Research Article - Biomedical Research (2017) Volume 28, Issue 4
Imageology in staging and treatment efficacy of transforaminal lumbar interbody debridement and fusion for Brucella spondylitis in lumbar spine
Yongfeng Wang1,2, Bin Zhao2, Jie Yuan2, Yibo Zhao2, Xiangdong Lu2, Xinyuan Wei2, Anmin Jin1*1Department of Orthopaedics, Zhu Jiang Hospital of Southern Medical University, China
2Department of Orthopaedics, the Second Hospital of Shanxi Medical University, China
- *Corresponding Author:
- Anmin Jin
Department of Orthopaedics
Zhu Jiang Hospital Southern Medical University, China
Accepted on September 26, 2016
Abstract
Objective: It was aimed to investigate the application of different imaging methodologies in the grading of lumbar Brucella spondylitis, and to evaluate the value of Transforaminal Lumbar Intervertebral Body Debridement and Fusion (TLIDF) in the treatment.
Methods: 22 patients who had undergone lumbar surgery for Brucella spondylitis were recruited in this study. Erythrocyte Sedimentation Rate (ESR), C-reactive Protein (CRP), Visual Analogue Scale (VAS) and the Oswestry Disability Index (ODI) were monitored before and after the surgery, and all patients were followed up after discharge to assess the treatment efficacy.
Results: 72.8% of the patients had middle stage Brucella spondylitis, with 22.7% and 4.5% of the patients fell into early and late stage, respectively. 20 out of 22 patients received TLIDF surgery. No relapse or complications were observed during follow-up in all patients. Compared with their presurgery levels, ESR and CRP decreased significantly in these patients after the surgery. Besides, postsurgery VAS and ODI scores also had a significantly improvement compared with the values before surgery (all p<0.05).
Conclusion: Lumbar Brucella spondylitis had different characteristics on image examinations at different stages of the disease. TLIDF was an effective surgical approach to eliminate symptoms and processed long-term clinical efficacy.
Keywords
Brucella spondylitis, TLIDF, Imaging.
Introduction
Brucella bacteria are intracellular bacterial pathogens which are capable of infecting both animals and humans. The pathogen is transmitted through the ingestion of infected food, direct contact with an infected animal or inhalation of Brucella bacteria-containing aerosols. Brucella commonly attacks tissues like bone and joints, and spine is the most common affected tissue by Brucella, leading to spinal brucellosis [1]. Spinal brucellosis is a spectrum of diseases affects various structures of the spine, including vertebral bodies (spondylitis), intervertebral discs (spondylodiscitis), facet joints (arthritis), ligaments, soft tissues, epidural space, meninges, subarachnoid space and the spinal cord (myelitis) [2]. Lumbar region was noted to be the most common site of the spinal brucellosis, counting for more than half of the cases. This is due to the fact that lumbar region had rich blood supply and higher likelihood of endplate degeneration once infected by pathogens [3].
Although defined diagnosis of spinal brucellosis is based on culture or serologic techniques, radiological methods are of great importance to locate the Brucella invaded lesion in the spine. Radiographic changes of spinal brucellosis may not be apparent until 2 to 8 weeks after the presentation of clinical symptoms. Anatomically, anterior and superior erosions of the vertebral bodies are the two common affected sides of the spine. As a result of the inflammation, formation of bone-like structure around the Brucella affected anterior vertebral end plates can produce a so-called parrot-beak appearance on radiological examinations [4]. Therefore, radiological examinations are very helpful in the diagnosis of spinal brucellosis. However, not every patient present typical imaging characteristic on routine radiological examinations like X-ray, Computed Tomography scan (CT) and Magnetic Resonance Imaging (MRI). This might count for the potential misdiagnosis or the delay of diagnosis. Therefore, how radiological examinations or techniques could help clinicians in the diagnosis or evaluation of lumbar Brucella spondylitis needs further investigation. In this study, we hypothesized that such uncertainty in the diagnostic role of radiological techniques of spinal brucellosis was due to the change of the features of lumbar Brucella spondylitis at its different stage. Therefore, one of the major aims of this study was to investigate the image characteristics on radiological examinations at different stages of lumbar Brucella spondylitis.
