Review Article - Biomedical Research (2016) Health Science and Bio Convergence Technology: Edition-I
Hyperglycemic memory and its long term effects in diabetes
Subhashree Venugopal*
Biomolecules and Genetics division, School of Biosciences and Technology, VIT University, Vellore, Tamilnadu, India
- *Corresponding Author:
- Subhashree Venugopal
Biomolecules and Genetics division
School of Biosciences and Technology
VIT University
India
Accepted date: April 12, 2016
Abstract
A number of randomized trials suggest that glycemic control in the initial stages decreases the macro vascular outcome and complications such as retinopathy, nephropathy and neuropathy due to micro vascular changes. Epidemiological studies indicate that memory effect continues and complications develop even after return to normoglycemia in hyperglycemic diabetic patients. Metabolic abnormalities that occur during diabetes stimulate the production of reactive oxygen species in mitochondria and hyperglycemic memory initiates a vicious cycle of ROS induced mutations in mitochondrial DNA. Interaction of advanced glycation end products (AGEs) with its receptor (RAGE) has played an important role in the pathogenesis of diabetes and its complications. AGEs upregulate RAGE mRNA levels by promoting intracellular ROS (reactive oxygen species) generation. Hence, to overcome the long term complications which develop due to hyperglycemia, therapeutic interventions to reduce the formation of oxygen radicals and block the formation of advanced glycation end products is crucial.
Keywords
Poor glycemic control, Good glycemic control, Hyperglycemic memory, Oxidative stress, Advanced glycation end products.
Introduction
The number of people affected by type 2 diabetes has widely risen over the past several years and has reached epidemic worldwide. It is estimated to reach 366 million throughout the world by 2030 [1]. Diabetes is a health problem of serious concern which is the cause for morbidity and decreased life anticipation due to the complications specific for the disorder. The main symptom which predominates is hyperglycemia, which can be controlled by administration of drugs that increases the insulin secretion or by administration of exogenous insulin that increases the use of glucose in the skeletal muscles, decrease release of glucose from the liver and delays the glucose absorption from intestine [2]. Observations made from various clinical studies of major importance that includes DCCT, EDIC, UKPDS and steno-2 have provided sufficient information that aggressive intensive treatment for restoring blood glucose to normal levels in the beginning stages of pre-diabetes and diabetes has far better impact over the long term to prevent micro vascular and macro vascular complications.
A profound decrease in the complications of chronic diabetes has been noticed in patients subjected to intense treatment in the initial stages of hyperglycemia. While for other patients, when the treatment was delayed, the vascular endothelial cells were subjected to an array of adverse vascular changes. Subsequent normoglycemic conditions cannot prevent or reverse the morphological alterations in vascular beds typical of micro or macroangiopathy [3-6]. The persistent negative effects of hyperglycemia and the long term beneficial effects of decreasing blood glucose in early stages of development of complications has been explained as metabolic memory [4]. The possible explanations for this phenomenon are the slow accumulation and subsequently slow degradation of advanced glycation end products. Enhanced oxidative stress and changes in antioxidant capacity, found in both clinical and experimental diabetes, are thought to be the main cause of chronic diabetic complications [7]. High levels of free radicals cause damage to vital cellular components such as proteins, membrane lipids, and nucleic acids, and finally lead to cell death [8].
