Review Article - Journal of Molecular Oncology Research (2017) Volume 1, Issue 1
Hyperglycosylated hCG drives malignancy in cancer cases.
Laurence A. Cole*
USA hCG Reference Service, 34 Broadmoor Way, P.O. Box 950 Angel Fire, New Mexico
- *Corresponding Author:
- Laurence A. Cole
USA hCG Reference Service, 34 Broadmoor Way
P.O. Box 950Angel Fire, New Mexico
Tel: 575-377-1330
E-mail: larry@hcglab.com
Accepted date: 03 August, 2017
Citation: Cole LA. Hyperglycosylated hCG drives malignancy in cancer cases. J Mol Oncol Res. 2017;1(1):53-63.
DOI: 10.35841/molecular-oncology.1.1.53-63
Visit for more related articles at Journal of Molecular Oncology ResearchAbstract
Purpose: Trophoblastic cancers (Choriocarcinoma, testicular and ovarian germ cell malignancies) secrete hyperglycosylated hCG and non-trophoblastic cancers secrete hyperglycosylated hCG free β- subunit.Both are structural variants of hCG independent to the hormone hCG. They are autocrines acting on a transforming growth factor β-II (TGF-β-II), receptor not acting on the hormone receptor. Here these molecules were examined as drivers of malignancy.
Methods: The cancer cell lines were examined, how they enzymatically invaded Matrigel chambers and how their growth responded to hyperglycosylated hCG and hyperglycosylated hCG free β-subunit signals. Use of hyperglycosylated hCG and hyperglycosylated hCG free β-subunit as tumor markers is examined in 959 cancer serum samples and 2257 cancer urine samples, plus 284 urines from benign disease patients. In addition, 56 new ovarian cancer urines were collected and tested in the supersensitive B204 assay.
Results: Hyperglycosylated hCG and its free β-subunit are both strong promoters of malignancy functions. A total of 100% of trophoblastic malignancies produced hyperglycosylated hCG markers in serum and urine. A total of 30% of non-trophoblastic cancers were detected in serum and 44% in urine. As shown, in reality, considering simple and complex autocrine secretion, 100% of nontrophoblastic malignancies produce hyperglycosylated hCG and hyperglycosylated hCG free β- subunit markers in urine. As demonstrated, hyperglycosylated hCG and its free β-subunit exist in all human cancers and promote malignancy. Multiple other researchers have confirmed these results. Discussion: It is inferred that hyperglycosylated hCG and its free β-subunit are produced by all or most human cancers, and are the promoters of malignancy.
Keywords
Hyperglycosylated hCG, Steroidogenesis, Ovulation, Luteogenesis
Introduction
What promotes malignancy in human cancers? A many times asked and never answered issue. Many authors have suggested oncogenic proteins, such as transforming growth factor β (TGF-β), TGF-β agonists and TGF-β antagonists.
The question still remains an issue and still remains unanswered [1-13]. This issue is addressed here, cancer hyperglycosylated hCG and hyperglycosylated hCG free β- subunit, both TGF-β antagonists, are seemingly the human malignancy promoter that differentiate benign and malignant cells.
As proposed in this article, cancer hyperglycosylated hCG is the most promising human cancer malignancy promoter. Hyperglycosylated hCG was discovered by me in 1997 [14]. It is a second form of hCG, a TGF-β antagonist, yet a completely independent and separate molecule to the hormone hCG.
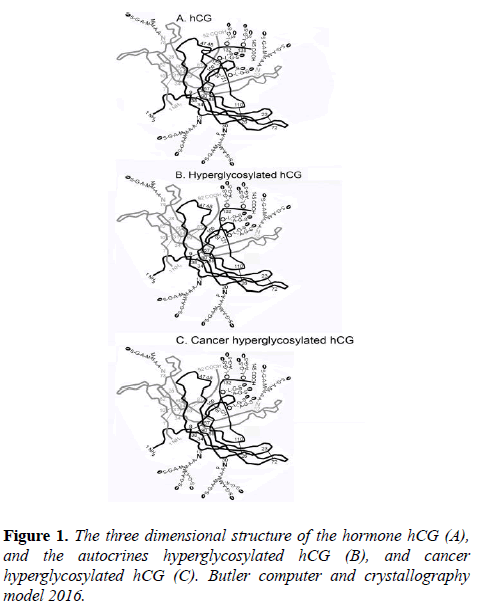
It shares an identical α-subunit and β-subunit amino acid sequence with hCG, and only differs from hCG by having three or four type 2 O-linked oligosaccharides on the hCG β-subunit C-terminal peptide, hexasexaccharides and pentasaccharide verses three of four sugar type 1, trisaccharide and tetrasaccharadide structures found on the hormone hCG (Figure 1).
The three dimensional structure of pregnancy and cancer hyperglycosylated hCG is virtually identical to the hormone hCG (Butler SA, Computer+X-ray 3D Models (Figure 1) [15]. Hyperglycosylated hCG is an autocrine binding a TGF-β-II receptor [16-18], and not acting at all on an hCG/luteinizing hormone (LH) receptor like the hormone hCG (Figure 1).
Hyperglycosylated hCG is made by invasive cytotrophoblast cells during pregnancy, while the hormone hCG is made by fused syncytiotrophoblast cells during pregnancy [19,20]. Hyperglycosylated hCG acts on the autocrine cytotrophoblast cell TGF-β-II receptor driving implantation of the blastocyst in the beginning of pregnancy [20,21], and deep implantation of hemochorial placentation at 10 weeks gestation [22,23], and during the menstrual cycle finalizes ovulation [24]. In all three processes, the autocrine TGF-β-II receptor drives invasive metalloproteinase and collagenase production [12,19], cell growth [19,25] and blocks cell apoptosis [26-28], three malignancy-like steps.
