- Biomedical Research (2016) Volume 27, Issue 3
Immunohistochemical GRP78 as a tumor biomarker may predict poor prognosis in patients with non-small cell lung cancers: a retrospective study.
Du-Juan Yu1, Yun-Gang Luo2*, Guo-Min Liu31The Department of Respiration, China Japan Union Hospital of Jilin University, ChangChun, Jilin 130033, PR. China
2Department of Stomatology, Jilin University Second Hospital, Changchun, Jilin 130041, PR. China
3Department of Orthopedics, Jilin University Second Hospital, Changchun, Jilin 130041, PR. China
- *Corresponding Author:
- Yun-Gang Luo
Department of Orthopedics
Second Hospital of Jilin University
PR. China
Accepted date: March 25, 2016
Abstract
Purpose: To examine the correlation of GRP78 and GRP94 protein levels with clinicopathological variables and prognosis in primary Non-Small Cell Lung Cancer (NSCLC) patients.
Methods: A total of 89 primary patients with NSCLC were included. Their surgically-resected tissues were studied by immunohistochemistry to assess GRP78 and GRP94 expression. Student's t-test, Kaplan-Meier estimator with log rank test and Cox regression analyses were performed to examine the associations of GRP78/GRP94 levels with clinicopathological variables and survival.
Results: GRP78 and GRP94 expressions increased significantly in tumor samples of NSCLC patients. High GRP78 positive was associated with pathologic TNM status (p=0.003) and pathologic N status (p=0.013); while high GRP94 positive was associated with squamous-cell carcinoma subtype (p=0.001). There is no correlation between GRP78 and GRP94 levels (p =0.086). Various levels of GRP78 proteins were associated with survival of NSCLC patients (p = 0.016). A higher GRP78 level marked a shorter survival time (p = 0.043). Various levels of GRP94 proteins were not associated with survival of NSCLC patients (p = 0.610).
Conclusions: High expression of GRP78 can be related to a poor prognosis. Expression level of GRP78 protein may be a potential predictor for NSCLC prognosis.
Keywords
GRP78, GRP94, NSCLC, Immunohistochemistry, Prognosis, Predictor.
Introduction
NonSmall Cell Lung Carcinoma (NSCLC) is one of the primary causes for cancerrelated death worldwide, in spite of obvious progress in surgery, chemotherapy, radiotherapy and biologically targeted therapy [1]. Research into novel prognostic biomarkers and therapeutic targets in NSCLC is still a focus of interest.
Glucose-Regulated Proteins (GRPs) are originally discovered as they are induced by glucose starvation, and GRPs belong to the Endoplasmatic Reticulum (ER) chaperone families [2]. Under pathological conditions, such as acidosis, hypoxia or hypothermia, expression levels of GRPs are up-regulated [3].
Recently, knowledge about the correlation of GRPs with cancer has been improved. GRPs have been studied in cultured cancer cells [4-7] and clinic cancers, for instance colorectal carcinoma [8], pediatric hepatoblastoma [9], hepatitis B virusrelated hepatocellular carcinomas [10], gastric carcinomas [11], esophagus adenocarcinomas [12] and lung cancer [13].
Novel GRPs-targeted therapeutic methods have been developed into preclinical tests and recommended for cancer treatment to some extent [14-17]. Among GRPs, either GRP78 or GRP94 is one of the central regulators of ER function because of their roles in protein folding and activation of transmembrane ER-stress sensors. High GRP78 and GRP94 levels have been found to relate with high pathologic TNM status and aggressive behavior of cancers [11].
Ma et al. suggest that serum GRP78 level may be a tumor marker that predicts poor prognosis in late-stage NSCLC patients [18]. This hints that GRPs have great potentials to be tumor markers. But this previous study only including small sample size fails to find the correlation of GRPs with clinicopathological variables. Therefore, more investigations are worthy of doing to reveal the correlation of GRPs with tumor, clinicopathological factors and prognosis.
