Research Article - Journal of Psychology and Cognition (2018) Volume 3, Issue 3
IL-1? polymorphisms and its interaction with Nrg-1 on the risk of intellectual disability.
Silvia Di Loreto1*, Anna Aureli1, Pierluigi Sebastiani1, Tiziana Del Beato1, Alessia Colanardi1, Marta Grannonico2, Anna Elvira Marimpietri3, Enzo Sechi31Institute of Translational Pharmacology (IFT), National Council of Research (CNR), L’Aquila, Italy
2Department of Life, Health and Environmental Sciences, University of L'Aquila, Italy
3Childhood and Adolescence Neuropsychiatric Clinic, University of L’Aquila, L’Aquila, Italy
- Corresponding Author:
- Silvia Di Loreto
Institute of Translational Pharmacology (IFT)
National Council of Research (CNR)
L’Aquila, Italy
Tel: +39 0862 318843
Fax: +39 0862 320750
ORCID: 0000-0002-8256-5819
E-mail: silvia.diloreto@cnr.it
Accepted on December 12 2018
Citation: Loreto SD, Aureli A, Sebastiani P, et al. IL-1β polymorphisms and its interaction with Nrg-1 on the risk of intellectual disability. J Psychol Cognition 2018;3(3):54-60.
DOI: 10.35841/psychology-cognition.3.54-60
Visit for more related articles at Journal of Psychology and CognitionAbstract
Aim: To evaluate the influence of pro-inflammatory cytokine interleukin-1β (IL-1β) and growth factor neuregulin-1 (NRG-1) and of their interactions in the risk of intellectual disability aetiology.
Methods: IL-1β rs16944(-511 T>C), rs1143634(+3962 C>T), NRG-1 rs6994992, NRG-1 rs35753505 and ErbB4 rs7598440 polymorphisms were studied by PCR-SSP and SBT methods in a population composed by 45 patients with mild/moderate intellectual disability and 31 healthy subjects perfectly matched for age, gender and ethnicity. IL-1β serum evaluation was done by enzyme-linked-immunosorbent- assay (ELISA).
Results: Our findings indicate that both the IL-1β variants are associated to ID. Moreover, analysis of gene-gene interaction suggests that there is an interaction between IL-1β and NRG-1 in ID risk. Measuring IL-1β levels we evidenced higher IL-1β serum concentration in ID patients than in controls. Conclusion: IL-1β and NRG-1 have been recognized to play a key role in activity-dependent development, and plasticity of synaptic structure and function. Genetic functional dysregulations of these molecules could impair neuronal processes influencing cognitive development and may have a wide range of neurological consequences. In this view our results can constitute a start point for further investigations and provide information to develop future projects and to planning strategies for early identify and managing ID.
Keywords
Intellectual disability, Cytokine, Neuregulin-1, Polymorphism, Neurodevelopmental disease.
Introduction
Intellectual disability (ID) (current term for mental retardation) is a complex neurodevelopmental disorder characterized by significant limitations in both intellectual functioning and adaptive behaviour. This disability originates before 18 years of age. A formal diagnosis classifies ID into four categories: mild, moderate, severe and profound. Severe form is mostly sporadic (3-4%), and about 95% of patients fall into the "mild/moderate" category. The exact aetiology of approximately 40% of cases still remains unknown. In the past, affected children were often grouped with other children affected by high incidence disabilities. This aggregation is also occurring in practice with a general preference not to label a child as having a mild intellectual disability [1]. Over the years there has been a change in the acceptance of the ID that has also passed through the name. The term ‘mental retardation’ (MR) is outdated and has changed to ‘intellectual disability’ (ID). The latter term, which is better suited to current professional practices focused on functional behavior and contextual factors, provides a coherent basis for the provision of individualized supports and is less offensive to people with disabilities [2]. In coincidence with the change of the name there has been a growing interest from the scientific community on the biomedical and genetic aspects of this condition. Many researcher team are now involved into a deep exploration of genetic mutations linked to this pathology with the aim to find the mechanism and a possible therapeutic fix for this syndrome. However, ID is the result of a set of known and unknown genetic, neurophysiological or environmental causes, trauma or combination thereof. Genetics trough mutation, deletion or reorganization of the genes that encode proteins, can indeed influence brain development or cognitive functioning. The environmental factors can concur to immunological dysfunctions, which are able to influence synaptic function through mechanisms implicated in neuroinflammation and mostly mediated by microglia.
