Special Issue Article - Biomedical Research (2017) Health Science and Bio Convergence Technology: Edition-II
Immunohistochemical screening for ALK fusion gene in signet-ring cell gastric carcinoma
Liang Chang, Bingjie Huo, Yalei Lv, Yujie Shan, Wei Liu*
Department of Medical Oncology, Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, PR China
- *Corresponding Author:
- Wei Liu
Department of Medical Oncology
Fourth Hospital of Hebei Medical University
PR China
Accepted date: January 10, 2017
Abstract
The aim of this study was to prescreen the expression of ALK-positive in signet-ring cell gastric carcinoma by IHC assay. We selected 84 FFPE samples with signet-ring cell gastric carcinoma (55 cases of GC, 29 cases of EGJ) and performed the detection of ALK-positive using IHC. The correlation of ALK-positive and clinicopathologic characteristics was statistically analyzed. The results showed, of 84 cases prescreened, 11 (13.09%) were ALK-positive. For the 6 cases with IHC 2+ (7.14%), and the 5 cases with IHC 1+ (5.95%). We noted that 8 (72.73%) cases were never smoker, 8 (72.73%) cases were >5 cm tumor size and 9 (81.82%) cases were T4 in invasive depth. All of the 11 cases were III of pathologic TNM stage. The ALK-positive patients showed significantly statistical difference in lymph node metastasis (p=0.0285) and TNM stage (p=0.0497), compared with the ALK-negative patients. In conclusion, the expression of ALK fusion is found in signet-ring cell gastric carcinoma by IHC assay.
Keywords
Immunohistochemistry, Screening, Signet-ring cell gastric carcinoma, ALK fusion gene
Introduction
The anaplastic lymphoma kinase (ALK) gene rearrangements were initially identified in anaplastic large cell lymphomas (ALCL) for over 20 years [1]. Subsequently, ALK gene was also recognized in diffuse large B-cell lymphoma (DLBCL) and in inflammatory myofibroblastic tumors (IMT) [2-4]. In 2007, the fusion of ALK gene with echinoderm microtubule associated protein [4] like (EML4) gene locus was detected in a subset of non-small cell lung cancer (NSCLC) in Japan [5]. The incidences of EML4-ALK fusions in NSCLC, mostly involving East Asian patients, were around 3%-13% with no significant differences between Asian and western countries [6-9]. By extrapolation, recent estimates indicate that approximately 5% of NSCLC contain an EML4-ALK fusion, which would mean that over 70,000 patients will get clinical benefit from crizotinib. It was a small molecule inhibitor of ALK and c-MET receptor tyrosine kinases and approved by the U.S. FDA for the treatment of EML4–ALK positive NSCLC patients [10-12]. Clinical characteristics associated with EML4-ALK fusion are younger age, never/light smoking status, or adenocarcinoma histology. Meanwhile, the majority of EML4-ALK fusion tumors were demonstrated a solid growth pattern with >10% signet-ring cells [13-15].
This distinct cytologic characteristic is reminiscent of the signet-ring cells more commonly seen in gastric carcinoma than in lung cancer. Signet-ring cell gastric carcinoma, characterized by cells with abundant mucin in the cytoplasm and nuclei located at the cell periphery, is a histologic diagnosis by the World Health Organization [16,17]. It has long been thought to have a worse prognosis than other forms of gastric cancer. However, several studies have begun to question this idea and found that is not the case [18-20]. The molecular basis of their growth and metastasis still remains unclear, and the genetic background has rarely been researched. Therefore, efficient screening for the ALK fusion gene is a crucial issue in molecular basis and clinical practice of signet-ring cell gastric carcinoma.
Currently, there are three methods for the EML4-ALK fusion gene detecting in NSCLC: 21 fluorescent in situ hybridization (FISH), reverse transcriptase-polymerase chain reaction (RTPCR) and Immunohistochemistry (IHC). FISH is sensitive and specific to detect EML4-ALK as an eligibility criterion in NSCLC, but it is not readily available due to technical and financial problems in routine pathology practice in China. Theoretically, although RT-PCR is a standard method for testing the EML4-ALK, it requires fresh frozen tissues for extracting RNA, so limited its clinical application. Compared with FISH and RT-PCR, the conventional IHC assay is more cost-effective and convenient screening test in most pathology practices, especially in pathology labs without a VENTANA IHC platform. Many research groups also reported good correlations between IHC stain and FISH for detection of EML4-ALK fusion gene [22-24]. For these reasons, conventional IHC seems suitable for a large-scale screening of patients with ALK positive carcinoma.