The hyperglycosylated Hcg -TGF-β-II pathway is the solitary enzymatic invasion pathway and cellular growth pathway coded in the human genome. Cancer cells steal this hyperglycosylated hCG and use it to drive human malignancy, to drive enzymatic invasion, growth and blockage of apoptosis in cancer cells [26-28].
This is cancer hyperglycosylated hCG produced by trophoblastic cancers, and hyperglycosylated hCG free β- subunit produced by non-trophoblastic cancers [29], the molecules that drives implantation in pregnancy, seemingly drives human malignancy.
Under medical dictionary definition, malignancy is the “process of cancer advancement, cancer growth, and cancer tissue invasion.” In the Merriam-Webster dictionary malignancy, it is defined as “aggressively malicious, tending to produce death or deterioration, tending to infiltrate, metastasize, and terminate fatally.”
As interpreted in this article, malignancy means the process of cancer advancement, cancer growth promotion, and cancer tissue invasion. We test hyperglycosylated hCG and its free β- subunit for these malignancy properties, and how a malignancy promoter absolutely differentiates cancers and benign diseases.
The ability of trophoblastic cancers to produce hyperglycosylated hCG was confirmed and non-trophoblastic cancers produce hyperglycosylated hCG free β-subunit. Then show that both the molecules produced by cancers have malignancy properties. Finally, the occurrence of these tumor agents was examined to show that 100% of cancers produce these malignancy agents.
Materials and Methods
Serum samples, 959, and urine samples 2257 were collected from men and women attending the oncology, thoracic oncology and gynecologic oncology clinics at Yale New- Haven Hospital in New Haven CT. Samples collection was started in 1992, multiple tubes of serum and urine were libraried in -80 C Revco freezers with the certification of Yale Clinical Research Internal Review Board seeking full patient approval. Additionally, 283 urine samples from women and men with benign disease were collected at Yale New Haven Hospital Oncology clinics and 154 serum and urine samples were at Oncology clinic from healthy and treated individuals.
A further group of 56 subjects with ovarian cancer supplied urines in 1999 for super sensitive B204 test examination with full IRB approval. At Yale University verbal consent was considered sufficient for collecting urine samples for tumor marker studies, signed consent was needed for serum samples.
Cell lines, JEG-3 choriocarcinoma cell line, JAr choriocarcinoma cell line, NTERA testicular germ cell cancer, T24 epithelial bladder carcinoma cell line, KLE endometrial adenocarcinoma cell line, CaSki epidermoid cervical cancer cells, ScaBER squamous bladder cancer cell, Hec-1-a endometrial cancer cells, SK-MES-1 lung epithelial cancer cells and KM-H2 Hodgkin’s lymphoma cancer cells were all maintained continuously in culture in T75 flasks using high glucose Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Cells were sub-cultured using 1X trypsin.
In the experiment the cells were cultured in flasks 3-5 days in quadruplicate until they reach approximately 70% flask confidence. Then medium was changed and replaced with medium containing 0 ng/ml, 20 ng/ml and 200 ng/ml hyperglycosylated hCG (JAr, JEG-3 and NTERA cell lines), or 0 ng/ml, 20 ng/ml and 200 ng/ml hyperglycosylated hCG free β-subunit (T24, KLE, CaSki, ScaBER, Hec-1-a, SK-MES-1 and KM-H2 cell lines). Cells were cultured for 24 h and the cell number determined in each flask using a hemocytometer.
Matrigel basement membrane chambers were purchased from Corning Inc. (Corning, NY). Matrigel chambers were managed according to manufacturer’s instructions, and manufacturer suggested calculation of proportion penetration. Cells, 70% confluent flasks of cells were suspended and transferred to Matrigel chambers, and cultured in Matrigel chambers for 24 h and proportion penetration or invasion determined.
Zero time culture cells were plated onto Matrigel membranes and control inserts (Biocoat Matrigel invasion membranes, BD Biosciences, Bedford, MA 01730), and cultured at 37ºC for 24 h in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum in quadruplicate. Matrigel membranes were processed and percentage invasion calculated. Briefly, membranes were rehydrated in the incubator for 2 h before use. Membranes and control inserts were then plated (25,000 cells in 0.5 medium per plate).
Plates were cultured for 24 h, and membranes removed from inserts using a scalpel. Membranes were transferred to a slide using Cytoseal mounting medium (Stephens Scientific Inc., Riverdale NJ), exposing the under surface and invaded cells. Cells were stained with DIF-Quick Stain (IMEB Inc., Chicago IL) to mark nuclei. Invaded cells were counted at 9 marked positions and were averaged for each insert. Cell penetration or invasion of membranes is directly compared to that of correspondingly cultured control inserts and percentage invasion calculated using the formula provided by the manufacturer.
All samples were tested by immunometric 96 well microtiter plate assays using Immulon-1 flat bottomed plates. For B152 hyperglycosylated hCG and hyperglycosylated hCG free β- subunit plates were coated with 1/1000 B152, or 15 μl antibody in 22 ml coating buffer. For B204 urine hyperglycosylated hCG free β-subunit plus β-core fragment plates were coated with B204 4 mg/ml in 22 ml coating buffer. For the super sensitive B204 assay plates were coated at 0.40 mg/ml in 22 ml coating buffer. For the FBT11 free β-subunit assay plates were coated with 0.033 ml FBT11 antibody concentrate in 22 ml coating buffer.
The secondary antibody used was 4001-POD for all assays, a monoclonal antibody specific to core hCG β-subunit. Coated plates were incubated with 0.2 ml sample or standard and incubated 2 h at room temperature with shaking for the B204 and FBT11 assay, 6 h at room temperature for the super sensitive B204 assay, and 4 h at room temperature for the B152 assay. In all 3 cases, the coating with the peroxidase labeled secondary antibody, 4001 POD was 2 h at room temperature. Finally, 200 μl of substrate (TMB reagent, dilute 50% with water [Sigma part#T8665]) is added to each well. After approximately 15 min incubation reaction is topped by the addition of 50 μl of 2 N HCl. Absorbance is then read on the titerplate reader at 450 nm.
