry for brain were little modified and performed [18]. The brain of larvae was dissected in 0.1M sodium phosphate buffer (PB, pH 7.4) and then fixed in 4% paraformaldehyde (PFA) in 0.1M PB for 5-9 hrs at 4°C, depending on the size of brain and stage. The fixed tissues were immersed in 0.01M phosphate- buffered saline (PBS) with 1% Triton X-100 at 4°C for overnight. Blockage of peroxidae activity was performed in 10% methanol with 3% H2O2 for 25 min. Wash in 0.1M Tris-HCl buffer (pH 7.6-8.6) containing 1% Triton X-100 and 4% NaCl were followed by incubation with a primary anti-GFAP (Sigma) diluted to 1:1000 in dilution buffer (0.01M PBS with 1% Triton X-100 and 10% normal serum) for 4-5 days with gentle shaking. After wash in 0.01M PBS with 1% Triton X-100, tissues were incubated in peroxidase-conjugated swine antirabbit IgG (DAKO), diluted to 1:200 for 2 days at 4°C. Following preincubation in 0.03% diaminobenzidine (DAB, Sigma) in 0.05M Tris-HCl buffer for 1 hr at 4°C, the tissues were treated with 0.03% DAB in 0.05M Tris- HCl buffer for 5-10 min containing 0.01% H2O2 . After rinses in 0.05M Tris-HCl buffer, tissues were embedded in glycerin, examined and photographed.
Fluorescence immunocytochemistry
This method was also followed by Park and Lee [19]. Brains of larval stage were isolated in 0.1M PB, fixed in 4% PFA for 4 hrs at 4°C, and washed with 80% ethanol (8 x 10 min). Additional wash in 0.01M PBS with 1% Triton X-100 (4 x 10 min) were followed, and tissues were then incubated with anti-GFAP (diluted to 1:1000 in 0.01M PBS with 1% Triton X-100 and 10% normal serum) for overnight at room temperature. Tissues were rinsed in 0.01M PBS (5x 10min) and then incubated with swine anti-rabbit IgG conjugated with Rhodamine (Sigma) for 4 hrs at room temperature in the dark room. Tissues were finally washed in 0.01M PBS with 1% Triton X-100 (3x10 min), embedded in glycerin, examined and photographed with a fluorescence microscope.
Immunoblotting analysis
About 500 brains from each stage of Bombyx mori were homogenized in 100? of 2% NaCl with a glass-glass homogenizer cooled in an ice bath. The homogenate was allowed to stand for 1 hr in the ice bath, heated for 2 min in boiling water, rapidly cooled, and centrifuged at 10,000xg for 10 min. The resultant supernatant was mixed with a buffer containing 2% SDS, 10% (v/v) glycerol, 0.001% (w/v) bromophenol blue, and 100mM Tris- HCl (pH 6.8), and heated in boiling water for 4 min. This sample was electrophoresed according to the method of Laemmli on a 12% polyacrylamide slab gel (80X70X1 mm) and then were transferred to HybondTM nitrocellulose membrane (Amersham Pharmacia Biotech). The nitrocellulose membrane containing the immobilized proteins was first blocked with non-fat dry milk (5%) plus BSA (1%) in Tris buffered saline containing Tween (TBS-T) for 90 min. After the blockage, the membrane was washed twice in TBS-T under constant stirring for 3 min. The membrane was incubated with the monoclonal antibody mouse anti-GFAP (Millipore) under constant strirring for 2 h at room temperature. The membrane washed again with TBS-T. The secondary antibody used was an anti-mouse HPR (1:1500) which was incubated with the membrane for 90 min at room temperature and washed. The GFAP was detected using the chemiluminescence ECLTM (Amersham Pharmacia Biotech) and a HyperfilmTM (Amersham Pharmacia Biotech) diagnostic film.