Moreover an inflammatory event due by infection occurring during the perinatal period might have long lasting consequences on cognition and behaviour and might also increase the susceptibility to disorder.
In central nervous system (CNS) cytokines take part in brain functions involved in molecular and cellular mechanisms mediating complex cognitive processes [3,4], playing a key role in synaptic plasticity, long-term potentiation (LTP), neurogenesis and memory consolidation [5,6]. Cytokine production is under genetic control. Different individual levels of interleukins can be linked to gene polymorphisms within the coding, intron, or promoter regions. Epidemiological evidence show that the onset of cognitive decline [7] and dementia [8] are characterized by increased peripheral cytokine levels. Furthermore, some genetic studies support associations between pro-inflammatory cytokine gene variations and cognitive impairment [9] or decline in an elderly population [10]. Among cytokines, IL-1β is one of the most prominent candidates for these genetic studies since it can affect the expression of other cytokines and trophic factors that in many physiological functions they modulate each other [11]. Moreover, IL-1β produces dose-dependent decreases in the number of neurons in embryonic rat cortical slices, suggesting decreased cerebral cortical neurons survival and, at high levels, it reduces dendrite development and neuron survival in vitro [11].
The association between the IL-1β SNPs and cognitive impairment has been studied mainly in memory performance, working memory, attention/processing speed or motor function of adults [12] with conflicting results recently reviewed by Stacey [13]. The NRG-1 is part of the growth factor family and influences neuro-developmental processes such as myelination, synapse formation, neuronal migration. NRG-1 and its receptor ErbB4, as regulator of N-methyl-d-aspartic acid (NMDA) and GABA receptors, have been associated with schizophrenia [14]. Importantly, memory impairment is a feature of schizophrenia as patients experience deficits in cognition, executive function, and memory processes [15]. In addition, NRG-1 also appears to be involved in both short-term and long-term plasticity [16,17] suggesting that it plays a role in cognitive processes [18]. In 2013 Kukshal [19] reported the association between two SNPs of NGR-1 (rs35753505 and rs6994992) and cognitive functions in Indian population confirming prior reports on Caucasian individuals [18] and in rodent models [20]. The interaction between cytokines and growth factors has been largely demonstrated and many works established a connection between neurological and neurodevelopmental disorders and peripheral levels of proinflammatory cytokines [21,22]. Desbonnet et al. in a recent review reported altered serum levels of pro-inflammatory cytokines associated with mutation of NRG-1 [23] besides, their results show the influence of IL-1β SNPs on the serum level of this cytokine [24,25].
NRG-1 signalling can regulate brain microvascular endothelial cells decreasing both pro-inflammatory stimuli and IL-1β increase [26] and can have a neuroprotective role in neuronal detrimental insults trough IL-1β expression regulation [27]. Regarding their genetic interaction, the NGR-1/ IL-1β association was described, as far as we know, only in schizophrenia [28].
Here we extend a previous study [9] with the aim of determining if there is an association of IL-1β and NRG-1 SNPs and the susceptibility to ID. To do this, we considered a children population with a diagnosis of mild/moderate ID without comorbidity or neurological impairment.
Materials and Methods
Patients and diagnostic assessment
A case control study was conducted. The study was approved by local hospital ethics committees (registration number 1931/17-11-2011) and was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The ID patients enclosed in this study were 45 (25 males and 20 females), at age between 3-18 years. All patients were Italians, Caucasians and have been enrolled at the Child Development and Neuropsychiatric Clinic of the S. Salvatore Hospital, L’Aquila, Italy.
Thirty-one healthy people matched with the patients for age, gender and ethnicity were used as controls and underwent a routine medical check-up in the Department of Paediatric of the Civil Hospital of L’Aquila. A detailed clinical examination was performed in all cases and the diagnosis of intellectual disability was made from clinicians of the Child Development and Neuropsychiatric Clinic of the S. Salvatore Hospital, L’Aquila, Italy. Written parental and child (in older subjects) consent was obtained.