In this study, we examined the ALK fusion gene using conventional IHC in signet-ring cell gastric carcinoma and analyzed the correlation between ALK fusion gene and clinicopathologic characteristics of the disease. The present study was aimed at preliminary screening for ALK fusion gene in signet-ring cell gastric carcinoma.
Materials and Methods
Patients and tissues
This study included 84 archival formalin-fixed paraffinembedded (FFPE) samples with signet-ring cell gastric carcinoma (55 cases of gastric cancer, 29 cases of esophagogastric junction carcinoma) at the Fourth Hospital of Hebei Medical University from 2011 to 2013. It was approved by the Ethics Review Board at the Fourth Hospital of Hebei Medical University. All samples were surgically resected tissues and were proved to the signet-ring cell carcinoma by experienced pathologists. They were collected and used after obtaining informed consent from the patients. According to the TNM staging for carcinoma of the stomach by AJCC (7th ed., 2010), the TNM stage was postoperative pathologic stage. Medical records were reviewed to extract data on clinicopathologic characteristics, including age, gender, smoking history, tumor size, invasive depth, lymph node metastasis and cancerembolus.
Immunohistochemistry
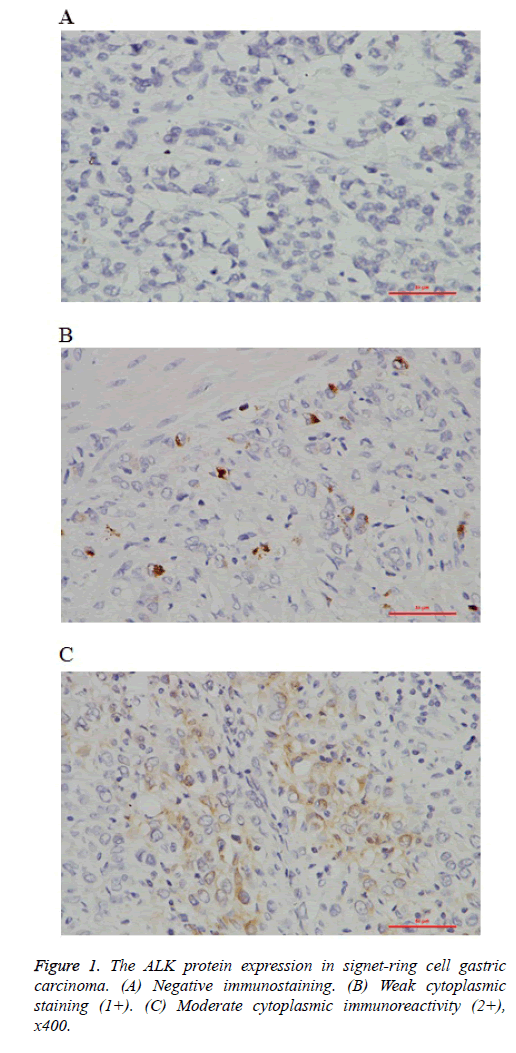
Immunohistochemical staining was performed on unstained 4 μm thick FFPE tissue sections. Briefly, after deparaffinization and rehydration, the slides were heated for antigen retrieval in steam cooker for 2 minutes in 1mM EDTA, pH 9.0(ZSGB-BIO, China). Then the slides were incubated with monoclonal for ALK (Clone SP8, Abcam, USA) and overnight at room temperature with a dilution of 1:100. Immunoreactivity was visualized with DAB detection kit (Dako, CA) according to the manufacturer’s protocol. The IHC stains were evaluated for the expression of ALK protein by two pathologists. The criteria for scoring ALK fusion gene were as follows with reference to ALK-positive lung cancer: 23 No staining (0); Faint or weak staining intensity with >5% tumor cells or any staining intensity with ≤ 5% tumor cells (1+); Moderate staining intensity with >5% tumor cells (2+); Strong and granular staining intensity with >5% tumor cells (3+).