The B152 hyperglycosylated hCG and hyperglycosylated hCG free β-subunit assay is tested with 5 C5 hyperglycosylated hCG standards at 2.0 fmol/ml, 8.0 fmol/ml, 32 fmol/ml, 128 fmol/ml and 512 fmol/ml. The B204 assay is tested with FEDE β-core fragment standard at 2.0 fmol/ml, 8.0 fmol/ml, 32 fmol/ml, 128 fmol/ml and 512 fmol/ml. The super sensitive B204 assay uses standards at 0.1 fmol/ml, 0.4 fmol/ml, 1.6 fmol/ml, 6.4 fmol/ml and 25.6 fmol/ml. The FBT11 assay is calibrated with recombinant hCG β-subunit standards at 4 ng/ml, 1 ng/ml, 0.24 ng/ml, 0.1 ng/ml and 0.04 ng/ml.
Specificity studies show that the B152 assay detect hyperglycosylated hCG, hyperglycosylated hCG β-subunit, nicked hyperglycosylated hCG and nicked hyperglycosylated hCG β-subunit. With<0.2% detection of the hormone hCG, and its dissociated hCG β-subunit. The B204 assay detects β-core fragment, hCG free β-subunit, nicked hCG free β-subunit, hyperglycosylated hCG β-subunit and nicked hyperglycosylated hCG β-subunit. The FBT11 assay detects hCG free β-subunit, nicked hCG free β-subunit, hyperglycosylated hCG β-subunit and nicked hyperglycosylated hCG β-subunit [19].
Serum samples were also detected for total hCG (hCG, +nicked hCG, +nicked hCG missing β-subunit C-terminal peptide, +hCG free β-subunit, +nicked β-subunit, +nicked β- subunit missing β-subunit C-terminal peptide, +hyperglycosylated hCG, +nicked hyperglycosylated hCG, +nicked hyperglycosylated hCG free β-subunit) using the automated Siemens Immulite assay. Tests were run according to manufacturer’s instruction using a total hCG standard mass curve, recombinant hCG (4 ng/ml, 1 ng/ml, 0.25 ng/ml, 0.5 ng/ml, 0.2 ng/ml and 0.09 ng/ml), run with every group of samples. All tumor marker data was entered into Microsoft Excel spreadsheets and analyzed and sorted by diagnosis code.
Results and Discussion
1. What do cancer cells secrete?
The freezers were searched for stored cancer patient serum and used for tumor marker studies and found 32 trophoblastic cancer (choriocarcinoma, ovarian germ cell and testicular germ cell cancers) serum samples and 92 non-trophoblastic cancer serum samples. All were tested for total hCG in the Siemens Immulite assay, for hyperglycosylated hCG and hyperglycosylated hCG β-subunit in the B152 assay, and for free β-subunit verses α-β- dimer using the FBT11 assay (Table 1).
| Cells | Effect (mean ± standard deviation) |
|---|---|
| JEG-3 choriocarcinoma cells | |
| Control cultures | 48 ± 11% invasion of basement membrane |
| Control+100 ng/ml hyperglycosylated hCG | 88 ± 6.0% invasion of basement membrane |
| Ttest P=0.010 | |
| NTERA testicular germ cells | |
| Control culture | 36 ± 12% invasion of basement membrane |
| Control+100 ng/ml hyperglycosylated hCG | 78 ± 8.1% invasion of basement membrane |
| Ttest P=0.022 | |
| CaSki epidermoid cervical cancer cells | |
| Control culture | 49 ± 15% invasion of basement membrane |
| Control+100 ng/ml hyperglycosylated hCG β-subunit | 69 ± 6.5% invasion of basement membrane |
| Ttest P=0.020 | |
| SK-MES-1 Epithelial lung cancer | |
| Control culture | 48 ± 11% invasion of basement membrane |
| Control+100 ng/ml hyperglycosylated hCG β-subunit | 76 ± 6.9% invasion of basement membrane |
| Ttest P=0.0055 | |
Table 1. What is produced by cancer cells?
The standards are testsed first, recombinant hCG (Serono) (Table 1). This is 100% hormone hCG. As found, B152 assay only detected 0.13% confirming that this assay only detects hyperglycosylated molecules, and does not detect the hormone hCG. Secondly, the recombinant hCG dissociated β-subunit was tested. As found, B152 only detected 0.11% confirming one again that the hormone hCG β-subunit is also not detected (Table 2).