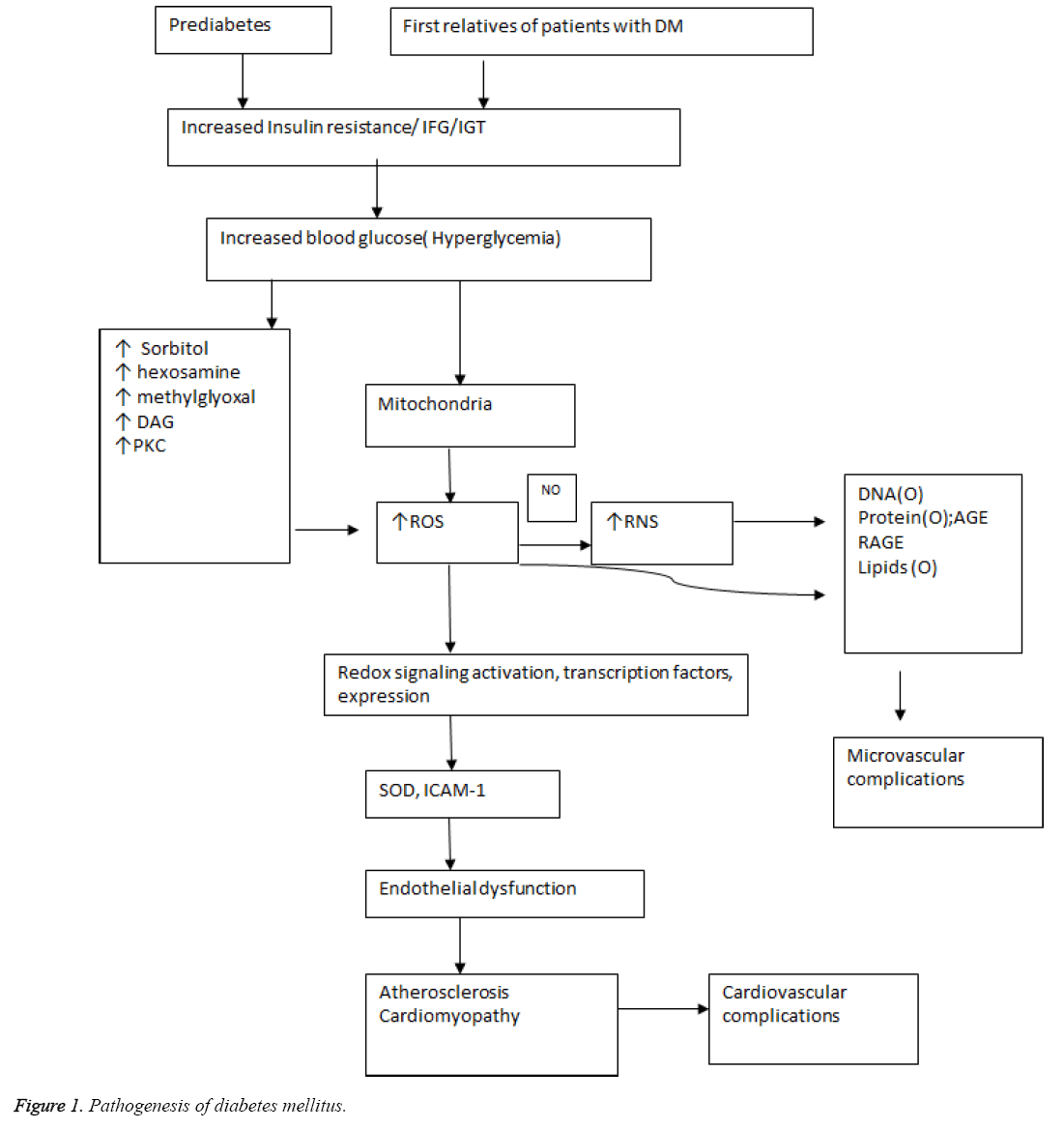
Hyperglycemia activates protein kinase C which increase levels of NADPH oxidase and triggers the production of excess ROS that leads to oxidative stress [9]. Production of AGE also activates NADP oxidase and ROS generation that culminates in oxidative stress and tissue factor release. Excess glucose also activates hexosamine and polyol pathway that augments the ROS production that induces the over-expression of growth factors and cytokines [10]. The pathogenesis of diabetes mellitus and its complications is outlined in Figure 1. Prediabetes which proceeds to hyperglycemic diabetic state progresses further to micro or macrovascular complications with increase in common carotid intima media thickening, increased brachial-ankle pulse wave velocity that increases stiffening of arteries with impaired coronary vessel endothelial function that increases risk for cardiovascular disease. Similarly, a family history of diabetes with first degree relatives suffering from diabetes mellitus develop insulin resistance with decreased NO production that causes endothelial dysfunction and predisposes to diabetic cardiomyopathy with greater susceptibility to cardiovascular damage [11]. Numerous studies have demonstrated that calcium (Ca2+) plays a pivotal role in insulin secretion from the islets of Langerhans and that altered cellular Ca2+ homeostasis may be involved in defective insulin release [12].