The current investigation was designed to detect the expression levels of GRP78 and GRP94 proteins by immunohistochemistry, and analyze the association with clinicopathological variables and prognosis.
Materials and Methods
Patients
A total of 89 patients with primary NSCLC underwent surgical resection in China Japan Union Hospital of Jilin University in June 2011 to October 2013. All patients did not undergo any anti-tumor therapy prior to surgery. The follow-up period was defined as the time from surgery to the last observation for censored cases, or death for complete observations. Patients without a study end date and who were lost to follow-up were considered censored. The minimum postoperative follow-up time was 15 months. The median survival time was 32 months. According to the current pathological Tumor Node Metastasis (TNM) classification, pT status and pN status of tumors were determined [19]. According to the WHO classification, histological subtype was determined [20]. All patients gave written informed consent. Study was approved by the Jilin University Ethics Committee.
Immunochemistry
Specimens (paired normal tissue and cancer mass) were removed from each of 89 NSCLC patients and were embedded by paraffin. Typical regions of paraffin-embedded specimens were marked. Using a manual tissue arrayer (Beecher Instruments, Sun Prairie, WI), specimens were cut into 2 μm thick slices of 0.6 mm diameter. Then slices were dewaxed and rehydrated. Heat-induced antigen retrieval was performed routinely. Slices were cooled, and incubated with the primary antibodies at a final concentration of 0.20 mg/ml: either GRP78 monoclonal antibodies (Cell Signaling Biotechnology, Inc., Beverly, MA) or GRP94 monoclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Using fast red as the reaction indicator, the color was developed with the labeled streptavidin-biotinylated alkaline phosphatase system. Slices were counterstained with hematoxylin, followed by dehydration. Plasma cell staining was used as internal positive control. The immunoreactivity of either GRP94 or GRP78 was classified into five categories: -, no positive cells or only single positive cell; +, positive cells accounted for less than 10%; ++, positive cells accounted for 10-50%; +++, positive cells accounted for 50-80%; and ++++, positive cells accounted for above 80% [21]. The + and ++ protein levels denoted lower expression; the +++ and ++++ protein levels denoted higher expression.
Statistical analysis
SPSS 19.0 statistical software was used for statistical analysis. Spearman correlation analysis was used to assess correlation. Student's t-test was used for comparison between groups. Kaplan-Meier estimator and log rank test was used for survival analysis. Univariate and multivariate Cox regression analyses were performed using the Cox proportional hazards model. A two-tailed p<0.05 was considered statistically significant difference.
Results
GRP78 and GRP94 expressions
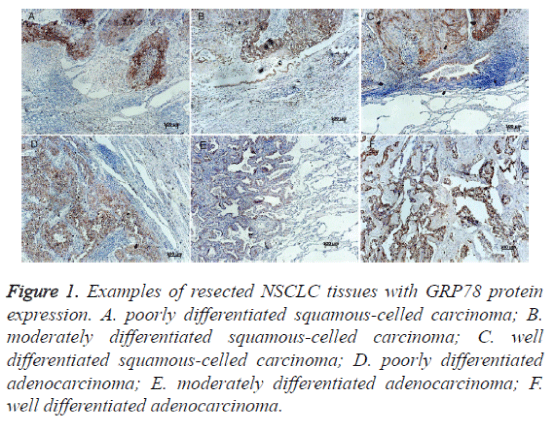
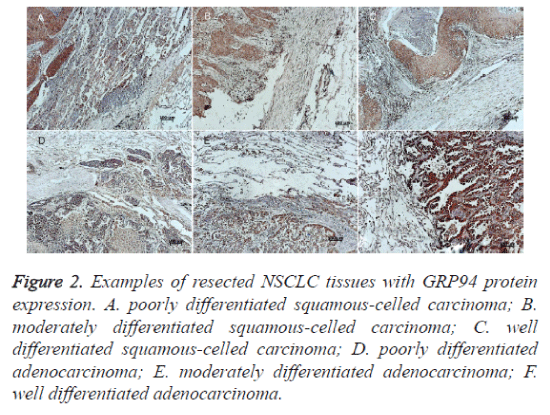
Immunohistochemistry was performed to examine the expression levels of GRP78 and GRP94 proteins. Both GRP78 and GRP94 were seen positive expression in cytoplasm and the staining intensities in cancer tissues were stronger than in normal tissues (Figures 1 and 2). The staining intensities of cancer tissues were ++ to ++++, while the intensities of the paired normal tissues were + to ++. There were statistically significant differences between them (p=0.000 and p=0.034. respectively) (see Table 1). GRP78 expression was not associated with GRP94 expression (Spearman correlation coefficient 0.421, p =0.086) (Table 2).