| Case or sample | Immulite 1000 assay | B152 assay | B152 assay | FBT11 assay |
|---|---|---|---|---|
| Standards | ||||
| Recombinant hCG (Ovidrel), 1000 ng/ml | 1007 | 0.13 | 0.01% | 1.17 |
| Recombinant hCG β-subunit, 600 ng/ml | 602 | 0.11 | 0.01% | 615 |
| Hyperglycosylated hCG standard C5, 1000 ng/ml | 997 | 995 | 100.00% | 14 |
| C5 free β-subunit standard, 600 ng/ml | 578 | 687 | 100.00% | 610 |
| Trophoblastic cancers | ||||
| Choriocarcinoma case 3, pre-therapy | 21200 | 23300 | 110.00% | 1172 |
| Choriocarcinoma case 7, pre-therapy | 33700 | 34700 | 103.00% | 1610 |
| Choriocarcinoma case 8, pre-therapy | 57400 | 54600 | 95.00% | 5680 |
| Choriocarcinoma case 11, pre-therapy | 20600 | 19900 | 97.00% | 2010 |
| Choriocarcinoma case 12, pre-therapy | 27500 | 30100 | 109.00% | 5330 |
| Choriocarcinoma case 23, pre-therapy | 153400 | 155000 | 101.00% | 15740 |
| Choriocarcinoma case 25, pre-therapy | 34700 | 36800 | 106.00% | 1230 |
| Choriocarcinoma case 26, pre-therapy | 43400 | 47700 | 110.00% | 1250 |
| Choriocarcinoma case 32, pre-therapy | 210500 | 210200 | 100.00% | 15750 |
| Choriocarcinoma case 36, during therapy | 2500 | 2100 | 84.00% | 155 |
| Choriocarcinoma case 41, during therapy | 3700 | 2900 | 78.00% | 505 |
| Choriocarcinoma case 42, during therapy | 1600 | 870 | 54.00% | 360 |
| Choriocarcinoma case 47, during therapy | 9900 | 9900 | 100.00% | 1260 |
| Choriocarcinoma case 48, during therapy | 20400 | 19000 | 93.00% | 1880 |
| Choriocarcinoma case 49, during therapy | 1600 | 1010 | 63.00% | 29 |
| Choriocarcinoma case 50, during therapy | 1100 | 1010 | 91.00% | 106 |
| Choriocarcinoma case 54, during therapy | 3300 | 2500 | 76.00% | 230 |
| Choriocarcinoma case 57, during therapy | 3600 | 3100 | 86.00% | 410 |
| Choriocarcinoma case 69, during therapy | 1050 | 1050 | 100.00% | 89 |
| Choriocarcinoma case 61, during therapy | 6300 | 5900 | 94.00% | 311 |
| Ovarian germ cell cancer case 1, pre-therapy | 13400 | 14000 | 104.00% | 2230 |
| Ovarian germ cell cancer case 4, pre-therapy | 8800 | 8800 | 100.00% | 547 |
| Ovarian germ cell cancer case 5, pre-therapy | 17600 | 17100 | 97.00% | 3120 |
| Ovarian germ cell cancer case 6, pre-therapy | 33400 | 34300 | 102.00% | 11560 |
| Ovarian germ cell cancer case 7, pre-therapy | 18900 | 18700 | 99.00% | 196 |
| Ovarian germ cell cancer case 11, pre-therapy | 16200 | 16100 | 99.00% | 151 |
| Testicular germ cell cancer case 7, pre-therapy | 10400 | 10600 | 102.00% | 655 |
| Testicular germ cell cancer case 9, pre-therapy | 14500 | 14700 | 99.00% | 907 |
| Testicular germ cell cancer case 10, pre-therapy | 19100 | 19300 | 101.00% | 430 |
| Testicular germ cell cancer case 11, pre-therapy | 11000 | 11100 | 101.00% | 1220 |
| Testicular germ cell cancer case 14, pre-therapy | 9200 | 9500 | 103.00% | 103 |
| Testicular germ cell cancer case 17, pre-therapy | 13700 | 14300 | 104.00% | 2240 |
| Mean ± standard deviation | 26,364±43,526 | 26,427±43,729 | 96%±12.6% | 2,492±4,227 |
| Non-trophoblastic cancers | ||||
| Bladder cancer case 14 | 0.09 | 0.09 | 100.00% | 0.1 |
| Bladder cancer case 77 | 0.09 | 0.1 | 111.00% | 0.09 |
| Bladder cancer case 120 | 0.12 | 0.12 | 100.0% | 0.11 |
| Bladder cancer case 121 | 0.15 | 0.15 | 100.00% | 0.15 |
| Bladder cancer case 169 | 1.3 | 1.4 | 108.00% | 1.4 |
| Breast cancer case 1 | 1.3 | 1.3 | 100.00% | 1 |
| Breast cancer case 6 | 0.09 | 0.1 | 111.00% | 1 |
| Breast cancer case 23 | 0.11 | 0.11 | 100.00% | 0.12 |
| Breast cancer case 32 | 0.66 | 0.62 | 94.00% | 0.71 |
| Breast cancer case 33 | 0.11 | 0.12 | 109.00% | 0.11 |
| Breast cancer case 37 | 0.14 | 0.14 | 100.00% | 0.15 |
| Breast cancer case 48 | 0.11 | 0.1 | 91.00% | 0.1 |
| Breast cancer case 40 | 0.09 | 0.09 | 100.00% | 0.1 |
| Breast cancer case 42 | 0.12 | 0.13 | 108.00% | 0.1 |
| Cervical cancer case 5 | 0.13 | 0.13 | 100.00% | 0.13 |
| Cervical cancer case 8 | 0.17 | 0.18 | 106.00% | 0.18 |
| Cervical cancer case 14 | 0.14 | 0.15 | 107.00% | 0.14 |
| Cervical cancer case 23 | 0.18 | 0.19 | 106.00% | 0.18 |
| Cervical cancer case 26 | 0.17 | 0.16 | 94.00% | 0.17 |
| Cervical cancer case 32 | 0.34 | 0.36 | 106.00% | 0.35 |
| Cervical cancer case 36 | 0.18 | 0.18 | 100.00% | 0.2 |
| Cervical cancer case 41 | 0.44 | 0.46 | 105.00% | 0.4 |
| Cervical cancer case 48 | 0.18 | 0.19 | 106.00% | 0.18 |
| Cervical cancer case 60 | 0.14 | 0.16 | 114.00% | 0.12 |
| Endometrial cancer case 11 | 0.16 | 0.16 | 100.00% | 0.14 |
| Endometrial cancer case 21 | 0.36 | 0.38 | 106.00% | 0.32 |
| Endometrial cancer case 53 | 0.09 | 0.09 | 100.00% | 0.1 |
| Ovarian cancer case 2 | 0.23 | 0.24 | 104.00% | 0.23 |
| Ovarian cancer case 25 | 0.14 | 0.13 | 93.00% | 0.15 |
| Ovarian cancer case 48 | 0.11 | 0.1 | 91.00% | 0.1 |
| Ovarian cancer case 82 | 0.1 | 0.11 | 110.00% | 0.1 |
| Ovarian cancer case 143 | 0.1 | 0.09 | 90.00% | 0.08 |
| Vulvar cancer case 13 | 0.27 | 0.28 | 104.00% | 0.23 |
| Vulvae cancer case 39 | 0.13 | 0.13 | 100.00% | 0.12 |
| Mean ± standard deviation | 0.24 ± 0.29 | 0.25 ± 0.30 | 102% ± 6.2% | 0.26 ± 0.31 |
| Acetone concentrated pool of 58 cancer samples | 0.11 | 0.12 | 109.00% | 0.1 |
Table 2: Promotion of cancer cell invasion in quadruplicate 70% confluent Matrigel basement membrane invasion chambers by C5 hyperglycosylated hCG and its β-subunit.