Although most patients suffered from spinal brucellosis was able to recover completely through non-surgical treatment [5], spinal surgery is usually unavoidable in patients of spinal brucellosis with persistent or progressive neurologic deficits due to compression of spinal cord or nerve roots, progressive vertebral collapse or spinal instability [6]. One of the major surgical approaches for Brucella spondylitis was posterior debridement and interbody bone grafting fusion combined with internal fixation [7]. However, such surgical approach would inevitably destroy the posterior ligamentous complex. Therefore, we further investigated the surgical efficacy of different surgical approaches, including Transforaminal Lumbar Interbody Debridement and Fusion (TLIDF), Anterior and Posterior Lumbar Fusion surgery (APLF) or lumbar spine revision surgery in preserving posterior ligamentous complex.
Materials and Methods
Patients
22 cases of spinal brucellosis who received surgery in Department of Orthopaedics at our hospital from February 2010 to June 2013 were recruited in this study. All patients have given their consent to using their clinical data for this study. 17 of them were male and the remaining 5 were female patients. The average age in this population was 53.9 ± 9.9 years old. The disease courses of them ranged from 0.26 month to 12 months, with an average of 3.1 ± 2.4 months. All patients reported cattle and sheep contact history. All of these patients had a significant increased titer in Standard Agglutination Test (SAT) (>1:160) and were positive for Coombs anti-Brucella or immune-capture agglutination test (>1:320).
Radiological examinations
All patients received lumbar spine X-ray, CT and MRI to examine the sized of the infection, to evaluate the size of abscess if possible, and to assess the degree of destroy in vertebral body and intervertebral areas. The images were analyzed and the Spinal brucellosis of each patient was staged.
Surgery intervention
All patients received oral therapy of Doxycycline (0.1 g, twice every day) and rifampicin (0.6 g, once every day) for at least 21 days before the surgery. This regiment helped to reduce the toxic symptoms brought by brucellosis infection and improved the status of malnutrition.
Patients received general anaesthesia for the surgery and lied in a prone position. After taking a median incision from the posterior spine, the corresponding surgery was performed in each patient. In those who received TLIDF, vertebral spinous process, lamina and facet were clearly exposed and c-arm Xray machine was used to make sure that pedicle screws were implanted at the accurate position. Bilateral transforaminal approach was chosen at the infected vertebral segment, and the lamina and spinous process were retained. After successful resection of the inferior articular process of the upper vertebra and the superior articular processes of the lower vertebrae, dural sac and nerve root of the infected vertebral segment were exposed. Yellow-green pus inside and outside the spinal canal and inflammatory granulation tissue around were thoroughly cleaned. Then, the annular fibrosus of the infected intervertebral disc were cut, and necrotic tissue and destroyed cartilage were removed completely. 3000 ml of normal saline was used to flush and clean the whole area thoroughly and repeatedly. Last, bone particles were implanted into intervertebral space and hybrid cage was then inserted followed by pedicle screw connection rod and drainage tube implantation. Patients who was planned to receive lumbar fusion surgery were then changed to lateral position after the completion of TLIDF surgery. An oblique incision was made to expose greater psoas muscle and the abscess was cleaned.
Post-surgery care and follow-up
Within the first 12 hours after surgery, single dose of antibiotics was used to prevent patients from infection. If white blood cell counts increased significantly within 2 days after surgery and patients have fever, antibiotics were continued. Mannitol (250 ml, IV drop, twice every day), dexamethasone (5 mg, in drop, twice every day) were used to facilitated the recovery of patients post-surgically. When the total drainage was <30 ml/day, the drainage tube was removed and antibrucellosis treatment was continued for another 6 months as a standard treatment for brucellosis. Since TLIDF performed in our department has reserved the structure of posterior ligamentous complex and spinal screw was also used, these approaches we have chosen for our patients have greatly stabilized the endogenous ligament and preserved the function of the spine. Therefore, short term (3-5 days) of immobilization was suggested to patients, and all patients were encouraged to walk with the help of back brace after 3-5 days of surgery. And back brace was obligated to be used in all patients for 6-8 weeks.