Figure 1. Examples of resected NSCLC tissues with GRP78 protein expression. A. poorly differentiated squamous-celled carcinoma; B. moderately differentiated squamous-celled carcinoma; C. well differentiated squamous-celled carcinoma; D. poorly differentiated adenocarcinoma; E. moderately differentiated adenocarcinoma; F. well differentiated adenocarcinoma.
Figure 2. Examples of resected NSCLC tissues with GRP94 protein expression. A. poorly differentiated squamous-celled carcinoma; B. moderately differentiated squamous-celled carcinoma; C. well differentiated squamous-celled carcinoma; D. poorly differentiated adenocarcinoma; E. moderately differentiated adenocarcinoma; F. well differentiated adenocarcinoma.
| Tissues | Cases | GRP78 | p value | GRP94 | p value | |||||||
| – | + | ++ | +++ | – | + | ++ | +++ | ++++ | ||||
| Tumor mass | 89 | 0 | 34 | 34 | 21 | 0.000* | 0 | 34 | 10 | 41 | 38 | 0.034* |
| Normal tissues | 89 | 65 | 24 | 0 | 0 | 38 | 43 | 8 | 0 | 0 | ||
Note:* p<0.05.Student's t -test.
Table 1. GRP78 and GRP94 expressions in paired NSCLC mass and normal tissues.
| GRP78 | Correlationcoefficient | p value | ||||
| + | ++ | +++ | ||||
| GRP94 | ++ | 5 | 2 | 3 | 0.421 | 0.086 |
| +++ | 14 | 22 | 5 | |||
| ++++ | 15 | 10 | 13 | |||
Table 2. Spearman correlation between GRP94 and GRP78 protein in NSCLC patients.
Clinicopathological variables
Clinicopathological data were listed in table 3. The correlation of clinicopathological factors with either GRP78 or GRP94 expressions was tested. Positive expression of GRP78 was associated with pathologic TNM status (p=0.003) and pathologic N status (p=0.013). Positive expression of GRP94 was associated with squamous cell carcinoma (SCC) subtype (p=0.001) (Table 3).