Then the C5 hyperglycosylated hCG standard was tested, this was 100% detected by the B152 assay, confirming that this assay is specific for hyperglycosylated molecules. Finally, the assays were tested with C5 hyperglycosylated hCG dissociated β-subunit, this was also 100% detected by the B152 assay. Similarly, it was found that the FBT11 assay only detected a separated β-subunit, detecting recombinant hCG dissociated β- subunit and C5 dissociated β-subunit.
As published previously [30], trophoblastic cancers produce cancer hyperglycosylated hCG, and non-trophoblastic cancers produce hyperglycosylated hCG free β-subunit. Cancer hyperglycosylated hCG and cancer hyperglycosylated hCG free β-subunit has a β-subunit with triantennary N-linked oligosaccharides (Figure 1), while pregnancy hyperglycosylated hCG has bi-antennary N-linked oligosaccharides.
The trophoblastic cancers produce a hyperglycosylated hCG was confirmed by Cole LA, and that non-trophoblastic cancers produce a hyperglycosylated hCG free β-subunit. As shown (Table 1), pre-therapy, 32 trophoblastic cancers just produced hyperglycosylated hCG, B152 hyperglycosylated hCG assay mean detection 96% ± 12.5% of total hCG.
Non-trophoblastic cancers produced a free β-subunit (Table 1). 92 non-trophoblastic cancer serum samples were tested. Only 34 of the 92 cancer serum samples was detected by the Siemens Immulite total hCG assay. The balance were assumed to be producing low concentrations of this tumor agent. The B152 hyperglycosylated hCG and β-subunit assay detected 102% ± 6.2% of the total hCG immunoreactivity in the remaining 34 serum samples. This indicated that nontrophoblastic cancers produce a hyperglycosylated hCG β- subunit. It is concluded that all cancers secrete hyperglycosylated hCG or hyperglycosylated hCG β-subunit. As shown, both hyperglycosylated hCG and hyperglycosylated hCG β-subunit both act on a TGF-β-II receptor [16-18]. There was a concern about the 58 of 92 samples missed by this testing as having very low concentrations of hyperglycosylated hCG free β-subunit. Do those producing low concentrations also produce primarily hyperglycosylated hCG free β-subunit. The 58 samples were pooled, 3 ml of each, this gave me 174 ml. Using a method described previously [14], the 174 ml was concentrated using 3X acetone. This precipitated the urine. The precipitate was completely dissolved in 10 ml phosphatebuffered saline buffer and then tested as a 17.4X concentrate. The concentrate was tested in the Immulite, B152 and FBT11 assays (Table 1). It contained 0.11 ng/ml according to the Siemens Immulite total hCG assay, 0.12 ng/ml by the B152 hyperglycosylated hCG assay, and 0.10 ng/ml using the FBT11 free β-subunit assay. This confirmed that the low concentration Samples also primarily contained a hyperglycosylated hCG free β-subunit.
Many of the laboratories demonstrating that non-trophoblastic cancers respond to hyperglycosylated hCG free β-subunit, or to the molecule secreted by the cancers [31-34], have used hCG β-subunit, not hyperglycosylated hCG β-subunit, to promote a cancer response. As published, hyperglycosylated hCG is nicked and dissociated into a β-subunit to bind the TGF-β-II receptor [15]. The receptor will respond to hyperglycosylated hCG β-subunit and to hCG β-subunit [15]. The LH-hCG hormone receptor, however will not respond to hCG β-subunit.
It was concluded that trophoblastic cancers secrete cancer hyperglycosylated hCG dimer. It was also concluded that all non-trophoblastic cancers primarily secrete a hyperglycosylated hCG free β-subunit. As demonstrated previously, both cancer molecules act on a TGF-β-II receptor to elicit a growth, anti-apoptosis or invasion response [16,17,28].
2. Cancer hyperglycosylated hCG and hyperglycosylated hCG free β-subunit
Why is hyperglycosylated hCG two molecules, hyperglycosylated hCG and hyperglycosylated hCG free β- subunit? Trophoblastic cancer produce hyperglycosylated hCG α-β subunit dimer, and non-trophoblastic malignancies produce hyperglycosylated hCG free β-subunit. Both molecules are TGF-β-II receptor autocrines promoting cancer cell malignancy. The reason needed to deal with both the dimer and the free β-subunit is very much explained by Beebe et al. [29], and by Ruddon et al. [30]. To complete the synthesis of the β- subunit requires completion of 6 disulfide bridges. The last two disulfide bridges, β26-110 and β93-100, requires a specialized disulfide isomerase, only found in trophoblast cells and pituitary cells. Without this isomerase, construction of the β- subunit is not completed, and β-subunit does not combine with α-subunit. This produces a free β-subunit as made in non trophoblastic cancers. Only trophoblastic cancers make an α-β subunit dimer [29,30].