All patients received a carefully designed follow-up plan at 3, 6, and 12 months after the surgery, and once every 6 months afterwards. The follow up parameters included complications and relapse of brucellosis evaluation, ESR and CRP levels, backache and lower extremity neuralgia symptoms improvements evaluation using Visual Analogue Scales (VAS) and Oswestry Disability Index (ODI). Pain was scaled into 10 degrees in VAS methodology, with 0 being defined as no pain existing and 10 being defined as the most severe pain. Daily activities functional status in ODI methodology included 9 different parameters with 5 scores assigned to each parameter (sexual life quality was not included). Final ODI was calculated using the formula ODI=(actual score/45) × 100%.
Statistics
SPSS16.0 was used in all statistical analysis in this study. Data were presented as mean ± Standard deviation (SD). X2 test was used in the comparison of ESR or CRP level before and after surgery. Paired t test was used in the analysis of VAS and ODI before and after surgery. P <0.05 was defined as statistically significant.
Results
Clinical characteristics of patients with Brucella spondylitis
The location of the affected spine segments included 4 cases of L2~3, 3 cases of L3~4, 3 cases of L4~5 and 6 cases of L5~S1 infection. 6 cases had more than 2 vertebral bodies infected, including 1 case of multiple L3~5 infection, 4 cases of multiple L4~S1 infection and 1 case of infection in both L2~3 and L4~S1 infection. Symptoms of these 22 patients included backache (22 cases), lower extremities pain (19 cases), fever (18 cases) and night sweat (10 cases) (Table 1).
| Lesion location | Case number | Gender (male/female) | Age (years) | Course of the disease (months) |
|---|---|---|---|---|
| L2-3 | 4 | 3/1 | 60.8 ± 11.3 | 3.0 ± 0.8 |
| L3-4 | 3 | 1/2 | 59.0 ± 4.6 | 3.0 ± 1.0 |
| L4-5 | 3 | 3/0 | 56.0 ± 4.4 | 2.0 ± 1.0 |
| L5-S1 | 6 | 5/1 | 46.3 ± 8.0 | 2.2 ± 1.1 |
| L3-5 | 1 | 0/1 | 38.0 ± 0.0 | 12.0 ± 0.0 |
| L4-S1 | 4 | 4/0 | 58.3 ± 10.2 | 2.6 ± 2.4 |
| L2-3 and L4-S1 | 1 | 1/0 | 49.0 ± 0.0 | 5.0 ± 0.0 |
Table 1. Clinical characteristics of 22 patients recruited in this study.
Imaging characteristics of patients with Brucella spondylitis
All patients received lumbar spine X-ray, CT and MRI examinations. Since Brucella spondylitis in lumbar spine had various manifestations on imaging examination, we stratified the stage of the diseases according to the criteria listed in Table 2. Only 1 patient (4.5%) was staged at an early stage in all subjects. 16 patients (72.8%) had a middle stage imaging manifestation of Brucella spondylitis while the remaining 5 subjects were categorized into late stage Brucella spondylitis (22.7%). Middle stage patients had less than 5 months of Brucella spondylitis medical history while late stage patients had 3.5-12 months Brucellosis. Representative images one case of early-middle stage, one case of middle stage and one case of late stage Brucella spondylitis were shown in Figure 1, Figure 2 and Figure 3, respectively.
| X-ray | CT | MRI | |
|---|---|---|---|
| Early Stage | Normal | Normal | Short Tau Inversion Recovery (STIR) sequence showed abnormally high signalling in vertebral body and paravertebral area |
| Middle Stage | normal or narrowed intervertebral space height | small osteolytic lesion and sclerosis lesion in intervertebral endplate | inflammatory signalling in vertebral body and intervertebral space, or intra-spinal or para-spinal abscess |
| Late Stage | Obvious narrowing of intervertebral space in the infected spine segment. Osteophyte proliferation was observed on the margin of vertebral body | vertebral body sclerosis, osteolytic and inflammatory osteophytosis co-existed and margins of vertebral body showed lace-like appearance | inflammatory signalling in intra-spinal and/or para-spinal areas with extensive abscesses formation in intra-spinal or para-spinal area and greater psoas muscle |
Table 2. Characteristics of imaging examinations on different stage of Brucellosis.