Preclinical Evidence
Evidence from experimental models
One of the studies which suggested that metabolic derangement and changes in tissue architecture occurring during good metabolic control can have long lasting sequelae in the system was conducted by Roy et al. The aim of the study was to evaluate whether insulin administration could reduce the expression of fibronectin in Sprague dawley rats. The levels of fibronectin mRNA were elevated in renal cortex and heart of insulin treated animals compared to diabetic animals despite near normalization of blood glucose. In cultured human endothelial cells compared in three groups, cells exposed to normal glucose, high glucose and shift from high to normal glucose at third subculture, levels of collagen IV and fibrinogen was higher in the cells shifted to normal glucose compared to control. Thus, some of the effect of high glucose in the early stages persists beyond its withdrawal and the persistently elevated mRNA levels may reflect heritable cellular changes [13].
In a study conducted by Hammes et al. syngeneic transplantation of islets after 6 weeks of diabetes prevented endothelial cell proliferation and diminished pericyte loss, but transplantation after 12 weeks of diabetes only partially restored capillary cell composition and did not prevent retinal vessel occlusion. Animals which were untreated, suffered acellular occluded vessels, micro aneurysm, increase in capillary endothelial cells. Hence the findings suggest that metabolic control in earlier stages decreases the effect of metabolic memory and complications [14]. The investigation performed by Kowluru in wistar rats, the objective of the study was to find the effect of good glycemic control on oxidative and nitrative stress in hyperglycemic conditions. Diabetes was induced by streptozotocin administration intraperitoneally and rats which were maintained in poor control for 13 months received 1-2 units of insulin 4-5 times a week, whereas rats maintained in good control received 8-10 units of insulin to maintain blood glucose and ketosis. In other two groups, rats were maintained in poor control (HbA1c>11%) for 2-6 months followed by good control for 7 months (HbA1c<5.5%). The rats with poor control exhibited elevated lipoperoxide, NO, iNOS and peroxynitrite but decreased glutathione.
However, 2 months poor control followed by 7 months GC, showed significant decrease in glutathione and a vast decrease in lipoperoxide levels and 70% decrease in NO and iNOS. However, reinstitution of good control after 6 months did not show significant decrease in glutathione or NO. Thus, reversal of blood glucose to normal conditions did not reverse the stress marker released due to oxidative stress. Nitrotyrosylated proteins and iNOS expression was not varied in retina of GC. Thus the variation in time duration for achieving GC in PC groups contributes to difference in oxidative stress marker expression. The modifications in retina due to oxidative or nitrative stress which begins early in retinopathy is not reversible by GC reinstitution [7]. Engerman and Kern, in an experiment examined to find the extent to which glycemic control could inhibit the progression of diabetic neuropathy. Dogs were randomly distributed into three groups with alloxan induced diabetes mellitus. The first group comprised of poor control for 5 years, the second group being good control for 5 years, and the third group with poor control for 2.5 years and good control for 2.5 years by administration of insulin. The control group was non-diabetic. In poorly controlled dogs, retinal capillary aneurysms and other histological lesions were observed, but if good control was initiated within the first two months, such morphological changes were inhibited.
But no retinopathy was observed in the first 2.5 years of poor control, but later developed during good control and thus retinopathy resists treatment even in the beginning stages before aneurysms develop [15]. In a study conducted by Yorek et al. it was aimed at finding the impact of hyperglycemic period and treatment on corneal nerve fiber density and neuropathy end points. The study design consisted of C57B1/6J mice designed as PC, GC and PC switched to GC. Conduction velocity of motor and sensory nerves slowed down after 4 and 8 weeks of hyperglycemia in diabetic mice, whereas loss of corneal nerves in corneal epithelium required 20 weeks to reach significant level. Though the average blood glucose levels were decreased in mice with GC, it was not sufficient in preventing diabetic neuropathy end points [8].