3. Hyperglycosylated hCG and hyperglycosylated hCG free β-subunit the malignancy molecules.
The malignancy properties of hyperglycosylated hCG and it free β-subunit were investigated by Cole LA. Multiple cancer cell lines were cultured on top of a Matrigel (Corning, Corning NY) basement membrane and the extent of penetration of the membrane by the cells determined. A wide variety of cancers was investigated (Table 3), ranging from choriocarcinoma cells to testicular germ cell malignancy cells, to cervical endometriod cancer cells, to lung epithelial cancer cells to Hodgkin lymphoma cancer cells. In each cell line control cells with no additive placed in medium (cells produce hyperglycosylated hCG or its free β-subunit naturally) were compared to cells with 100 ng/ml additive (Table 3).
| Cells Ttest vs 0 ng/ml | Supplement added to culture fluid % effect on cell count | % effect on cell count | P value |
|---|---|---|---|
| Jar choriocarcinoma | Hyperglycosylated hCG 20 ng/ml | 112% cell count after 24 h | P=0.0123 |
| Hyperglycosylated hCG 200 ng/ml | 130% cell count after 24 h | ||
| JEG-3 choriocarcinoma | Hyperglycosylated hCG 20 ng/ml | 110% cell count after 24 h | P=0.0018 |
| Hyperglycosylated hCG 200 ng/ml | 128% cell count after 24 h | ||
| NTERA Testicular germ cell | Hyperglycosylated hCG 20 ng/ml | 118% cell count after 24 h | P=0.0018 |
| Hyperglycosylated hCG 200 ng/ml | 132% cell count after 24 h | ||
| Hec-1-a endometrial cancer | Hyperglycosylated hCG β-subunit 20 ng/ml | 138% cell count after 24 h | P=0.0021 |
| Hyperglycosylated hCG β-subunit 200 ng/ml | 166% cell count after 24h | ||
| ScaBER squamous bladder cancer | Hyperglycosylated hCG β-subunit 20 ng/ml | 150% cell count after 24 h | P=0.010 |
| Hyperglycosylated hCG β-subunit 200 ng/ml | 156% cell count after 24h | ||
| KLE Endometrial adenocarcinoma | Hyperglycosylated hCG β-subunit 20 ng/ml | 117% cell count after 24 h | P=0.0.001 |
| Hyperglycosylated hCG β-subunit 200 ng/ml | 132% cell count after 24 h | ||
| SK-MES-1 Epithelial lung cancer | Hyperglycosylated hCG β-subunit 20 ng/ml | 134% cell count after 24 h | P=0.005 |
| Hyperglycosylated hCG β-subunit 200 ng/ml | 163% cell count after 24 h | ||
| KM-H2 Hodgkin’s Lymphoma cells | Hyperglycosylated hCG β-subunit 20 ng/ml | 120% cell count after 24 h | P=0011 |
| Hyperglycosylated hCG β -subunit 200 ng/ml | 145% cell count after 24 h | ||
| T24 Bladder epithelial carcinoma | Hyperglycosylated hCG β -subunit 20 ng/ml | 110% cell count after 24 h | P=0.123 |
| Hyperglycosylated hCG β -subunit 200 ng/ml | 128% cell count after 24 h | ||
| Caski epithelial cervical carcinoma | Hyperglycosylated hCG β -subunit 20 ng/ml | 115% cell count after 24 h | P=0.008 |
| Hyperglycosylated hCG β -subunit 200 ng/m | 142% cell count after 24 h |
Table 3. Promotion of cancer cell 24 h growth at 70% confluency by C5 hyperglycosylated hCG and its β-subunit.
Control cells penetrated the basement membrane, 36 ± 12% to 49 ± 15%. Additive cells penetrated the basement membrane to a much more significant extent 69 ± 6.5% to 88 ± 6.0%. Each cell line showed a significant difference between control cultures and additive cultures.
Multiple studies show that molecules acting on TGF-β-II receptors promote enzymatic cell to cell invasion by promoting production of metalloproteinases and collagenases [31-35]. It is concluded that hyperglycosylated hCG and it free β-subunit drive cancer cell metalloproteinases and collagenases production.
The action of hyperglycosylated hCG and its free β-subunit on cancer grow the was investigated. Ten different cultured cancer cell lines were cultured to 70% confluency, and then cultured with 20 ng/ml hyperglycosylated hCG and hyperglycosylated hCG free β-subunit, and then with 200 mg/ml hyperglycosylated hCG and hyperglycosylated hCG free β- subunit supplement. Cell growth was compared to no supplement control cultures (Table 3). A wide variety of cells was investigated, ranging from Jar and JEG-3 choriocarcinoma cells, NTERA testicular germ cell cancer cells, Hec-1-a and KLE endometrial cancer cells, ScaBER and T24 bladder cancer cells, SK-MES-1 lung cancer, Caski cervical cancer and KM-H2 lymphoma cells. The addition of 20 ng/ml hyperglycosylated hCG and hyperglycosylated hCG free β- subunit caused 110% to 134% enhancement in cell population, the addition of 200 ng/ml hyperglycosylated hCG and hyperglycosylated hCG free β-subunit caused a significantly greater (all additives caused a significant advancement in cell growth) enhancement in cell population, 128% to 163% of the control population.
Clearly, hyperglycosylated hCG and its free β-subunit enhance cell growth and promote cell to cell invasion through the actions of metalloproteinases and collagenases. Most of these studies have been repeated and confirmed by the 8 independent other groups who have very much confirmed our studies using a wide range of alternative cell lines [35-38]. Multiple other authors have also shown that hyperglycosylated hCG and it free β-subunit also blocks apoptosis, another action in malignancy [39]. All told, hyperglycosylated hCG and hyperglycosylated hCG free β-subunit promote the production of the invasive enzymes collagenases and metalloproteinases, promote cell growth, and block cellular apoptosis, or have all malignancy properties.
Having shown that hyperglycosylated hCG and hyperglycosylated hCG β-subunit promote all malignancy properties, it was necessary to show the occurrence of these tumor agents.