Results
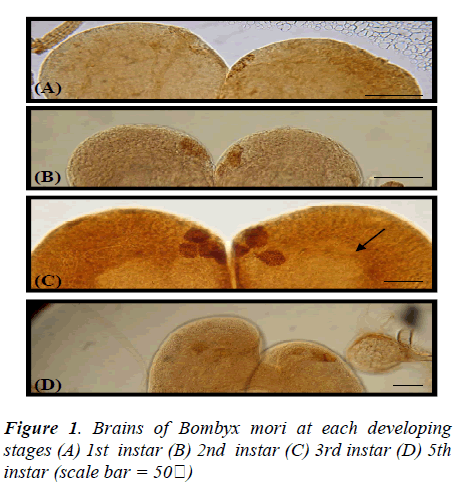
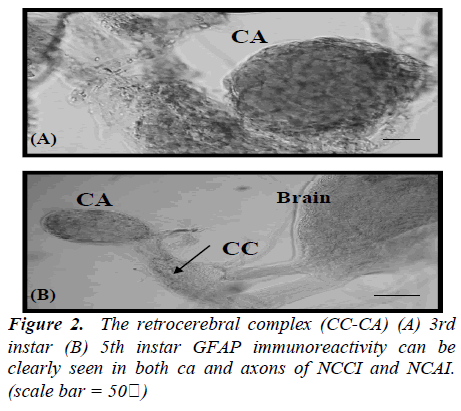
In the brain of 1st instar Bombyx larva, two pairs of dorsolateral NSCs (Fig.1A) and those GFAP immunoreactive cells became more clear and large in the 2nd instar larval brain (Fig.1B). Four pairs of more intense GFAP immunoreactive cells are shown at 3rd instar larva (Fig. 1C) which are median neurosecretory cells. However, this GFAP immunoreactivity is decreased and disappeared cells of median neurosecretory are in the 5th instar larval brain (Fig. 1D). The axons originated from those GFAP immunoreactive cells passed along the edge of the neuropile toward the midline of the 3rd instar larval brain. Whole-mount immunohistochemistry clearly showed the overall pathway of the axons by which the immunoreactive material was transported though the brain. The axons originating from the four lateral NSCs are fasciculated, traverse the midline of the brain to enter the contralateral lobe, and proceed posteriorly toward the retrocerebral nerve. However, axons from other stages could not be traced. Interestingly, all of GFAP immunoreactive cells are located up to 3rd instar larva in the median neurosecretory area but those are scattered in the 5th instar larval brain. The immunoreactive axons and their terminals were abundantly observed between the glandular cells of the CA, while few axons were seen in the CC (Fig. 2). In the retrocerebral nerve connecting the CC to the brain, immunostainable axon was also observed. In the 3rd instar brain, in particular, corpora allata contained abundant GFAP immunoreactivity but weak immunoreactivity was shown in the 5th instar.
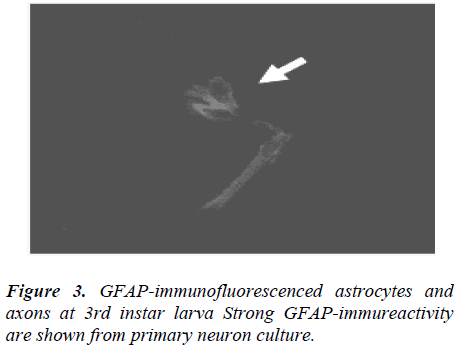
GFAP-immunofluorescenced astrocytes and axons are not appeared at 1st and 2nd instar larva but firstly shown from 3rd instar larva. In the course of 5th instar larval stages, GFAP immunofluorescence reactivity are not clearly detected (data not shown), comparing with 3rd instar stage (Fig. 3).
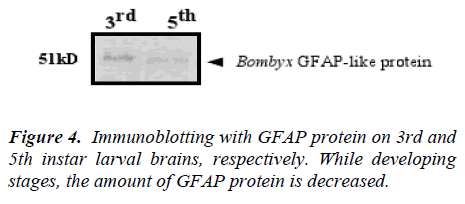
Components immunoreactive to the GFAP antibody in the Bombyx brain extract were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and immunblotted (Fig. 4).
The brain extracts of each stage (500-brain equivalent each) were loaded and immunoblotted with the monoclonal antibody mouse anti-GFAP. The amount of GFAP immunoreactivity was maximized in the 3rd instar larva but diminished it, approaching to 5th instar. Comparing with two bands, there are surely difference between two larval stages.
Discussion
GFAP is a classical marker for astorcytes in the vertebrate central nervous system, even though it has also been detected elsewhere in vertebrates [5,7,11,19].