Evidence from clinical studies
Tan et al. made a hypothesis on the role of activation of receptor for advanced glycation end products in development of vascular complications in diabetes mellitus. Diabetic patients were divided into categories based on proteinuria, microalbuminuria or normoalbuminuria. The soluble form of receptor for advanced glycation end products (sRAGE) was highest in proteinuric patients and there was a significant trend with nephropathy. Levels of advanced glycation end products and creatinine were the independent factors determining the concentration of sRAGE The study infers that further prospective studies are in need to find the importance of sRAGE in development of vascular complications [16]. The study contributed by Devangelio et al. focused on up regulation of ligand- RAGE axis with impairment of NO synthesis, due to increased oxidative stress, 86 caucasian patients with type 2 diabetes were studied against 43 control subjects for sRAGE , Asymmetric Dimethyl Arginine (ADMA) and urinary-8-iso prostaglandin. sRAGE levels were significantly decreased but ADMA levels were significantly increased in diabetic patients compared to controls. A statistically significant inverse correlation was found between HbA1c and sRAGE and a direct one between HbA1c and ADMA. Twenty-four of 86 patients with newly diagnosed diabetes and 12 patients in poor metabolic control were reevaluated after treatment with a hypoglycemic agent or insulin, respectively. Improvement in metabolic control by oral agents or insulin resulted in a significant increase in sRAGE and decrease in ADMA levels Thus, poor glycemic control reduces sRAGE levels, in association with enhanced oxidative stress and endothelial dysfunction in diabetes. This study suggests that these abnormalities are susceptible to modulation by improvement in metabolic control [17].
A study was performed by Mishra and Singh in type I diabetic subjects. The study aimed at investigating the effect of glycemic control on lipid profile and blood viscosity. The design included three groups of study with control subjects, poor glycemic control and good glycemic control. All the diabetic patients were on insulin therapy for 5 to 15 years. The malondialdehyde levels were significantly increased in poor glycemic control compared to nonsignificant changes in good glycemic control, but viscosity changes were not significant in both the groups compared to control. Hence good glycemic control helps in decreasing the lipid profile and blood viscosity that might help to prevent the onset of diabetic complications [18]. In another investigation performed by Kostolanska et al. oxidative stress parameters were evaluated in hyperglycemic conditions. The protein glycation levels and advance oxidation protein products were measured in patients with poor glycemic control, good glycemic control against controls. The levels of serum advanced glycation end products, fructosamine and glycated hemoglobin were significantly elevated in patients with poor glycemic control than with good glycemic control compared to controls though advanced oxidation protein products increase was not significant in poor glycemic control. Hence the oxidative and glycative parameters vary with extent of glycemic control and are a major contributing factor in the development of diabetic complications [19].
Nakamura et al. examined whether sRAGE were correlated to circulating levels of AGEs and soluble forms of vascular cell adhesion molecule-1 (sVCAM-1) and intercellular adhesion molecule-1 (sICAM-1) in patients with type 2 diabetes. Eightytwo Japanese type 2 diabetic patients underwent a complete history and physical examination, determination of blood chemistries, sRAGE, AGEs, sVCAM-1 and sICAM-1. When the diabetic patients with diabetic nephropathy and those without diabetic nephropathy were analyzed separately, circulating sVCAM-1 in patients without diabetic nephropathy, and AGEs and sVCAM-1 in patients with diabetic nephropathy were identified as independent determinants of sRAGE. Hence, the observations suggest that sRAGE level may be enhanced depending on circulating AGEs, thus being a novel marker of vascular injury in patients with type 2 diabetes [20].
In an another study by Motawi et al. which was designed to evaluate the effect of glycemic control on sRAGE and oxidative stress markers in type 2 diabetic patients, seventy patients with type 2 diabetes and 20 healthy subjects participated in the study. Blood glutathione, plasma total nitric oxide, superoxide dismutase activity, plasma C-peptide, oxidized LDL, sRAGE, and VCAM-1 levels were measured. Plasma sRAGE levels, blood glutathione were significantly lower while VCAM-1 levels were significantly higher in poorly controlled diabetic patients compared with healthy control. Plasma C-peptide, nitric oxide, oxidized LDL levels, and SOD activity were not significantly different in diabetic patients compared with healthy control. Plasma levels of sRAGE were negatively associated with circulating VCAM-1 levels in diabetic patients. Thus, poor glycemic control decreases plasma sRAGE and increases VCAM-1 levels while good glycemic control improves these abnormalities which provide benefit to diabetic patients [21].