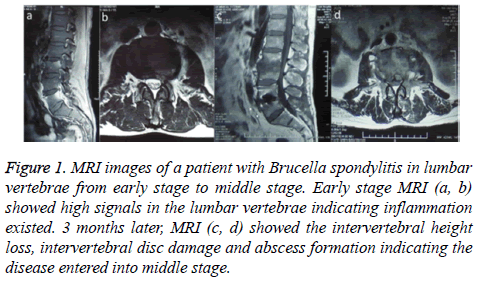
Figure 1 : MRI images of a patient with Brucella spondylitis in lumbar vertebrae from early stage to middle stage. Early stage MRI (a, b) showed high signals in the lumbar vertebrae indicating inflammation existed. 3 months later, MRI (c, d) showed the intervertebral height loss, intervertebral disc damage and abscess formation indicating the disease entered into middle stage.
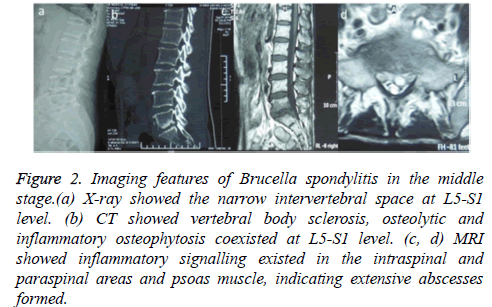
Figure 2: Imaging features of Brucella spondylitis in the middle stage.(a) X-ray showed the narrow intervertebral space at L5-S1 level. (b) CT showed vertebral body sclerosis, osteolytic and inflammatory osteophytosis coexisted at L5-S1 level. (c, d) MRI showed inflammatory signalling existed in the intraspinal and paraspinal areas and psoas muscle, indicating extensive abscesses formed.
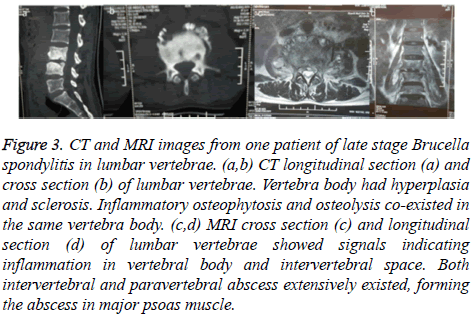
Figure 3: CT and MRI images from one patient of late stage Brucella spondylitis in lumbar vertebrae. (a,b) CT longitudinal section (a) and cross section (b) of lumbar vertebrae. Vertebra body had hyperplasia and sclerosis. Inflammatory osteophytosis and osteolysis co-existed in the same vertebra body. (c,d) MRI cross section (c) and longitudinal section (d) of lumbar vertebrae showed signals indicating inflammation in vertebral body and intervertebral space. Both intervertebral and paravertebral abscess extensively existed, forming the abscess in major psoas muscle.
Surgery summary of patients with Brucella spondylitis
20 patients received TLIDF surgery, and remaining two received TLIDF plus APLF surgery and lumbar spinal revision surgery, respectively. The surgery time for TLIDF was 152.0 ± 29.3 minutes, while that for patients received TLIDF+APLF or TLIDF+lumbar spinal revision surgery were 215 minutes and 136 minutes, respectively.
TLIDF and lumbar spinal revision surgery had similar bleeding volume, post-surgery drainage and in-patient admission time, while TLIDF+APLF surgery seemed to have a higher bleeding volume, more post-surgery drainage volume and longer inpatient admission time (Table 3).
However, statistical analysis could not be performed since sample size equaled to one in either group (Table 3). MRI images of one patient received TLIDF surgery was shown in Figure 4. MRI before and after surgery of one patient and Xray of spine from the same patient after surgery is shown in Figure 5.
| Surgical approach | Case number | Rationale for the chosen surgical approach | surgery time (minutes) | Bleeding volume during the surgery (ml) | Post-surgery drainage volume (ml) | In-patient admission time (days) |
|---|---|---|---|---|---|---|
| TLIDF | 20 | Destroy of intervertebral disc, intra-spinal abscess formation, compression of dura mater and nerve root. | 152.0 ± 29.3 | 258.0 ± 37.3 | 196.8 ± 46.7 | 9.3 ± 3.7 |
| APLF | 1 | Destroy of both intervertebral body and intervertebral disc, multiple intra-spinal or paraspinal abscesses, and psoas abscess | 215 | 450 | 258 | 13 |
| Lumbar spinal revision surgery | 1 | Multiple segment of spinal cord infection, including L2-3,L4-S1, L4-S1 unilateral fixation and lumbar fusion surgery was performed due to the misdiagnosis?and L2-3 TLIDF was performed 3 months after diagnosis | 136 | 230 | 153 | 11 |
Table 3. Summary of surgical approach and para-operative parameters in all 22 patients.