| Clinicopathological variables | Cases | GRP78 | P value | GRP94 | P value | |||||
| + | ++ | +++ | ++ | +++ | ++++ | |||||
| Number | 89 | 34 | 34 | 21 | 10 | 41 | 38 | |||
| Sex | Male | 67 | 27 | 26 | 14 | 0.322 | 10 | 31 | 26 | 0.082 |
| Female | 22 | 7 | 8 | 7 | 0 | 10 | 12 | |||
| Smoking | Yes | 47 | 15 | 20 | 12 | 0.258 | 7 | 21 | 19 | 0.263 |
| No | 32 | 13 | 11 | 8 | 1 | 15 | 16 | |||
| Unknown | 10 | 6 | 3 | 1 | 2 | 5 | 3 | |||
| pTNM status | I | 40 | 15 | 14 | 11 | 0.003 | 5 | 14 | 21 | 0.425 |
| II | 32 | 12 | 14 | 6 | 4 | 16 | 12 | |||
| III | 17 | 7 | 6 | 4 | 1 | 11 | 5 | |||
| pT status | T1 | 31 | 11 | 12 | 8 | 0.779 | 4 | 15 | 12 | 0.055 |
| T2 | 47 | 21 | 14 | 12 | 3 | 20 | 24 | |||
| T3 | 11 | 2 | 8 | 1 | 3 | 6 | 2 | |||
| pN status | N0 | 53 | 15 | 24 | 14 | 0.013 | 6 | 25 | 22 | 0.823 |
| N1 | 21 | 8 | 6 | 7 | 3 | 8 | 10 | |||
| N2 | 13 | 10 | 3 | 0 | 1 | 6 | 6 | |||
| N3 | 2 | 1 | 1 | 0 | 0 | 2 | 0 | |||
| Differentiation | Low | 21 | 8 | 9 | 4 | 0.153 | 2 | 12 | 7 | 0.742 |
| Moderate | 55 | 23 | 21 | 11 | 6 | 23 | 26 | |||
| Well | 8 | 3 | 2 | 3 | 2 | 3 | 3 | |||
| Unknown | 5 | 0 | 2 | 3 | 0 | 3 | 2 | |||
| Pathology | SCC | 55 | 24 | 22 | 9 | 0.057 | 9 | 30 | 16 | 0.001 |
| AC | 34 | 10 | 12 | 12 | 1 | 11 | 22 | |||
| Local infiltration | Yes | 26 | 8 | 11 | 7 | 0.388 | 0 | 13 | 13 | 0.137 |
| No | 63 | 26 | 23 | 14 | 10 | 28 | 25 | |||
pTNM: pathological tumor-node metastasis, pT: pathological tumor, pN: pathological node, SCC: squamous cell carcinoma, AC: adenocarcinoma.
Table 3. Clinicopathological variables of NSCLC patients and correlation with either GRP78 or GRP94 protein levels. (*p<0.05)
Survival analysis
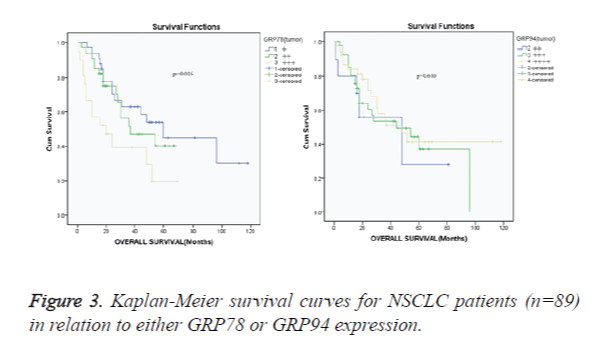
The minimum postoperative follow-up time was 15 months. The median survival time was 32 months. The 1-, 2-, 3-, and 5- year survival rates were 88.7%, 67.3%, 53.7% and 38.7% for overall NSCLC patients with +, ++ and +++ GRP78; 86.7%, 66.1%, 57.3% and 36.7% for overall NSCLC patients with ++, +++ and ++++ GRP94. Correlation of either GRP78 or GRP94 protein expressions with survival of NSCLC patients was analyzed as shown in Kaplan-Meier survival curve (Figure 3). Various levels of GRP78 proteins were associated with survival of NSCLC patients (p=0.016. Figure 3A). According to the setting of the current study, the + and ++ protein levels denoted lower expression; the +++ and ++++ protein levels denoted higher expression. The patients with a higher GRP78 protein level had shorter survival times compared with the patients with a lower GRP78 level (median overall survival, 36.1 [+++ patients] versus 47.2 months [+ and ++ patients]; RR: 0.371, 95% CI: 0.135-1.019; p=0.043). Various levels of GRP94 proteins were not associated with survival of NSCLC patients (p=0.610) (Figure 3B).