Statistical analysis
The statistical analyses were carried out by using Statistical Analysis System V8 (SAS Institute Inc. USA). To analyze correlations between ALK status and clinicopathologic features, we used the conventional chi-square association test or Fisher’s exact test. All statistical tests were two-sided, and a value of P<0.05 was considered to be statistically significant.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the medical ethics committee of fourth hospital of Hebei medical university.
Results
ALK protein expression by IHC in signet-ring cell gastric carcinoma
In the preliminary study, we analyzed a panel of 84 signet-ring cell gastric carcinoma samples, comprising 55 cases of gastric cancer, and 29 cases of esophagogastric junction carcinoma (EGJ). ALK rearrangement was detected in 11 of 84 cases (13.09%). All the ALK-positive cases exhibited cytoplasmic staining pattern in tumor cells (Figures 1A-1C). For the 6 cases with IHC 2+ (7.14%), and the 5 cases with IHC 1+ (5.95%). Of the 11 ALK-positive cases, 8 were gastric cancer, and 3 were EGJ. Individual patient characteristics of ALK-positive cases were listed in Table 1.
| N | ALK (IHC) |
Gender | Age | Smoking | Location | Tumor size (cm) |
Invasive depth | Lymph node metastasis | TNM stage | Cancerembolus |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1+ | male | 53 | smoker | EGJ | 6 | T3 | N3a | IIIB | negative |
| 2 | 2+ | male | 36 | never | EGJ | 7 | T4a | N3a | IIIC | negative |
| 3 | 1+ | female | 72 | never | EGJ | 3.5 | T4a | N1 | IIIA | negative |
| 4 | 2+ | female | 58 | never | GC | 3.5 | T4a | N3a | IIIC | negative |
| 5 | 1+ | female | 55 | never | GC | 8 | T3 | N3a | IIIB | negative |
| 6 | 2+ | female | 48 | never | GC | 13 | T4a | N3a | IIIC | negative |
| 7 | 2+ | male | 67 | smoker | GC | 6 | T4a | N1 | IIIA | negative |
| 8 | 2+ | male | 59 | smoker | GC | 3 | T4a | N2 | IIIB | negative |
| 9 | 2+ | female | 66 | never | GC | 11.5 | T4a | N2 | IIIB | negative |
| 10 | 1+ | male | 74 | never | GC | 6 | T4a | N3a | IIIC | positive |
| 11 | 1+ | male | 45 | never | GC | 6 | T4a | N1 | IIIA | negative |
| EGJ: Esophagogastric Junction Carcinoma; GC: Gastric Cancer | ||||||||||
Table 1: Individual patient characteristics of ALK Fusion-positive Patients in Signet-ring Cell Gastric Carcinoma.
As summarized in Table 2, the cases composed of 6 (54.55%) men and 5 (45.45%) women with a median age 57.5 years (from 36 to 74). Smoking, 3 (27.27%) were smoker, and 8 (72.73%) were never. The tumor size was ≤ 5 cm in 3 (27.27%), and >5 cm in 8 (72.73%). In invasive depth, 2 (18.18%) were T3, and 9 (81.82%) were T4. The lymph node metastasis was N1 in 3 (27.27%), N2 in 2 (18.18), and N3 in 6 (54.55%). All of the 11 cases were III of pathologic TNM stage, and only 1 case was cancerembolus positive.
| Clinicopathologic features (N=11) | ALK-positive | |
|---|---|---|
| (n) | (%) | |
| Gender | ||
| Male | 6 | 54.55 |
| Female | 5 | 45.45 |
| Age (median age 57.5) | ||
| ≥ 60 | 4 | 36.36 |
| <60 | 7 | 63.64 |
| Smoking | ||
| Smoker | 3 | 27.27 |
| never | 8 | 72.73 |
| Tumor size | ||
| ≤ 5 cm | 3 | 27.27 |
| >5 cm | 8 | 72.73 |
| Invasive depth | ||
| T3 | 2 | 18.18 |
| T4 | 9 | 81.82 |
| Lymph node metastasis | ||
| N1 | 3 | 27.27 |
| N2 | 2 | 18.18 |
| N3 | 6 | 54.55 |
| TNM stage | ||
| III | 11 | 100 |
| cancerembolus | ||
| Negative | 10 | 90.91 |
| Positive | 1 | 9.09 |
Table 2: Clinicopathologic Features of ALK Fusion-positive Patients in Signet-ring Cell.