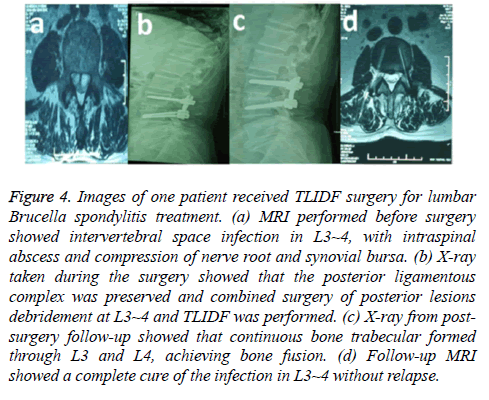
Figure 4: Images of one patient received TLIDF surgery for lumbar Brucella spondylitis treatment. (a) MRI performed before surgery showed intervertebral space infection in L3~4, with intraspinal abscess and compression of nerve root and synovial bursa. (b) X-ray taken during the surgery showed that the posterior ligamentous complex was preserved and combined surgery of posterior lesions debridement at L3~4 and TLIDF was performed. (c) X-ray from postsurgery follow-up showed that continuous bone trabecular formed through L3 and L4, achieving bone fusion. (d) Follow-up MRI showed a complete cure of the infection in L3~4 without relapse.
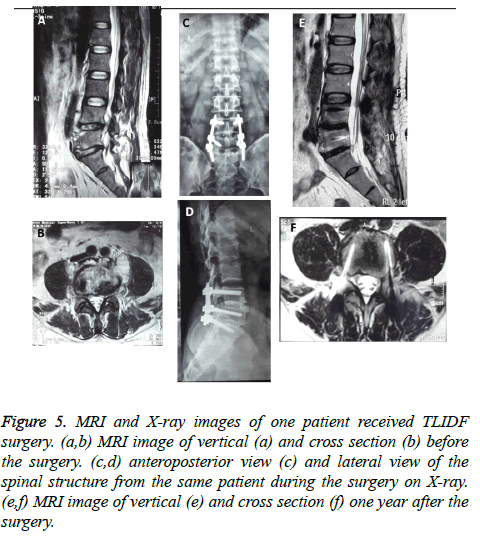
Figure 5: MRI and X-ray images of one patient received TLIDF surgery. (a,b) MRI image of vertical (a) and cross section (b) before the surgery. (c,d) anteroposterior view (c) and lateral view of the spinal structure from the same patient during the surgery on X-ray. (e,f) MRI image of vertical (e) and cross section (f) one year after the surgery.
Comparison of ESR and CRP levels, and VAS and ODI scores before and after surgery
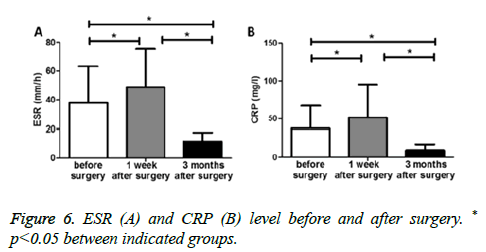
Erythrocyte Sedimentation Rate (ESR) level when they were firstly admitted to hospital ranged from 4-104 mm/h, with an average of 37.7 ± 25.4 mm/h. C-reactive Protein (CRP) ranged from 3.8-78.4 mg/L, with an average of 33.1 ± 29.3 mg/L. Patients had a significantly higher ESR level 1 week after surgery (48.7 ± 26.4 mm/h vs. 37.7 ± 25.4 mm/h before surgery, p<0.05) (Figure 6A), which most probably due to the stress of surgery. In contrast, ESR level decreased significantly 3 months after surgery (11.4 ± 6.3 mm/h) compared with the levels before surgery and or 1 week after surgery (both p<0.05) (Figure 6A). Similar phenomenon was also observed in CRP levels before and after surgery (Figure 6B).