Prognostic factors for NSCLC patients
The + and ++ protein levels denoted lower expression; the +++ and ++++ protein levels denoted higher expression. Therefore, patients were divided into lower and higher groups. A univariate analysis was performed to examine the clinicopathological factors that predict prognosis (Table 4).
| Clinicopathological variables | Hazard ratio | 95%CI | p value |
| Sex (male vs. female) | 3.264 | 1.188-8.966 | 0.015 |
| Smoking (yes vs. nope) | 1.258 | 0.458-3.456 | 0.026 |
| pTNM status (stages I-II vs. III) | 0.533 | 0.194-1.464 | 0.425 |
| pT status(T1-2 vs. T3) | 0.297 | 0.108-0.816 | 0.035 |
| pN status(N0 vs. N1-3) | 0.682 | 0.248-1.873 | 0.523 |
| Differentiation(well and moderate vs. low) | 3.252 | 1.184-8.933 | 0.014 |
| Pathology (SCC vs. AC) | 0.154 | 0.056-0.423 | 0.131 |
| Local infiltration (yes vs. nope) | 0.268 | 0.098-0.736 | 0.037 |
| GRP78 level (low vs. high) | 0.371 | 0.135-1.019 | 0.043 |
| GRP94 level (low vs. high) | 0.285 | 0.104-0.783 | 0.254 |
pTNM: pathological tumor-node metastasis, pT: pathological tumor, pN: pathological node.
Table 4. Univariate analysis of prognostic factors for NSCLC patients (n=89).
Among the factors analyzed, sex, smoking, pathological T, differentiation, local infiltration and GRP78 level were significant prognostic factors (p=0.015, p=0.026, p=0.035, p=0.014, p=0.037 and p=0.043, Table 4). Then six factors were subjected to multivariate analysis to evaluate independent prognostic factors, including sex, smoking, pathological T, differentiation, local infiltration and GRP78 level. The results of multivariate analysis illustrated that sex, smoking, differentiation and GRP78 level were four independent prognostic factors in these NSCLC patients (Table 5).
| Clinicopathological variables | Hazard ratio | 95%CI | p value |
| Sex (male vs. female) | 3.042 | 1.107-8.356 | 0.042 |
| Smoking (yes vs. no) | 1.865 | 0.679-5.123 | 0.006 |
| pT status(T1-2 vs. T3) | 0.493 | 0.179-1.354 | 0.105 |
| Differentiation(low vs. well and moderate) | 0.168 | 0.061-0.461 | 0.004 |
| Local infiltration (Yes vs. no) | 0.121 | 0.044-0.332 | 0.138 |
| GRP78 level (low vs. high) | 0.276 | 0.101-0.758 | 0.043 |
pT: pathological tumor,
Table 5. Multivariate analysis of independent prognostic factors for NSCLC patients (n=89).
Discussion
There were 3,093,039 new lung cancer patients and 1,956,622 lung cancer deaths in China, 2010 [22]. Most patients are advanced lung cancer at the time they are diagnosed [23]. Thus we need specific biomarkers to evaluate the prognosis of NSCLC patients.
GRP78 and GRP94 are ER-retentive chaperones that are induced by glucose starvation [24]. They play an important role in tumorigenesis [25]. GRPs’ expression is associated with tumorous development. Overexpression of GRP78 and GRP94 can be detected in oesophageal adenocarcinomas, and the coexpression is related with poor prognosis [21]. In colorectal carcinoma cell lines, GRP78 and GRP94 expressions seem to be associated with malignancy [8]. In gastric cancer cells, GRP78 and GRP94 expressions are correlated with BCL2, BAX, ERBB2 and CASP3 protein expressions, and involve in the process of apoptosis [26]. In gastric carcinomas, overexpression of GRP78 and GRP94 is associated with aggressive behavior and poor prognosis [11].
In Japanese gastric adenocarcinomas, GRP78 and GRP94 expressions are weaker in signet ring cell carcinoma [27]. In children with hepatoblastoma, overexpression of GRP78 and GRP94 is more frequently present in the patients with lymph node metastasis and higher clinic stage [28]. In esophagus adenocarcinoma, higher GRP78 is related with early pT stage, well differentiation and good prognosis; GRP94 expression is significantly associated with earlier tumor stage and less lymph node involvement; GRP94 seems to be correlated with GRP78 protein expression inversely [29].