GFAP is not strictly confined to astrocytes in the vetebrate central nervous system. GFAP-like immunoreactivity has been shown in enteric glia [5], Schwann cells of unmylinated peripheral nerve fibers [7,11], lens epithelium [8], and Kupffer cells in the liver [19]. Recently, it was shown that GFAP is also expressed by other cell types in CNS, including ependymal cells. GFAP has also been located in rat kidney glomeruli [20] and skin keratinocytes [21], osteocytes of bones, chondrocytes [22] and stellate-shaped cells of the pancreas and liver. Due to various distribution of GFAP expression in various locations, GFAP is thought to help to maintain astrocyte mechanical strength, as well as the shape of cells but its exact function remains poorly understood, despite the number of studies using it as a cell marker. In insects, intermediate filament-like proteins were biochemically identified within the olfactory dendrites in the antennae of two types of silkmoths [23]. More than one form of GFAP has also been observed in Xenopus [24] and axolotl [25], it has been suggested that GFAP may exist in more than one form in animals that retain radial glia throughout life [25].
In the brain, GFAP did not immunoreactivity in the whole distributive area, rather, it was distributed in median NSC area only. In amphibians, localized expression of GFAP in the distal portion of cells has been reported in both adult spinal cord [25] and embryonic brain [24,26,27]. In contrast to the limited disbribution of GFAP immunoreactivity in the brain, GFAP immunoreactivity in the Bombyx mori was shown different amount at developing larval stages. However, we did not shown this GFAP immuno reactivity in the pupal and adult stage so further study will be necessary to investigate the correct distribution at more developed stages. It is interesting to speculate that the different amount of distribution of GFAP immunoreactivity depends on developing stage may reflect differential or specific functions. Four pairs of dorso-lateral NSCs of brain were found at 3rd instar larval brains, suggesting that a possible release of GFAP as a neurotransmitter of neuromodulator at this site.
The distribution in the Bombyx brain-CC-CA complex of GFAP nerves in CA might suggest that GFAP acts as a neurotransmitter or neuromodulator that affects the CA activity to synthesize and release the juvenile hormone, in addition to being simply released to haemolymph from CA. Furthermore, it is speculated that GFAP production from NSCs might be involved in the control of the CA activity. This also suggests that GFAP immunoreactivity produced in the brain neurons are transported via the axons within NCC I+II and NCA I to the corpora allata that appears to be a main accumulation and release site for the GFAP immunoreactivity in some brain cells produces. We found a similar molecular weight of 51kD from Bombyx mori brains with monoclonal antibody mouse anti- GFAP and Paula et al. [13] confirmed 55kDa from snails. These results suggest that GFAP has not only been conserved in vertebrate evolution, but also appear to be expressed in the nervous system of some lower and higher invertebrate.
In this paper, we could conclude that GFAP probably can be functioned and presumed as neurotransmitter of neuromodulator in the central nervous system as well as conservative evolution even though there is no direct evidences nor related functions between vetebrates and invertebrates.
Acknowledgement
Authors cordially appreciate the priceless comment and advice of Dr. Bong Hee LEE of Korea University.
References
- Pentreath VW, Kai-Kai MA. Significance of the postassium signal from neurons to glial cells. Nature 1982; 295: 56-61.
- Silver J, Lorenz SE, Wahlstein D, Coughline J. Axonal guidance during development of the great cerebral commissures: Descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J. Comp. Neurol. 1982; 210: 10-29.
- Eng LF, Vanderhaegen JJ, Bignami, A, Gerstl B. As acidic protein isolated from fibrous astrocytes. Brain Res. 1971; 28: 351-354.
- Bignami, A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972; 43: 429-435.
- Jessen KR. and Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980; 286: 736-737.
- Yen SH. and, Fields KL. Antibodies to neurofilament, glial filament, and fibroblast intermediate proteins bind to different cell types of the nervous system. J. Cell Biol. 1981; 88: 115-126.
- Dahl D., Chi, NH, Miles LE. and Nguyen BT. and Bignami A. Glial fibrillary acidic (GFA) protein in Schwann cells: fact or artifact? J. Histochem. Cytochem. 1982; 30: 912-918.
- Hatfield JS., Skoff RP., Maisel, H. and Eng, LF. Glial fibrillary acidic protein is localized in the lens epithelium. J. Cell Biol. 1984; 98: 1895-1898.
- Ahboucha S, Laalaoui A, Didier-Bazes M, Montange M., Cooper HM and Gamrani H. Differential patterns of glial fibrillary acidic protein-immunolabeling in the brain of adult lizards. J Comp Neurol. 2003; 464 (2): 159-171.