Physical activity plays a role in delaying and even prevent the progression of type 2 diabetes by improving the antioxidative levels and thereby lowering systemic oxidative stress. Peroxiredoxins (PRDX) are highly abundant in RBCs. In a study which examines the influence of glycemic control and physical fitness on oxidative stress and the peroxiredoxin system in the RBCs of 22 type 2 diabetic men at rest, oxidative stress was measured by 8-iso-prostaglandin-F2α (8-Iso-PGF) and the overoxidized form of peroxiredoxins. Peroxiredoxin isoforms PRDX1 and PRDX2 were also measured. A positive relationship was found between 8-Iso-PGF plotted against HbA1c, fasting glucose. Poor glycemic control may increase oxidative stress in the erythrocytes of type 2 diabetic men. Good physical fitness is associated with increased peroxiredoxin. Hence, it can be speculated that physical training can contribute to the improvement of the erythrocyte peroxiredoxin system to overcome free radical stress in type 2 diabetic patients [22].
Oxidative Stress and Hyperglycemic Memory
Excess production of superoxide radicals in hyperglycemia has been suggested as the “unifying hypothesis” for the development of complications of diabetes. This suggests that mitochondria are also important contributors in propagating the “metabolic memory”. Methylglyoxal (MGO) levels, a highly reactive α-dicarbonyl by-product of glycolysis, are enhanced in diabetes. MGO readily reacts with arginine, lysine and sulfhydryl groups of proteins in addition to nucleic acids, inducing the formation of a variety of structurally identified AGEs, both in target cells and in the plasma. Advanced glycation end products accumulate due to excess blood glucose, oxygen radicals, enhanced activity of polyol pathway which mediates inflammation and glycation of proteins and lipids that promotes vascular damage, Hence, AGE acting through the receptors also is a main contributing factor in hyperglycemic memory.
A study was conducted to investigate the status of oxidative stress and nitric oxide related parameters in type II Diabetes Mellitus (DM) patients with micro and macrovascular complications. To find the effect of extent of glycemic control, erythrocyte copper zinc-superoxide dismutase (Cu Zn-SOD), erythrocyte and plasma selenium dependent glutathione peroxidase (Se-GPx), erythrocyte catalase (CAT) activities, erythrocyte and plasma thiobarbituric acid reactive substances (TBARS) levels; nitrite/nitrate (NO2-/NO3-), cyclic guanosine monophosphate (cGMP) and nitrotyrosine levels were measured in plasma of type II DM patients. Erythrocyte CuZn-SOD activities and TBARS levels in type II DM were significantly higher than those of the control subjects.
Plasma NO2-/NO3- levels in type II DM patients both during poor glycemic control and after three months of oral antidiabetic treatment were significantly higher than those of the control subjects. Plasma cGMP levels in type II DM patients during poor glycemic control were significantly lower than those of control. Increased Cu Zn-SOD activity in type II DM patients activity could not protect the patients against the Reactive Oxygen Species (ROS), since lipid peroxidation (defined by erythrocyte and plasma TBARS levels) still occurs in DM patients. After the therapy with oral antidiabetic agents for three months, erythrocyte SE-GPx and CAT activities were found to be reduced below the control values. The results suggested that oxidative status and nitric oxide metabolism are affected in type II DM patients and the decreased cGMP levels in the study may be a good marker of endothelium dysfunction in DM [23].
Karima et al. performed a clinical study to find the role of inflammation and oxidative stress in the pathogenesis of diabetic complications. The study was conducted in 50 diabetic patients, 45 non-diabetic subjects and 6 patients with chronic periodontitis. The patients were grouped based on glycemic control as Good Control (GC), Moderate Control (MC) and Poor Control (PC). Neutrophils from diabetic patients with GC released increased amounts of superoxide which was not statistically significant. Superoxide released by moderate or poor control patients were 2 to 3 times higher than normal neutrophils stimulated with phorbol myristyl acetate. Also, neutrophils of moderate or poor control exhibited higher protein kinase C (PKC) activity and increased diglyceride and enhanced phosphorylation of p47-phox, during cell stimulation leading to increased superoxide generation. There is a significant correlation between glycemic control (HbA1c) levels) and the severity of periodontitis in diabetic patients, suggesting that enhanced oxidative stress and increased inflammation exacerbate both diseases. Thus, hyperglycemia can lead to a novel form of neutrophil priming, where elevated PKC (Protein Kinase C) activity results in increased phosphorylation of p47-phox and superoxide release [24].