4. Tumor markers
The occurrence of hyperglycosylated hCG and its free β- subunit as cancer markers were investigated (Table 4). The serum hyperglycosylated hCG and its free β-subunit were measured using the B152 immunoassay, and the urine degradation products of hyperglycosylated hCG and hyperglycosylated hCG β-subunit, the terminal degradation product β-core fragment using the B204 immunoassay. The B204 immunoassay detects β-core fragment and nicked hyperglycosylated hCG β-subunit. The serum and urine on 110 trophoblastic cancer cases were examined, and serum on 849 non-trophoblastic cancer cases, and urine on 2,167 nontrophoblastic cancer cases (Table 4).
| Source | Serum, B152 assay | Urine B204 assay | ||||
|---|---|---|---|---|---|---|
| #Cases | #Detected | Sensitivity | #Cases | #Positive | Sensitivity | |
| Trophoblastic malignancies | ||||||
| Choriocarcinoma | 63 | 63 | 100.00% | 63 | 63 | 100.00% |
| Ovarian germ cell cancer | 30 | 30 | 100.00% | 11 | 11 | 100.00% |
| Testicular germ cell cancer | 17 | 17 | 100.00% | 17 | 17 | 100.00% |
| Total | 110 | 110 | 100.0% | 110 | 110 | 100.0% |
| Non-trophoblastic malignancy | ||||||
| Bladder cancer | 170 | 60 | 35.00% | 140 | 62 | 44.00% |
| Breast cancer | 42 | 15 | 36.00% | 456 | 156 | 34.00% |
| Cervical cancer | 60 | 37.00% | 410 | 197 | 48.00% | |
| Colorectal cancer | 136 | 23 | 17.00% | 80 | 29 | 36.00% |
| Endometrial cancer | 55 | 18 | 33.00% | 233 | 103 | 44.00% |
| Gastric cancer | 205 | 90 | 44.00% | |||
| Hepatic cancer | 46 | 21 | 44.00% | |||
| Lung cancer | 143 | 26 | 18.00% | 154 | 38 | 25.00% |
| Intestinal cancer | 17 | 8 | 47.00% | |||
| Lymphoma | 41 | 13 | 32.00% | |||
| Ovarian cancer | 150 | 57 | 38.00% | 207 | 145 | 70.00% |
| Pancreatic cancer | 29 | 10 | 33.00% | 29 | 16 | 55.00% |
| Prostate cancer | 12 | 9 | 75.00% | |||
| Renal cancer | 66 | 32 | 48.00% | |||
| Uterine cancer | 63 | 26 | 41.00% | |||
| Vulvar cancer | 64 | 26 | 41.00% | 8 | 4 | 50.00% |
| Total | 849 | 257 | 30.00% | 2167 | 949 | 44.00% |
| Healthy | ||||||
| NED, post cancer chemotherapy | 33 | 33 | 2 | 6.00% | ||
| NED, post cancer surgery | 21 | 21 | 1 | 5.00% | ||
| Healthy female, no cancer history | 72 | 72 | 2 | 3.00% | ||
| Healthy male, no cancer history | 28 | 28 | 1 | 4.00% | ||
| Total | 154 | 154 | 6 | 4.00% | ||
| Benign Disease | ||||||
| Benign gynecological lesion, tumor | 28 | 0 | 0.00% | |||
| Benign lung lesion | 4 | 0 | 0.00% | |||
| Follicular ovarian cyst, benign | 67 | 0 | 0.00% | |||
| Benign endometrial wart | 53 | 0 | 0.00% | |||
| Benign ovarian cyst, non-functional | 26 | 0 | 0.00% | |||
| Benign prostate hyperplasia | 8 | 0 | 0.00% | |||
| Cervical carcinoma in-situ | 12 | 0 | 0.00% | |||
| Cervical dyskaryosis | 66 | 0 | 0.00% | |||
| Condyloma | 30 | 0 | 0.00% | |||
| Endometriosis | 16 | 0 | 0.00% | |||
| Myoma | 27 | 0 | 0.00% | |||
| Total | 337 | 0 | 0.00% | |||
Table 4. Serum hyperglycosylated hCG+β-subunit (B152 assay) and urine degradation product hyperglycosylated hCG β-subunit+β-core fragment (B204 assay) as tumor markers.
As found, 100% of trophoblastic cancer case were detected in serum and urine (Table 4). In contrast, an average of 30% of non-trophoblastic cancer cases were detected in serum, and 44% of cases in urine samples. The higher detection in urine may be due to the rapid blood clearance of hyperglycosylated hCG free β-subunit and its degradation products.
Detection of non-trophoblastic cancers varied greatly according to cancer site. In serum, detection ranged from 17% with colorectal cancers to 38% with ovarian cancers. In urine, using the B204 assay, detection ranged from 34% with breast cancers to 70% with ovarian malignancies (Table 4). Questions have been raised on why 34% to 70% and not 100%, since 100% of cancers show malignancy?
5. Simple and complex autocrines
There are two kinds of TGF-β autocrines, “simple autocrines” and “complex autocrines” [40-43]. Simple autocrines are produced at low concentration, are secreted and act directly on the cancer cell TGF-β-II receptor without necessarily circulating [44] Complex autocrines are produced at higher concentration. They are secreted, and circulate in the blood stream before acting back on the original cancer cell TGF-β-II receptor. Are the cancers simple autocrine or complex autocrine TGF-β-II receptor cases? Does simple autocrine production explain the lower detection, 34%-70% in nontrophoblastic cancers (Table 4)?