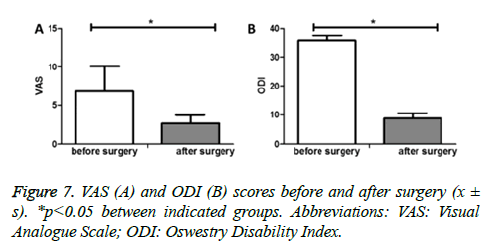
A further follow-up of the manifestation of Brucellosis showed that VAS (assessment of backache and low extremity neuralgia) was significantly lower after surgery compared with before surgery (2.66 ± 1.04 vs. 6.82 ± 2.14, p<0.05) (Figure 7A). During the last follow-up of each patient, the ODI score was significantly decreased when compared with that of before surgery (8.82 ± 1.73 vs. 35.72 ± 1.91, p<0.05) (Figure 7B).
Discussion
Brucellosis is an endemic zoonotic disease and spine is the most commonly affected tissue. About 60% of spinal brucellosis cases were lumbar brucellosis which invaded L4 or L5 [4]. In the current study, we recruited patients of spinal brucellosis not only had L4~5 lumbar spinal brucellosis, but also in all other segments of the spine except L1~2.
Compared with single lesion in spine, multiple-segment involvement of spinal brucellosis was relatively rare, counting for only 3.2-9% of diagnosed spinal brucellosis cases [8]. In this study, we recruited 6 of multi-segment affected spinal brucellosis cases (27%), the number of which was higher than reported numberMen were affected more frequently than women in this study (17 male and 5 female patients), which
was consistent with the reports from other [9]. Spondylodiscitis has been reported more frequently in adults and the elderly [10]. Consistent with the literatures, the mean age of patients this study was 53.9 ± 9.9 years old (range from 36 to 75 years old). In this cohort, the average of time from the first onset of symptom to final diagnosis was 3.1 ± 2.4 months, ranging from 0.26 to 12 months. Since serological diagnosis of brucellosis has been challenging, at least 2 serological tests, such as SAT and Coombs anti-Brucella test or immunocapture agglutination test, have been suggested in the diagnosis of spinal brucellosis with increased sensitivity [5]. Besides, moderately elevation of ESR and CRP were reported in brucellosis patients [11]. In this study, we found that both ESR and CRP elevated after the surgery, which probably reflected the post-surgery stress status of these patients. Interestingly, we observed a significantly decreased ESR and CRP levels 3 months after the surgery when compared with both their levels before and shortly after the surgery. The combined regimen of a thorough surgery to eliminate the infection source and a regular 6 months postsurgery anti-brucellosis treatment possibly counted for the correction of inflammatory status, leading to the reduced ESR and CRP levels. Clinically, Brucellosis usually has 4 different disease stages including acute, subacute, chronic and relapsing stages [6]. The clinical course of the disease correlated with the manifestation of Brucella spondylitis on radiological examinations to some degree. For example, severe inflammatory damage always existed with features of intervertebral space narrowing, vertebral body hyperplasia of sclerosis, osteolysis and abscess formation in intra-spinal and para-spinal areas in severe cases with chronic disease courses. In contrast, patients with shorter course of the disease usually presented with minimal X-ray, CT or MRI changes. Despite of the fact that Brucella spondylitis could have different manifestations on imaging examination; the radiological staging of Brucella spondylitis has not been well established.
Since Brucella spondylitis was a chronic disease, about 25% of the patients could have a featured X-ray abnormality of osteophyma of vertebral body and intervertebral space narrowing [12]. CT had more advantage in detecting bone inflammatory change since it was able to show the pathological change of vertebral disc, spinal canal and para-spinal areas. MR imaging has been currently recognized as the best imaging tool for diagnosis and follow-up of patients with spinal infections, due to its high sensitivity and specificity [13]. Fatsaturation technique with contrast enhancement has been impressive in displaying and delineating the changes in vertebral body, intervertebral disc, and paravertebral soft tissue involvement of spinal brucellosis [14]. Two radiologic forms of Brucella spondylitis exist so far, namely the focal form which was limited to the anterior part of the endplate, and the diffuse form which involved the entire vertebral body, intervertebral disc, adjacent vertebra, epidural space, meninges, and spinal cord [15].