Correlation with clinicopathologic features
In the present study, a total of 84 cases had successful IHC stain for detection of ALK fusion gene expression. Eight of 55 patients with gastric cancer were ALK-positive (14.55%), for 2 patients with IHC 1+ (3.64%), and 6 patients with IHC 2+ (10.91%). Three of 29 patients with EGJ were ALK-positive (10.35%), but all of 3 patients were IHC 1+. As the median ages of the ALK-positive and ALK-negative groups were 57.5 and 60.7 years, respectively, there were no significantly difference (p>0.05). The clinicopathologic features of the ALK-positive and ALK-negative patients were compared and the results were shown in Table 3. The ALK-positive patients showed significantly statistical difference in lymph node metastasis (p=0.0285) and TNM stage (p=0.0497), compared with the ALK-negative patients. No appreciable correlation was demonstrated between ALK-positive and ALK-negative groups in gender, smoking habit, tumor size, invasive depth, or cancerembolus.
| Clinicopathologic features (N=84) | ALK fusion gene | P-value | |
|---|---|---|---|
| Positive n=11(%) |
Negative n=73(%) |
||
| Gender Male Female |
6(9.68) 5(22.73) |
56(90.32) 17(77.27) |
0.2337 |
| Age ≥60 <60 |
4(10.0) 7(15.91) |
36(90.0) 37(84.09) |
0.4227 |
| Smoking Smoker never |
3(9.37) 8(15.38) |
29(90.63) 44(84.62) |
0.6456 |
| Tumor size ≤5cm >5cm |
3(7.69) 8(17.78) |
36(92.31) 37(82.22) |
0.1718 |
| Invasive depth T1 T2 T3 T4 |
0 0 2(20.0) 9(15.52) |
13 3 8(80.0) 49(84.48) |
0.3722 |
| Lymph node metastasis N0 N1 N2 N3 |
0 3(33.33) 2(6.90) 6(22.22) |
19 6(66.67) 27(93.1) 21(77.78) |
0.0285 |
| TNM stage I+II III+IV |
0 11(18.64) |
25 48(81.36) |
0.0497 |
| cancerembolus Negative Positive |
10(15.15) 1(5.56) |
56(84.85) 17(94.44) |
0.4993 |
Table 3: Frequency of the ALK Fusion Gene Expression in Signet-ring Cell Gastric Carcinoma and its Association with Clinicopathologic features./
Discussion
Signet-ring cell gastric carcinoma is a distinct entity. Its diagnosis is based on an adenocarcinoma containing a majority of signet-ring cells by the identification of pathologist. A study shows that signet-ring cells in EGJ have a more aggressive biological behaviour [25]. Meanwhile, signet-ring cells present a low sensitivity to chemotherapy [19,20]. Therefore, we would try to research the molecular basis and find the potent molecular targets of Signet-ring cell gastric carcinoma. Considering the majority of EML4-ALK fusion lung cancer was demonstrated a solid growth pattern with >10% signet-ring cells, it is reminiscent of the signet-ring cells more commonly seen in gastric carcinoma than in lung cancer [13,14]. In this preliminary study, we show that ALK fusion gene was detected in 11 of 84 (13.09%) patients with Signet-ring cell gastric carcinoma. By using IHC stain, of the 11 ALK-positive cases, 6 cases were IHC 2+ (7.14%) and 5 cases IHC 1+ (5.95%). It seems to bring the hope and surprise to the disease.
Searching for targetable oncogenes in lung cancer have not been stopped and made steady progress in recently years. The EML4-ALK fusion gene represents one of the newest molecular targets in NSCLC. Although it is a minor genetic abnormality in approximately 5% of NSCLC, the incidence of NSCLC is increasing in Asian and western countries, so the absolute number of ALK-positive lung cancer is not trivial. The potent oncogenic activity of EML4-ALK fusion can be effectively blocked by crizotinib, a small molecular ALK tyrosine kinase inhibitor, which demonstrated dramatic response and longer PFS in ALK-positive lung cancer [26,27]. The patients with ALK-positive in NSCLC are more likely to be young, never/light smoker, and adenocarcinoma histology [7-9]. In our study, we note that 8 (72.73%) cases were never smoker, 8 (72.73%) cases were >5 cm tumor size and 9 (81.82%) cases were T4 in invasive depth. Meanwhile, all of the 11 cases were III of pathologic TNM stage. Although ALK-positive cases share several clinicopathologic features, compared with the ALK-negative groups, there is no appreciable correlation was demonstrated. We also find that lymph node metastasis (p=0.0285) and TNM stage (p=0.0497) show significantly statistical difference between ALK-positive and ALK-negative groups.