To address this issue 56 new non-treated ovarian cancer cases were tested providing urine samples with the B204 assay. The current assay used has a sensitivity of 1.4 fmol/ml, and the cut off in the tumor marker assay of 3.0 fmol/ml. A significantly more sensitive assay was developed by 10-fold reducing the plating concentration of B204 antibody, and by extending the first incubation time from 2 h to 6 h. The new super-sensitive assay detected down to 0.08 fmol/ml, and has a tumor marker cut-off of 0.10 fmol/ml. This assay was used to test the 56 ovarian cancer urines Table 5.
| Pathologic Diagnosis | Stage | Status | β-core fragment |
|---|---|---|---|
| Ovarian Endometrioid carcinoma | IV | Persistent | 0.12 |
| Ovarian Endometrioid carcinoma | IV | New, not treated | 0.15 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 0.2 |
| Ovarian Serous cystadenocarcinoma | IIIc | Persistent | 0.2 |
| Ovarian Serous cystadenocarcinoma | IIIc | Recurrent | 0.25 |
| Ovarian Serous cystadenocarcinoma | IV | Recurrent | 0.4 |
| Ovarian Serous cystadenocarcinoma | IV | Recurrent | 0.4 |
| Ovarian Brenner tumor | Ia | New, not treated | 0.58 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 0.6 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 0.75 |
| Ovarian Brenner tumor | Ia | New, not treated | 0.88 |
| Ovarian Serous cystadenocarcinomacucystcancerccancercystadenocarcinoma | IIIc | Recurrent | 1.2 |
| Serous cystadenocarcinoma | II | Recurrent | 1.6 |
| Ovarian Serous cystadenocarcinoma | IIIc | Recurrent | 2.1 |
| Ovarian Endometrioid carcinoma | III | Recurrent | 2,3 |
| Ovarian Serous cystadenocarcinoma | IIIc | Persistent | 2.5 |
| Ovarian Mucinous ccarcinomacarcinomacystadenocarcinoma | Ia | New, not treated | 2.9 |
| Ovarian Endometrioid carcinoma | I | New, not treated | 3.1 |
| Ovarian Granulosa-theca cell malignancy | IIIc | Recurrent | 3.1 |
| Ovarian Serous cystadenocarcinoma | II | New, not treated | 3.5 |
| Ovarian Granulosa-theca cell malignancy | IIIc | Persisent | 3.7 |
| Ovarian Mucinous cystadenocarcinoma | III | Recurrent | 4.2 |
| Ovarian Serous cystadenocarcinoma | III | New, not treated | 4.5 |
| Ovarian Serous cystadenocarcinoma | III | New, not treated | 4.3 |
| Ovarian Clear cell carcinoma | IIIc | New, not treated | 4.8 |
| Ovarian Clear cell carcinoma | IIIc | Recurrent | 5.5 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 6.3 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 6.6 |
| Ovarian Mixed epithelial tumor | IIIc | New, not treated | 7.1 |
| Ovarian Mixed epithelial tumor | III | New, not treated | 7.9 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 8.1 |
| Ovarian Serous cystadenocarcinoma | IIIc | Persistent | 8.8 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 9 |
| Ovarian Serous cystadenocarcinoma | III | Persistent | 9.5 |
| Ovarian Mixed epithelial tumor | IIc | New, not treated | 10 |
| Ovarian Mixed epithelial tumour | IIb | New, not treated | 11.3 |
| Ovarian Serous cystadenocarcinoma | III | New, not treated | 11.4 |
| Ovarian Serous cystadenocarcinoma | IV | Recurrent | 11.4 |
| Ovarian Serous cystadenocarcinoma | IV | Recurrent | 12 |
| Ovarian Serous cystadenocarcinoma | IIIc | Recurrent | 12 |
| Ovarian Serous cystadenocarcinoma | IIIc | Recurrent | 12 |
| Ovarian Endometrioid carcinoma | IIc | New, not treated | 12.2 |
| Ovarian Endometrioid carcinoma | III | Persistent | 13.1 |
| Ovarian Mixed mesodermal carcinoma | III | Recurrent | 14.4 |
| Ovarian Mixed mesodermal carcinoma | III | Recurrent | 16.3 |
| Ovarian Serous cystadenocarcinoma | IV | Recurrent | 16.9 |
| Ovarian Serous cystadenocarcinoma | IV | Recurrent | 17.5 |
| Ovarian Serous cystadenocarcinoma | IIIc | New, not treated | 18.8 |
| Ovarian Serous cystadenocarcinoma | IIIc | Persistent | 20 |
| Ovarian Mixed epithelial tumor | IIIc | Recurrent | 20 |
| Ovarian Mixed epithelial tumor | III | Recurrent | 21 |
| Ovarian Serous cystadenocarcinoma | IV | New, not treated | 28 |
| Ovarian Serous cystadenocarcinoma | IV | New, not treated | 29 |
| Ovarian Serous cystadenocarcinoma | III | New, not treated | 32 |
| Ovarian Serous cystadenocarcinoma | IIb | New, not treated | 41 |
| Ovarian Malignant dermoid cyst/teratoma | IIb | New, not treated | 54 |
Table 5. Ovarian cancer super-sensitive urine B204 assay. Tumor marker study of 56 ovarian cancer cases receiving no therapy using super-sensitive B204 assay (sensitivity>0.1 fmol/ml).
As shown in Table 5, 56 of 56 cancer urines were positive using the super-sensitive B204 assay. Thirty-nine (70%) of the urines would be detected by a tumor marker assay with 3.0 fmol/ml cut-off, and 56 of 56 urine (100%) would be detected in the assay using the new 0.1 fmol/ml cut-off.
It was concluded that thirty-nine cases probably produced a complex autocrine (>3.0 fmol/ml cut-off samples), and the balance, seventeen cases, probably produced tiny concentrations (<3.0 fmol/ml), or were simple autocrines. It was clear that 100% of cases produced hyperglycosylated hCG and its free β-subunit. Seemingly 100% of all cancers, as simple autocrine and complex autocrine TGF-β-II cancers.
In support of the concept that all cancers produced simple and complex autocrines, Acevedo et al. [45-47] used