In the current study, we carefully analyzed the imaging from X-ray, CT and MRI of all 22 patients and tried to stage the disease according to their radiological manifestations. According to the report, early radiological manifestations of Brucella spondylitis were usually non-specific. MRI, with the help of fat-suppression technique and contrast enhancement, has been reported as the most useful method for detecting the presence and extension of Brucella spondylitis [16]. In the middle stage spinal brucellosis, tissue injury becomes more severe and inflammatory injury or erosion was involved in not only soft tissues but also bones. At this stage, MRI, X-ray and CT were able to detect the pathological change. As the disease progressed, vertebral body became sclerotic, inflammatory osteophyte proliferation enhanced and para-spinal abscesses would extensively form such that the radiological manifestation of spinal brucellosis became more typical and characteristic (Table 2). Although the initial clinical signs of Brucella infection had 2-4 weeks latent period, the earliest radiological signs may appear as late as 12 weeks or just a bit later than the onset of clinical symptoms. Due to the high blood supply, the superior end plate was usually the starting point in most cases of spinal brucellosis, although the inferior end plate might also be involved. When the balance between the virulence of the organism and host defense mechanism is destroyed, the disease progresses. Finally, the infection could spread to the entire vertebral body, invading the neighbour intervertebral disc space and adjacent vertebrae [10].
Surgery for the treatment of spinal brucellosis has been done in 3-29% of the patients [7] and was usually advised when persistent or progressive neurologic deficits existed. Patients who had progressive vertebral collapse or spinal instability, and who did not respond to prolonged antibiotic treatments were also potential candidates for surgery [6]. Early surgical approach could provide some benefits to these patients including achieving neural decompression earlier, rapid relief of symptoms and fresh sample access for earlier diagnosis before the deterioration of neurological and clinical parameters [17]. Reported surgery approaches for spinal brucellosis included posterior lumbar laminectomy, debridement and infusion surgery and anterior lumbar fusion debridement internal fixation. Although these approaches had assured treatment effect, we had to admit that such extensive surgery itself was a huge injury to patients and some of these surgical approaches damaged the structure of posterior ligamentous complex.
TLIDF had its advantage in this aspect since it retained the structure of posterior spine complex which was composed of spine ligaments, spinous process, interspinous ligament, yellow ligament, and facet joint capsule. The preservation of this complex during the surgery could provide stable attachment point for erector spinae muscles, which greatly stabilize the endogenous ligament and preserved the function of spine. Liu et al. showed that patients received TLIDF had a significantly higher JOA score compared with those who received the laminectomy interbody fusion surgery, suggesting that posterior complex structure had an important role in stabilization of the spine structure and could potentially increase the clinical treatment effect of the surgery [18,19]. Considering the fact that the destruction of bone by Brucella bacteria has a relatively small impact on the stability of spine, our TLIDF surgery approach has greatly preserved the spinal stability and function, and the shorter spinal instrumentation would have a much smaller impact on the spinal mobility compared with longer spinal instrumentation, we have decided to use short spinal instrumentation for our patients in this study.
Therapeutic failure was defined as the presence of persistent or worsening symptoms together with high levels of CRP or ESR 1 month after surgery [5]. Relapse rate of spinal brucellosis ranged between 4%-14% [13,20]. In the current study, we did not find any relapsed case and all patients had a significantly reduced ESR and CRP levels in their 3 months follow-up. VAS and ODI scores also indicated that backache and lower extremity neuralgia issues had been solved by surgery and the quality of life has been greatly improved. According to the report, relapse rate could be as high as 55% if antibiotic treatment was administered for only 6 weeks [21]. Thus, we prolonged the administration of antibiotics for at least 6 months [7] to reduce the relapse rate.
In summary, TLIDF surgery approach was an effective treatment choice for patients suffered from Brucella spondylitis. The combined treatment of surgery intervention and antibiotics processed a long-term benefit to these patients.
References
- Zhao YT, Yang JS, Liu TJ, He LM, Hao DJ. Sclerosing vertebra in the spine:typical sign of spinal brucellosis. Spine J 2015; 15: 550-551.
- Tali ET, Gultekin S. Spinal infections. Eur Radiol 2005; 15; 599-607.