Current diagnostic methods to detect ALK fusion gene include IHC, FISH and RT-PCR. Duo to the higher requirements for fresh frozen tissue, RT-PCR unlikely becomes the standard test in clinical practice. Although a criterion method for ALK-positive in NSCLC had not been established, FISH has been used as the current gold standard for detecting ALK fusion gene in clinical trials with crizotinib [28-30]. However, FISH for the routine large-scale detection in China remains to be challenging because of the technological complexity, time consumption and high cost. Meanwhile, interpretation of FISH in ALK-positive lung cancer tends to be more difficult than in ALCL or DLBCL, because ALK gene variants in NSCLC is an intrachromosomal rearrangement, so the break-apart probes can be subtle and relatively difficult to recognize [22-24,30]. Rodig and colleagues have also shown that FISH as a pre-screening method did not detect all cases with ALK-positive in NSCLC [8]. Since IHC is rapid, easy, sensitive and relatively inexpensive for detecting ALK fusion protein in NSCLC by pathologists, it remains a mainstay of routine surgical pathology diagnosis. A few validated antibodies in IHC for ALK protein have been widely used to diagnose ALK-rearranged ALCL today. However, the IHC used to ALCL is inadequate for detecting the majority of ALK-positive lung cancer. In an initial research, Rodig et al. found that only 4 ALK-positive in lung cancer by the ALCL standard test, and through FISH proved to be 10 cases [8]. Subsequently, some studies showed that ALK expression was detected in all ALK-positive lung cancer to be substantially lower than in ALKrearranged ALCL [31]. Therefore, the College of American Pathologists (CAP), International Association for the Study of Lung Cancer (IASLC), and Association for Molecular Pathology (AMP) established the molecular testing guideline for selection of lung cancer patients for ALK tyrosine kinase inhibitors [8]. They found the interpretation criteria for ALK-positive lung cancer in IHC. In this preliminary study, according to the scoring criteria of ALK-positive lung cancer, we examined the ALK fusion gene using conventional IHC in signet-ring cell gastric carcinoma. Of the 11 ALK-positive cases, 6 cases were IHC 2+ (7.14%) and 5 cases IHC 1+ (5.95%). We thought that this was inappropriate in signet-ring cell gastric carcinoma. With the deep research and increased cases, we should find the diagnostic standard for ALK-positive in signet-ring cell gastric carcinoma.
Currently, the consistency of FISH and IHC is still controversial in detecting ALK-positive NSCLC. Some studies have found the good concordance between FISH and IHC [32,33]. Paik et al. also showed that none of IHC 1+ and 30% of IHC 2+ was FISH-positive and the specificity of IHC 2+ or more was 95.2% in detecting ALK-positive NSCLC [34]. However, Park et al. reported that 83.3% (5/6) of IHC 1+ and 84.6% (11/13) of IHC 2+ were FISH-positive, respectively. They thought that the high FISH-positive rate of IHC 1+ and 2+ in this study may result from different cutoff value of fluorescence, but there is no found standard for separation distance to define spilt signal [35]. In our present study, we performed the prescreening of ALK-positive in signet-ring cell gastric carcinoma by IHC assay, but we have not finished the further confirmation using FISH or RT-PCR. This is just a preliminary result, and don’t rule out the possibility of false positive. One important goal of this preliminary study was to prescreen the expression of ALK-positive in signet-ring cell gastric carcinoma. In conclusion, our data show that the expression of ALK fusion gene is found in signet-ring cell gastric carcinoma by IHC assay. The further study will be required to performed FISH and RT-PCR assay as ALKpositive confirmation.
Informed Consent
Informed consent was obtained from all individual participants included in this study.
References
- Morris SW, Kirstein MN, Valentine MB. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994; 263: 1281-1284.
- Delsol G, Lamant L, Mariamé B, Pulford K, Dastugue N. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2; 5 translocation. Blood 1997; 89: 1483-1490.