Ma et al. suggest that GRP78 is highly enriched in late-stage lung cancer and may be a prognostic marker for non-small cell lung cancer [18]. Inconsistence of GRP78 or GRP94 expression in various tumors demonstrates that either GRP94 or GRP78 is tumor type-specific in various tumors. Thus the correlation of GRP78 and GRP94 with grade of differentiation, stage of cancer and prognosis in NSCLC patients should be explained specifically.
In the current investigation, we detected GRP78 and GRP94 protein expressions in NSCLC patients by immunohistochemisty. The results showed that overexpression of GRP78 and GRP94 proteins was seen in tumor tissues, but there was no correlation between them. GRP78 was associated with pTNM and pN. GRP94 was correlated with SCC subtype. While, only GRP78 was correlated with poor survival. These results suggest that GRP94 may be a biomarker to distinguish pathological subtype, and GRP78 may be a biomarker to assess prognosis. The inconsistence between our results and others may imply a different GRP regulation due to the pathology subtype of tumors or the heterogeneity of patients included.
The current investigation had another interesting finding that GRP78 protein expression was associated with pTNM status and pN status, suggesting that GRP78 may be involved in tumor development and lymph node metastasis. Further analysis illustrated that high GRP78 protein expression was observed more frequently in pTNM II stage and pN0/N1 stage, and high GRP94 protein expression was detected more frequently in SCC subtype. This finding may be elucidated by different mechanisms of GRP regulation: overexpression in early stages of lung cancer may indicate an early response of the host's immune system, while overexpression in advanced stages may be correlated to dissimilar stress factors such as glucose starvation, hypoxia or other immune reactions towards lung cancer [30].
Furthermore, the current investigation examined the prognostic factors via univariate and multivariate analyses. We found that GRP78 level was one of four independent prognostic factors for NSCLC and a higher GRP78 expression was associated with a shorter overall survival time in the NSCLC patients included. This result was consistent with the results of Ma et al.’s study [18].
In the current investigation, the valid of outcome was limited by the small size of cases included. We did not track postoperative treatment, which may impact the survival of the patients. The operation manner that we did not discriminate may also affect prognosis. In addition, protein expression can also be analyzed using ELISA that provides more quantitative data. In the next investigation, we would investigate how the activated GRP78 and/or GRP94 impact the tumor subtyping and patients’ outcome. A larger number of NSCLC patients would be included. ELISA would be preferred to quantify GRP78/GRP94 expression. The response of GRP78 to chemotherapy and radiotherapy in late-stage NSCLC would also be examined so that guides the treatment of NSCLC.
In conclusion, high expression of GRP78 protein is related to a poor prognosis, and GRP78 may be a predictor for NSCLC prognosis. Larger and more detailed follow-up studies are required.
References
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non‑small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008; 83: 584‑594.
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 2001; 26:504-510.
- Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr 1994; 4:1-18.
- Dey A, Kessova IG, Cederbaum AI. Decreased protein and mRNA expression of ER stress proteins GRP78 and GRP94 in HepG2 cells over-expressing CYP2E1. Arch Biochem Biophys. 2006; 447:155-166.
- Gazit G, Lu J, Lee AS. De-regulation of GRP stress protein expression in human breast cancer cell lines. Breast Cancer Res Treat. 1999; 54:135-146.
- Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin,but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000; 77:396-408.
- Qian Y, Harris ED, Zheng Y, Tiffany-Castiglioni E. Lead targets GRP78, a molecular chaperone, in C6 rat glioma cells. Toxicol Appl Pharmacol. 2000; 163:260-266.
- Takahashi H, Wang JP, Zheng HC, Masuda S, Takano Y. Overexpression of GRP78 and GRP94 is involved in colorectal carcinogenesis. Histol Histopathol 2011;26:663-671.