Cognitive measures
The evaluation of ID was done according to diagnostic criteria of the DSM-5 for intellectual disabilities (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, American Psychiatric Association, 2013). Based on age, the Wechsler Primary and Preschool Scale of Intelligence (WPPSIR) and Wechsler Intelligence Scale for children (WISC-III) were used to study intellectual functioning in verbal and performance cognitive domains and the child’s general intellectual ability. Vineland Adaptive Behavior Scales were used to measure personal and social skills required for everyday living. The Child Behavior Checklist (CBCL) was used to detect emotional and behavioral problems in children and adolescents and the Conners’ Continuous Performance Test (C-CPT), which measures and evaluates a child’s attention span and ability to maintain focus on a task, was used to support the conclusions. According to the criteria of Diagnostic and Statistical Manual of Mental Disorders- V Edition (DSMV) that recognizes four degrees of ID severity, the children included in this study had an IQ score comprised between 50 and 70 that reflect a mild to moderate level of intellectual impairment. However, the diagnosis was initially expressed based on clinical judgment, rather than on formal standardized assessments and the subjects had a significant functional impairment.
Genetic conditions such as Down syndrome, fragile X syndrome, and phenylketonuria were considered exclusion criteria from the study. Instead, the study includes environmental conditions that interfere with the growth and development of the brain such as prenatal (severe maternal malnutrition, alcohol and drug abuse), during birth (hypoxia, extreme prematurity) and after birth (severe head injury, malnutrition of the child, severe emotional neglect or abuse) factors. Clinical and neurophysiological data were collected by the clinicians.
DNA extraction
Genomic DNA was extracted from whole blood according to the manufacturer’s protocol (QIAamp DNA blood MiniKit, Qiagen, Courtaboeuf, France) and kept at −20°C until use. DNA concentration and purity was determined using a spectrophotometer (Beckman Instruments, Inc. Fullerton, CA. 92834-3100).
Genotyping
IL-1βrs16944 and rs1143634 gene polymorphisms were investigated in patients and controls using a polymerase chain reaction (PCR)-based method (Pel-Freez Cytokine Genotyping Kit) which provides for the use of forty-eight formulations of specific lyophilized primer sets to amplify genomic DNA. Briefly, as reported in Aureli et al. [9], PCR reactions were performed using a mixture composed of PCR Buffer, water and Taq DNA Polymerase. A volume of 50 μL of DNA sample (75-125 ng/μL) was added to the buffer mixture and 10 μL of the reaction mixture was dispensed into each well. The PCR products were then electrophoresed by 1.5% agarose gel containing 0.5 mg/ml ethidium bromide and documented by photography. The genotype specificity was determined by the presence or absence of a specific PCR product of known size.
The study of the NRG-1 and ErbB4 SNPs was conducted at high resolution by sequence-based typing (SBT) assay. DNA was amplified by PCR using the following primers pairs:
• NRG-1rs6994992 forward sequence, 5’- AGTAGGATTGGATGTTTG-3’; reverse sequence, 5’- TTAGCAGCATAGTTGGGCAG-3’;
• NRG-1 rs35753505 forward sequence, 5’- GCATTAGAACTAGAACTTGCGTGA-3’; reverse sequence, 5’-TGGGAACTCTCCATCTCTTTC-3’; and
• ErbB4 rs7598440 forward sequence, 5’- CTCAGCAGAGGCATCAAC-3’; reverse sequence, 5’- CTGGACACTCATGGGAAG-3’.
Amplifications were carried out on 50 ng of genomic DNA per 50 μl reaction. PCR products were identified on 1.5% agarose gel and then purified by a PCR clean-up reagent (EXOSAP). Sequence reactions were performed using the Big-Dye Terminator Chemistry v 1.1 (Applied Biosystems, Foster City, CA), processed on an Applied Biosystems 3130 Genetic Analyzer and then purified. Typing was obtained on the basis of alignment of the processed sequences with the sequences retrieved from the Genbank Database.