- Falini B, Mason DY. Proteins encoded by genes involved in chromosomal alterations in lymphoma and leukemia: clinical value of their detection by immunocytochemistry. Blood 2002; 99: 409-426.
- Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000; 157: 377-384.
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561-566.
- Choi YL, Takeuchi K, Soda M Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res 2008; 68: 4971-4976.
- Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008; 14: 4275-4283.
- Rodig SJ, Mino-Kenudson M, Dacic S Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009; 15: 5216-523.
- Mino-Kenudson M, Chirieac LR, Law K A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunehistochemistry. Clin Cancer Res 2010; 16: 1561-1571.
- Perner S, Wagner PL, Demichelis F, Mehra R, Lafargue CJ. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia 2008; 10: 298-302.
- Rodig SJ, Shapiro GI. Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr Opin Investig Drugs 2010; 11: 1477-1490.
- Gupta SK. Role of Crizotinib in previously treated non-small-cell lung cancer. South Asian J Cancer 2014; 3: 138-140.
- Shaw AT, Yeap BY, Mino-Kenudson M Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009; 27: 4247-4253.
- Inamura K, Takeuchi K, Togashi Y, Nomura K, Ninomiya H. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008; 3: 13-17.
- Popat S, Gonzalez D, Min T, Swansbury J, Dainton M. ALK translocation is associated with ALK immunoreactivity and extensive signet-ring morphology in primary lung adenocarcinoma. Lung Cancer 2012; 75: 300-305.
- Kim JY, Kim YY, Kim SJ, Park JC, Kwon YH. Predictive factors for lymph node metastasis in signet ring cell gastric cancer and the feasibility of endoscopic submucosal dissection. J Gastric Cancer 2013; 13: 93-97.
- Kim BS, Oh ST, Yook JH Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery 2014; 155: 1030-1035.
- Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol 1994; 3: 221-227.
- Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol 2012; 30: 3493-3498.
- Li C, Kim S, Lai JF, Hyung WJ, Choi WH. Advanced gastric carcinoma with signet ring cell histology. Oncology 2007; 72: 64-68.
- Murakami Y, Mitsudomi T, Yatabe Y. A Screening Method for the ALK Fusion Gene in NSCLC. Front Oncol 2012; 2: 24.
- Wu YC, Chang IC, Wang CL, Chen TD, Chen YT. Comparison of IHC, FISH and RT-PCR methods for detection of ALK rearrangements in 312 non-small cell lung cancer patients in Taiwan. PLoS One 2013; 8: e70839.
- Thunnissen E, Bubendorf L, Dietel M, Elmberger G, Kerr K. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch 2012; 461: 245-257.
- Shan L, Lian F, Guo L Combination of conventional immunohistochemistry and qRT-PCR to detect ALK rearrangement. Diagn Pathol 2014; 9: 3.
- Nafteux PR, Lerut TE, Villeneuve PJ Signet Ring Cells in Esophageal and Gastroesophageal Junction Carcinomas Have a More Aggressive Biological Behavior. Ann Surg 2014; 260: 1023-1029.
- Yoshida A, Tsuta K, Nakamura H, Kohno T, Takahashi F. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011; 35: 1226-1234.
- Choi YL1, Soda M, Yamashita Y, Ueno T, Takashima J. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010; 363: 1734-1739.
- Martelli MP, Sozzi G, Hernandez L, Pettirossi V, Navarro A. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 2009; 174: 661-670.
- Zhang YG, Jin ML, Li L Evaluation of ALK rearrangement in Chinese non-small cell lung cancer using FISH, immunohistochemistry, and real-time quantitative RT- PCR on paraffin-embedded tissues. PLoS One 2013; 8: e64821.
- Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol 2009; 27: 4232-4235.
- Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer 2010; 46: 1773-1780.
- Jokoji R, Yamasaki T, Minami S, Komuta K, Sakamaki Y. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol 2010; 63: 1066-1070.
- McLeer-Florin A, Moro-Sibilot D, Melis A, Salameire D, Lefebvre C. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol 2012; 7: 348-354.
- Paik JH, Choe G, Kim H. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011; 6: 466-472.
- Park HS, Lee JK, Kim DW. Immunohistochemical screening for anaplastic lymphoma kinase (ALK) rearrangement in advanced non-small cell lung cancer patients. Lung Cancer 2012; 77: 288-292.