- Simon FS., Clynton LC., Giovane GT., Marcelo E, and Ana MBM Silvana A. Glial fibrillary acidic protein (GFAP)-like immunoreactivity in the visual system of the crab Ucides cordatus (Crustecea, Decapoda). Biol. of the Cell 2004; 96: 724-734.
- Dahl D. Crosby CJ., Sethi JS. and Bignami A. Glial fibrillary acidic protein (GFAP) in vertebrates: immunofluorescence and immunoblotting study with monoclonal and polyclonal antibodies. J Comp Neurol. 1985; 239: 75-88.
- Marcus RC., Stephen S. and Easter JR. Expression of Glial Fibrillary Acidic Protein and Its Relation to Tract Formation in Embryonic Zebrafish (Danio rerio) J. Comp. Neurol. 1995; 359:365-381.
- Paula CS., Günther G. Gristina FH and Matilde A. Detection of glial fibriallary acidic protein (GFAP) and vimentin (Vim) by immunoelectron microscopy of the glial cells in the central nervous system of the snail Megalobulimus abbreviatus. Acta Zoologica 2005; 86: 135-144.
- Radojcic, T. and Pentreath WR. Invertebrate glia. Prog. Neurobiol. 1979; 12: 115-179.
- Cardone B and Roots BI. Comparative immunohistochemical study of glia filament protein (glial fibrillary acidic protein and vimentin) in goldfish, octopus, and snail. Glia 1990; 3: 180-192.
- Riehl, B. and Schulue WR. Morphological organization of neuropile glial cells in the central nervous system of the medicinal leech (Hirudo medicinalis). Tissue Cell. 1998; 30: 177-186.
- Dos Santos P. Gehlen G., Faccioni-Heuser MC Zancan DM and Achaval M. Distribution of glial cells in the central nervous system of the pulmonate snail Megalobulimus oblongus identified by means of glial fibrillary acidic protein marker. Acta Zool. (Stockholm) 2002; 83: 345-351.
- Park C and Lee BH. Localization of Manduca sexta Allatotropin Neuropeptides in developing ventral nerve cord of the silk moth Bombyx mori. Zool. Sci. 2001; 3 (2): 177-185.
- Gard AL, White FP, Dutton GR. Extra-neural glial fibrillary acidic protein (GFAP) immunoreactivity in perisinusoidal stellate cells of the rat liver. J Neuroimmunol. 1985; 8: 359-375.
- Buniatian GH, Hartmann HJ, Traub P, Wiesinger H, Albinus M, Nagel W, Shoeman R, Mecke D, Weser U. Glial fibrillary acidic protein-positive cells of the kidney are capable of raising a protective biochemical barrier similar to astrocytes: expression of metallothionein in podocytes Anat Rec. 2002; 267 (4): 296-306.
- Danielyan L, Tolstonog G, Traub P, Salvetter J, Gleiter CH, Reisig D, Gebhardt R, Buniatian GH. Colocalization of glial fibrillary acidic protein, metallothionein, and MHC II in human, rat, NOD/SCID, and nude mouse skin keratinocytes and fibroblasts. J Invest Dermatol. 2007; 127 (3): 555-563.
- Kasantikul V, Shuangshoti S. Positivity to glial fibrillary acidic protein in bone, cartilage, and chordoma J. Surgical Oncol. 1989; 41: 22-26.
- Kumar GL, Maida R, Keil TA. Identification of cytoskeletal proteins in the antennae of the silkmoths Antheraea polyphemus and A. pernyi. Neuroreport 1996; 7: 1985-1989.
- Szaro BG, Gainer H. Immunocytochemical identification of non-neuronal intermediate filament proteins in the developing Xenopus lueuis nervous system. Dev. Brain Res. 1998; 43: 207-224.
- Holder N, Clarke JDW, Kamalati T, Lane EB. Heterogeneity in spinal radial glia demonstrated by intermediate filament expression and HRP labelling. J. Neurocytol. 1990; 19: 915-928.
- Godsave SF, Anderton BH, Wylie CC. The appearance and distribution of intermediate filament proteins during differentiation of the central nervous system, skin and notochord of Xenopus laeuis. J Embryol Exp Morph 1986; 97: 201-223.
- Messenger NJ., and Warner AE. The appearance of neural and glial cell markers during early development of the nervous system in the amphibian embryo. Development 1989; 07: 3-54.