IL1- β measurements
Serum samples from patients and healthy controls were separated from venous blood and stored at -80°C until cytokine analysis. IL1- β levels were evaluated by enzyme-linkedimmuno- sorbent- assay (ELISA) using commercially available kits (Boster Biological Technology) and performed in accordance with the manufacturer's protocol. The sensitivity of the assays was <0.15 pg/ml. Samples were run in duplicate and the mean value was used for further statistical analysis.
Statistical analysis
Person’s chi-square test and Fisher’s exact test were used to evaluate genotype and allele frequencies among cases and controls. The odds ratio (OR) was calculated to assess the relative risk conferred by a particular allele and genotype. Comparison of serum IL-1β level between cases and control group was done with one-way analysis of variance (ANOVA). Statistical significance was set at p<0.05. Calculations were performed using the SPSS/Win software (Version 12.0, SPSS Inc., Chicago, IL).
Results
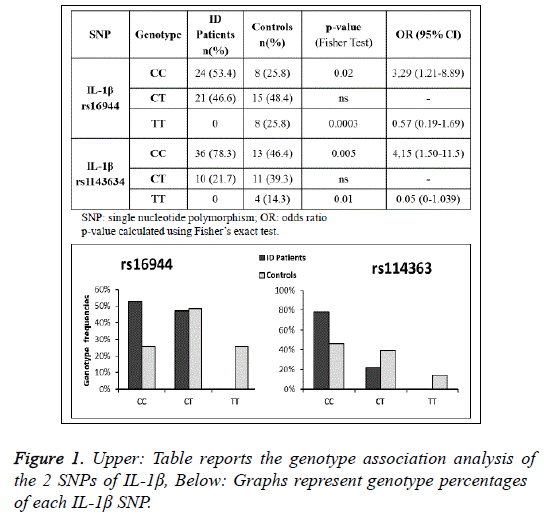
The genotype and allele frequencies of the IL-1β rs16944 and IL-1β rs1143634 polymorphisms in ID patients and control subjects are shown in Figures 1 and 2. Our findings indicate that both the IL-1β variants are associated to ID. We found that subjects carrying the rs16944 CC genotype had an increased risk to develop ID (p=0.02; OR:3.29) compared to those with the rs16944 TT genotype (p=0.0003; OR:0.57). We also found that C allele was more frequent in patients than in controls (p=0.0007 OR:3.29) and on the contrary, T allele was more frequent in controls than in patients (p=0.0007 OR:0.30). The analysis of theIL-1β rs1143634 revealed a positive association with ID for the CC genotype and a negative one for the TT genotype (p=0.005; OR:4.15 and p=0.01; OR:0.05).
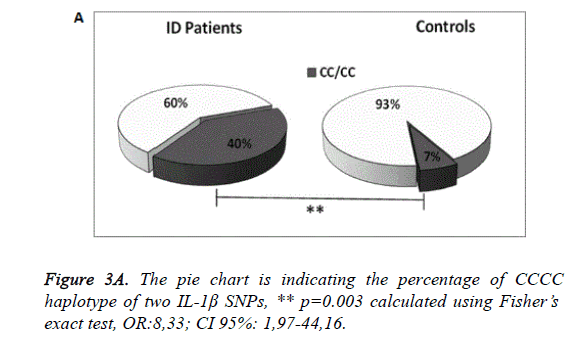
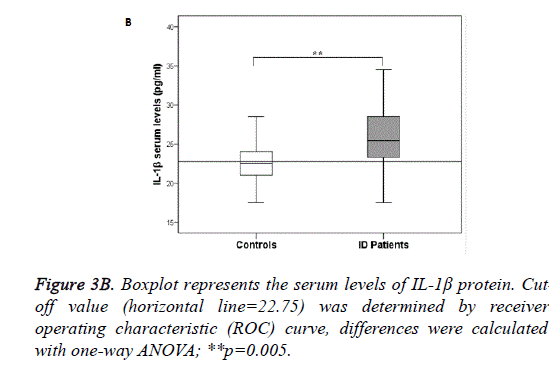
A significant difference was also observed in the frequency of C and T alleles ofrs1143634 in patients and controls (p=0.0006 for C allele, OR: 4.21 and p=0.0006 for T allele, OR:0.24). Considering together the two SNPs of IL-1β we noted that CC CC combination was associated with an increased risk to develop ID (p=0.003 OR:8.33), Figure 3A. Examining the genotype and allele frequencies of the NRG-1 rs6994992, NRG-1 rs35753505 and ErbB4 rs7598440 polymorphisms, we did not observe statistically significant differences between patients and controls. From the study of the IL-1β serum concentration we detected a significantly higher level of IL-1β in patients with ID than in control subjects (p=0.005), Figure 3B.