- Bozgeyik Z, Ozdemir H, Demirdag K, Ozden M, Sonmezgoz F, Ozgocmen S. Clinical and MRI findings of brucellar spondylodiscitis. Eur J Radiol 2008; 67; 153-158 .
- Drapkin MS, Kamath RS, Kim JY. Case records of the Massachusetts General Hospital. Case 26-2012. A 70-year-old woman with fever and back pain. N Engl J Med 2012; 367; 754-762.
- Colmenero JD, Ruiz-Mesa JD, Plata A, Bermúdez P,Martín-Rico P,Queipo-Ortuño MI, Reguera JM. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin Infect Dis 2008; 46: 426-433.
- Tali ET, Koc AM, Oner AY. Spinal brucellosis. Neuroimaging Clin N Am 2015; 25: 233-245.
- Katonis P, Tzermiadianos M, Gikas A. Surgical Treatment of Spinal Brucellosis. Clin Orthop Relat Res 2006; 444: 66-72.
- Raptopoulou A, Karantanas AH, Poumboulidis K, Grollios G, Raptopoulou-Gigi M, Garyfallos A. Brucellar spondylodiscitis: noncontiguous multifocal involvement of the cervical, thoracic, and lumbar spine. Clin Imaging 2006; 30: 214-217.
- Ceran N, Turkoglu R, Erdem I, Inan A,Engin D,Tireli H, Goktas P. Neurobrucellosis: clinical, diagnostic, therapeutic features and outcome. Unusual clinical presentations in an endemic region. Braz J Infect Dis 2011; 15: 52-59.
- Oztekin O, Calli C, Adibelli Z, Kitis O, Eren C, Altinok T. Brucellar spondylodiscitis: magnetic resonance imaging features with conventional sequences and diffusion-weighted imaging. Radiol Med 2010; 115: 794-803.
- Solera J, Lozano E, Martínez-Alfaro E, Espinosa A, Castillejos ML, Abad L. Brucellar spondylitis: review of 35 cases and literature survey. Clin Infect Dis 1999; 29: 1440-1449.
- Baleriaux DL, Neugroschl C. Spinal and spinal cord infection. Eur Radiol 2004; 14: E72-83.
- Hong SH, Choi JY, Lee JW, Kim NR,Choi JA,Kang HS. MR imaging assessment of the spine: infection or an imitation? Imagings 2009; 29: 599-612.
- Sharif HS, Clark DC, Aabed MY, Haddad MC,al Deeb SM,Yaqub B, al Moutaery KR. Granulomatous spinal infections: MR imaging. Radiology 1990; 177: 101-107.
- Chelli Bouaziz M, Ladeb MF, Chakroun M, Chaabane S. Spinal brucellosis: a review. Skeletal Radiol 2008; 37: 785-790.
- Ozaksoy D, Yucesoy K, Yucesoy M, Kovanlikaya I,Yüce A,Naderi S. Brucellar spondylitis: MRI findings. Eur Spine J 2001; 10: 529-533.
- Ekici MA, Ozbek Z, Göko?lu A, Kovanlikaya I, Yüce A, Naderi S. Surgical Management of Cervical Spinal Epidural Abscess Caused by Brucella Melitensis: Report of Two Cases and Review of the Literature. J Korean Neurosurg Soc 2012; 51: 383-387.
- Liu H, Wu W, Li Y, Liu J, Yang K, Chen Y. Protective effects of preserving the posterior complex on the development of adjacent-segment degeneration after lumbar fusion: clinical article. J Neurosurg Spine 2013; 19: 201-206.
- Mousa AM, Bahar RH, Araj GF, Koshy TS,Muhtaseb SA,al-Mudallal DS, Marafie AA. Neurological complications of brucella spondylitis. Acta Neurol Scand 1990; 81: 16-23.
- Colmenero JD, Jimenez-Mejias ME, Sánchez-Lora FJ, Reguera JM, Palomino-Nicás J,Martos F, García de las Heras J,Pachón J. Pyogenic, tuberculous and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis 1997; 56: 709-715.
- Lifeso RM, Harder E, McCorkell SJ. Spinal brucellosis. J Bone Joint Surg 1985; 67: 345-351 .