- Chen GN, Ma Y, Yang ZL. Expression of GRP78 and GRP94 in the liver tissues and their clinicopathological significance in children with hepatoblastoma. Zhongguo Dang Dai Er Ke Za Zhi 2010;12: 634-636.
- Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, Cho JW, Yoo BC, Jung GH, Park CK. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol 2005;11:2072-2079.
- Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol 2008; 39:1042-1049.
- Langer R, Feith M, Siewert JR, Wester HJ, Hoefler H. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer 2008; 8:70.
- Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, Wang L, Wang S, Chen Y. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev 2005; 29:544-551.
- Usmani SZ, Bona RD, Chiosis G, Li Z. The anti-myeloma activity of a novel purine scaffold HSP90 inhibitor PU-H71 is via inhibition of both HSP90A and HSP90B1. J Hematol Oncol 2010; 3: 40.
- Uckun FM, Qazi S, Ozer Z, Garner AL, Pitt J, Ma H, Janda KD. Inducing apoptosis in chemotherapy-resistant B-lineage acute lymphoblastic leukaemia cells by targeting HSPA5, a master regulator of the anti-apoptotic unfolded protein response signalling network. Br J Haematol 2011; 153: 741-752.
- Sharma V, Eng JK, Maccoss MJ, Riffle M. A mass spectrometry proteomics data management platform. Mol Cell Proteomics. 2012;11:824-831.
- Jego G, Hazoumé A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett 2013;332:275-285.
- Ma X, Guo W, Yang S, Zhu X, Xiang J, Li H. Serum GRP78 as a tumor marker and its prognostic significance in non-small cell lung cancers: A Retrospective Study. Dis Markers 2015; 2015: 814670.
- Izumi K, Ikeda H, Maolake A, Machioka K, Nohara T, Narimoto K, Ueno S, Kadono Y, Kitagawa Y, Konaka H, Mizokami A, Namiki M. The relationship between prostate-specific antigen and TNM classification or Gleason score in prostate cancer patients with low prostate-specific antigen levels. Prostate 2015;75:1034-1042.
- Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J 2001;18:1059-1068.
- Slotta-Huspenina J, Berg D, Bauer K, Wolff C, Malinowsky K, Bauer L. Evidence of prognostic relevant expression profiles of heat-shock proteins and glucose-regulated proteins in oesophageal adenocarcinomas. PLoS One 2012; 7:e41420.
- Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X. Report of cancer incidence and mortality in China, 2010. Ann Transl Med 2014;2: 61.
- Travis WD. Pathology of lung cancer. Clin Chest Med 2011; 32:669-692.
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 2001;26:504-510.
- Udono H, Levey DL, Srivastava PK. Cellular requirements for tumorspecific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+T cells in vivo. Proc Natl Acad Sci USA 1994;91:3077-3081.
- Bai Z, Ye Y, Liang B, Xu F, Zhang H, Zhang Y, Peng J, Shen D, Cui Z, Zhang Z, Wang S. Proteomics-based identification of a group of apoptosis-related proteins and biomarkers in gastric cancer. Int J Oncol 2011; 38: 375-383.
- Zheng HC, Zheng YS, Xia P, Xu XY, Xing YN, Takahashi H, Guan YF, Takano Y. The pathobiological behaviors and prognosis associated with Japanese gastric adenocarcinomas of pure WHO histological subtypes. Histol Histopathol 2010;25:445-452.
- Chen GN, Ma Y, Yang ZL. Expression of GRP78 and GRP94 in the liver tissues and their clinicopathological significance in children with hepatoblastoma. Zhongguo Dang Dai Er Ke Za Zhi 2010;12:634-636.
- Langer R, Feith M, Siewert JR, Wester HJ, Hoefler H. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer 2008; 8:70.
- Ciocca DR, Calderwood SK: Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005; 10:86-103.