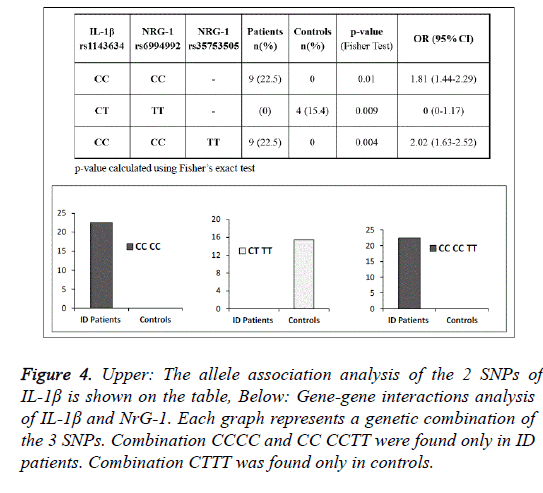
Analysis of gene-gene interaction revealed some combinations associated with ID. Given the complexity of the statistical analysis, only associations with p<0.05 (Fisher-test) were taken into account. The first significant combination, founded only in the group of patients, was between the CC genotype of IL-1βrs1143634 with the CC genotype of NRG-1 rs6994992 (p=0.01 OR:1.81). Instead, the combination CT of IL-1βrs1143634 with TT of NRG-1 rs6994992 was found only in controls (p=0.009 OR:0.6), Figure 4. The last significant combination, founded only in patients, was CC of IL-1βrs1143634 with CC of NRG-1 rs6994992 and TT of NRG-1rs35753505 (p=0.004 OR:2.02), Figure 4.
Figure 4: Upper: The allele association analysis of the 2 SNPs of IL-1Ã is shown on the table, Below: Gene-gene interactions analysis of IL-1Ã and NrG-1. Each graph represents a genetic combination of the 3 SNPs. Combination CCCC and CC CCTT were found only in ID patients. Combination CTTT was found only in controls.
Discussion
The ID is the most common developmental disorder characterised by a congenital limitation in intellectual functions and adaptive behaviour which include conceptual, social, and practical skills. It is assumed that this disorder, considered chronic, has a prenatal origin but often the children are subject to assessments only in school age and show learning and fitness disorders. Moreover, an overlap of gene dysfunction with other neurodevelopmental disorders such as autism, schizophrenia and epilepsy has been highlighted [29]. The severe ID is quite sporadic, whereas more often milder forms can occur and spread out in family and population. In this contest, it is important to elucidate genetic influences underlying this form of cognitive impairment. For the present investigation, we recruited only a children population whose clinical diagnosis was mild to moderate ID with the exclusion of other co-morbidity. Studying 2 SNPs located in the IL-1β gene (rs16944 and rs1143634) we demonstrated a positive association between both CC genotype and ID and a negative one with TT genotype. At allelic level, C allele of both SNPs was significantly associated with ID, confirming results obtained analysing genotypes. On the contrary, T-allele was significantly more frequent in control children. The involvement of inflammatory cytokines, especially IL-1, IL-6, and tumor necrosis factor (TNF-a) in the normal physiological regulation of hippocampal-dependent memory has been widely analysed [30] and it has been demonstrated that the influence of IL-1β on memory processes includes both detrimental and beneficial effects [31]. The key role of pro-inflammatory cytokines and specifically of IL1β in neuronal functionality seems to be thinly regulated in a dose-effect manner. Many research conducted mainly in rodent models, underlined that both peripherally and centrally altered levels of IL-1βmight be responsible for hippocampal-dependent memory impairments [32]. This fact is important because peripheral cytokines can affect the brain both through blood-borne and neural routes [33] and can lead to de novo production of pro-inflammatory brain cytokines [34]. The IL-1β gene displays many SNPs both in promoter and coding regions, which have been associated with IL-1β production. The rs16944 andrs1143634 SNPs are respectively a promoter polymorphism and a silent polymorphism in exon 5 of IL-1β and are able to influence circulating protein expression [35]. There are several pieces of evidence of an association between the C allele of IL-1β rs1143634 and higher serum IL-1β concentrations [36]. In our study, we show a significantly higher IL-1β serum level in ID than in controls. Besides, patients with CC genotype and C allele of both SNPs had higher IL-1β expression than controls even if not statistically significant (p=0.06) (data not shown). The post hoc statistical power analysis assessed that this is likely due to the small sample size. The children enrolled were selected according to binding exclusion criteria that inexorably reduced the sample size by improving, however, specificity. The initial aim of the present study was to evaluate the possible genetic associations between polymorphisms of NRG-1 and cognitive retard. NRG-1is a growth factor with multiple functions in the embryonic and postnatal brain and it is involved in a variety of physiological processes, including migration of embryonic interneurons, maturation and survival of oligodendrocytes [37] and modulation of synaptic plasticity [38]. Therefore it may influence cognitive function. However, in our sample, the analysis of the frequencies showed no association between ID and the two SNPs of NRG-1 nor with the SNP of its receptor ErbB4 but, logistic regression analysis demonstrated IL-1β and NRG-1 genes interaction. More specifically, our results provide evidence that IL-1β rs1143634 and its interaction with both NRG-1 SNPs affects the risk when CC genotype of IL-1β combines with CC and TT of NRG-1. On the contrary, CT TT combination of IL-1β rs1143634and NRG-1 rs6994992 appeared to be protective as it is present only in controls. Traditionally ID is categorized by severity but, another classification to facilitate the diagnostic approach is that between syndromic ID (S-ID) that is associated with dysmorphic or malformative features and non-syndromic ID (NS-ID) that is not associated with congenital defects or dimorphism.
While robust evidence supports a role for the genetic origin of S-ID mainly linked to chromosomal aberrations, about 59% of NS-ID doesn’t have identifiable causes [39]. Many environmental factors as teratogens, viruses, radiation or severe head trauma, perinatal injury, causing lack of oxygen to the brain, can be involved in ID aetiology and, in the case of mild-ID also exogenous environmental factors such as level of maternal education, access to education/opportunity and access to healthcare have to be considered. However, all these factors could explain only some cases of NS-ID, and it cannot be excluded the gene-environment interaction. For these reasons, it is also essential to explore genetic aetiology. The brain development requires a cellular and molecular network orchestration incredibly complex involving numerous genes and proteins. It is therefore likely that any gene rearrangement, mutation or deletion could happen and it is not inconceivable that will be severe consequences for brain development and cognitive functioning. The NS-ID patients show intellectual impairment as the only feature [40]. It is conceivable that the genes related to learning and memory processes might be involved. As widely reported above both IL-1β and NRG-1 are involved in the cognitive regulatory processes. While the role of IL-1β SNPs in intellectual impairment has been more explored [41-45].
Taking into account this state of the art, we hypothesised that there might be a relation between ID and both NRG-1 and IL-1 β SNPs. Here we demonstrated that both CC homozygous genotypes of the two SNPs of IL-1β considered are associated with ID risk, and that there are also some genotypic combinations of IL-1β and NRG-1 associated with ID. As far as we know, this is the first study on the interaction between genetic variants of IL-1β and NRG-1 in the ID risk.
As previously asserted, the small sample size represents a limitation of our investigation and further studies with larger groups are needed to confirm our findings. Nevertheless, finding and selecting candidate genes to be associated with NS-ID is relevant not only in research and clinically based studies but also in the management of families with affected individuals, particularly where consanguinity is involved.
Conclusion
This study reports data showing the influence of IL-1β polymorphisms and the IL-1β/NRG-1 interaction in the mild/ moderate ID risk. These results could be a start point for further investigations and can provide information to develop future projects on this topic and/or to planning strategies that identify ID early. All this with the aim of improve access to management interventions and specialized care for ID.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics Statement
All studies have been approved by the appropriate ethics committee (n. 1931/17.11.2011) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
References
- Beirne-Smith M, Patton JR, Kim SH. Mental retardation (7th ed.) Upper Saddle River, NJ: Pearson Education. 2006.
- Schalock RL, Luckasson RA, Shogren KA, et al. The renaming of mental retardation: Understanding the change to the term intellectual disability. Intellect Dev Disabil. 2007;45:116-24.
- Pickering M, O’Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339-54.
- Pugh CR, Fleshner M, Watkins LR, et al. The immune system and memory consolidation: A role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29-41.
- Pribiag H, Stellwagen D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology. 2014;78:13-22.
- Takemiya T, Fumizawa K, Yamagata K, et al. Brain interleukin-1 facilitates learning of a water maze spatial memory task in young mice. Front Behav Neuro Sci. 2017;11:202.
- Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76-80.
- Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam study. Arch Neurol. 2004;61:668-672.
- Aureli A, Sebastiani P, Del Beato T, et al. Involvement of IL-6 and IL-1 receptor antagonist on intellectual disability. Immunol Lett. 2014;162:124-31.
- Zhuang L, Liu X, Xu X, et al. Association of the interleukin 1 beta gene and brain spontaneous activity in amnestic mild cognitive impairment. J Neuroinflammation. 2012;9:263.
- Marx CE, Jarskog LF, Lauder JM, et al. Cytokine effects on cortical neuron MAP-2 immunoreactivity: Implications for schizophrenia. Biol Psychiatry. 2001;50:743-9.
- Voineagu I, Eapen V. Converging pathways in autism spectrum disorders: Interplay between synaptic dysfunction and immune responses. Front Hum Neurosci. 2013;7:738.
- Tsai SJ, Hong CJ, Liu ME, et al. Interleukin-1 beta (C-511T) genetic polymorphism is associated with cognitive performance in elderly males without dementia. Neurobiol Aging. 2010;31:1950-1955.
- Stacey D, Ciobanu LG, Baune BT. A systematic review on the association between inflammatory genes and cognitive decline in non-demented elderly individuals. Eur Neuropsychopharmacol. 2017;27:568-588.
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: Genetics, gene expression and neurobiology. Biol Psychiatry. 2006;60:132-40.
- Heinrichs RW, Vaz SM. Verbal memory errors and symptoms in schizophrenia. Cogn Behav Neurol. 2004;17:98-101.
- Li B, Woo RS, Mei L, et al. The neuregulin-1 receptor controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583-97.
- Liu X, Bates R, Yin DM, et al. Specific regulation of NRG-1 isoform expression by neuronal activity. J Neurosci. 2011;31:8491-501.
- Yokley JL, Prasad KM, Chowdari KV, et al. Genetic associations between neuregulin-1 SNPs and neurocognitive function in multigenerational, multiple schizophrenia families. Psychiatr Genet. 2012;22:70-81.
- Kukshal P, Kodavali VC, Srivastava V, et al. Dopaminergic gene polymorphisms and cognitive function in a north Indian schizophrenia cohort. J Psychiatr Res. 2013;47:1615-22.
- O'Tuathaigh CM, Babovic D, O'Sullivan GJ, et al. Phenotypic characterization of spatial cognition and social behavior in mice with 'knockout' of the schizophrenia risk gene neuregulin1. Neuroscience. 2007;147:18-27.
- Pinto JV, Passos IC, Librenza-Garcia D, et al. Neuron-glia interaction as a possible pathophysiological mechanism of bipolar disorder. Curr Neuropharmacol. 2018;16:519-32.
- Di Marco B, Bonaccorso CM, Aloisi E, et al. Neuro-inflammatory mechanisms in developmental disorders associated with intellectual disability and autism spectrum disorder: A neuro- immune perspective. CNS Neurol Disord Drug Targets. 2016;15:448-63.
- Desbonnet L, Cox R, Tighe O, et al. Altered cytokine profile, pain sensitivity and stress responsivity in mice with co-disruption of the developmental genes Neuregulin-1 × DISC1. Behav Brain Res. 2017;320:113-8.
- Hulkkonen J, Laippala P, Hurme M. A rare allele combination of the interleukin-1 gene complex is associated with high interleukin-1 beta plasma levels in healthy individuals. Eur Cytokine Netw. 2000;11:251-5.
- Hurme M, Santtila S. IL-1 receptor antagonist (IL-1 RA) plasma levels are co-ordinately regulated by both IL-1 RA and IL-1b genes. Eur J Immunol. 1998;28:2598-602
- Wu L, Ramirez SH, Andrews AM, et al. Neuregulin1-β decreases interleukin-1β-induced RhoA activation, myosin light chain phosphorylation and endothelial hyperpermeability. J Neurochem. 2016;136:250-7.
- Xu J, Hu C, Chen S, et al. Neuregulin-1 protects mouse cerebellum against oxidative stress and neuroinflammation. Brain Res. 2017;1670:32-43.
- Hänninen K, Katila H, Saarela M, et al. Interleukin-1 beta gene polymorphism and its interactions with neuregulin-1 gene polymorphism are associated with schizophrenia. Eur Arch Psychiatry ClinNeurosci. 2008;258:10-5.
- Vissers LE, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nat Rev Genet. 2016;17:9-18.
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain BehavImmun. 2011;25:181-213.
- Baartman TL, Swanepoel T, Barrientos RM, et al. Divergent effects of brain interleukin-1ß in mediating fever, lethargy, anorexia and conditioned fear memory. Behav Brain Res. 2017;324:155-63.
- Barrientos RM, Frank MG, Hein AM, et al. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46-54.
- Dantzer R. Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399-411.
- Dimov D, Vlaykova T, Kurzawski M, et al. Single Nucletide polymorphisms in gene of IL-1Beta in bronchial asthma. Biotechnology & Biotechnological Equipment. 2012;26:45-51.
- Lacruz-Guzmán D, Torres-Moreno D, Pedrero F, et al. Influence of polymorphisms and TNF and IL-1β serum concentration on the infliximab response in Crohn's disease and ulcerative colitis. Eur J ClinPharmacol 2013;69:431-8.
- Fernandez PA, Tang DG, Cheng L, et al. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron. 2000;28:81-90.
- Neddens J, Vullhorst D, Paredes D, et al. Neuregulin links dopaminergic and glutamatergic neurotransmission to control hippocampal synaptic plasticity. Commun Integr Biol. 2009;2:261-4.
- Rauch A, Hoyer J, Guth S, et al. Diagnostic yield of various genetic approaches in patients with unexplained developmental delay or mental retardation. Am J Med Genet A. 2006;140:2063-74.
- Kaufman L, Ayub M, Vincent JB. The genetic basis of non-syndromic intellectual disability: A review. J Neurodev Disord. 2010;2:182-209.
- Chaudhury AR, Gerecke KM, Wyss JM, et al. Neuregulin-1 and erbB4 immunoreactivity is associated with neuritic plaques in Alzheimer disease brain and in a transgenic model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:42-54.
- Chang KA, Shin KY, Nam E, et al. Plasma solubleneuregulin-1 as a diagnostic biomarker for Alzheimer's disease. Neurochem Int. 2016;97:1-7.
- Jiang Q, Chen S, Hu C, et al. Neuregulin-1 (Nrg1) signaling has a preventive role and is altered in the frontal cortex under the pathological conditions of Alzheimer's disease. Mol Med Rep. 2016;14:2614-24.
- Ryu J, Hong BH, Kim YJ, et al. Neuregulin-1 attenuates cognitive function impairments in a transgenic mouse model of Alzheimer's disease. Cell Death Dis. 2016;7:e2117.
- Xu J, De Winter F, Farrokhi C, et al. Neuregulin 1 improves cognitive deficits and neuropathology in an Alzheimer's disease model. Sci Rep. 2016;6:31692.