Cakatay, made an investigation to depict the role of plasma protein carbonyl (PCO) and Advanced oxidation protein product levels of diabetic patients (AOPP). AOPP with poor glycemic control (HbA1c>7%) were increased significantly compared with those of the diabetic patients with good glycemic control (HbA1c ≤ 7%). The decreased plasma T-SH (total thiol) level in the diabetic patients with poor glycemic control was not statistically significant. On the other hand, plasma LHP (lipid hydro peroxide) levels were increased significantly in the diabetic patients with poor GC compared with those of the diabetic patients with good glycemic control. This study supports the hypothesis that poor glycemic control is an important factor in generation of increased protein oxidation in type 2 diabetic patients clinically free of complications. Thus the outcome of the study is that, increase in plasma PCO, AOPP, and LHP levels in the diabetic patients with poor glycemic control may contribute to the development of diabetic complications [25].
The evaluation of oxidative stress markers in young type 1 diabetics suggested that Advanced Oxidation Protein Products (AOPP), Thiobarbituric Acid-Reactive Substances (TBARS) were increased and Total Antioxidant Status (TAS) were decreased. AOPP and TAS were good indicators of diabetes. AOPP and TBARS correlated with HbA1C (independent predictor), but were poor markers of non-adequate glycemic control. Hence the study concludes that AOPP accumulation and TAS decrease develop before diabetes and hence can be considered as susceptible indicators in relatives, but not as diabetic markers in general population [26]. In a study by Piwowar et al. Ischemia-Modified Albumin (IMA) was evaluated in patients with type 2 diabetes mellitus patients. The study was conducted in 76 diabetics and 25 control subjects and levels of IMA, glucose , fructosamine, HbA1c, total cholesterol and triglycerides were determined. Vascular late complications in diabetics were recognized and 19 had microangiopathy, 31 had macroangiopathy and 26 had both. The patients of last two groups had myocardial ischemia. Diabetic patients exhibited a higher level of IMA compared to control and patients with PC showed a higher IMA level than GC. A significant correlation was detected between IMA and HbA1c. Hence the higher IMA in PC of non-cardiac origin is provoked during oxidative stress conditions in diabetes by hyperglycemia [27].
Raffaelli et al. made an in vitro study of Syzygium cumini (L.) (Sc) on platelets in 48 PC and 29 GC patients with diabetes after in vitro Sc incubation and revealed that the activities of NO, total antioxidant capacity and SOD increased , the lipid peroxide levels decreased. A collagen induced decrease in aggregation of platelets was observed. Hence, the study suggests the possibility of Syzygium cumini (L.) in management of diabetes and protective effect in oxidative damage progression [28]. Lausten et al. examined the cause for poor glycemic control despite insulin administration. Wistar rats were subjected to insulopenic diabetes by streptozotocin. 6 randomly chosen rats were treated with insulin and controls were not subjected to insulin treatment. The markers of oxidative stress damage in kidney (TBARS and protein carbonyl) were similar in both the groups but plasma TBARS was decreased in diabetics receiving insulin. The increased pyruvate utilization in kidneys resulted in increased lactate to pyruvate ratio in diabetic insulin treated rats compared to untreated diabetic rats indicating increased anaerobic metabolic flux. Also an increased bicarbonate pyruvate ratio indicates increased aerobic flux in diabetic insulin treated rats. Thus, accelerated pyruvate use after insulin administration indicates that the metabolic disturbances in diabetic kidney during poor control are limited by substrate availability, indicating a novel therapeutic target to treat diabetic nephropathy [29].
Gohel and Chacko evaluated serum gamma glutamyl transferase and hsCRP (high sensitivity C reactive protein) in type 2 diabetes with good and poor control. The cross sectional study consisted of 150 patients with equal number of patients having type 2 DM with good control, with poor control and normal healthy control. Serum GGT, HbA1c, hsCRP and other biochemical parameters were measured. Mean serum GGT (gamma glutamyl transferase) and hsCRP concentration were statistically significantly higher in PC patients compared to GC and healthy control subjects. A significant positive correlation was observed between GGT and hsCRP concentration as well as both with HbA1c, fasting blood glucose and post prandial 2 hour blood glucose. Hence the study suggests a link between oxidative stress (increased GGT), inflammation (increased hsCRP) and glycemic control (type 2 DM). Hence, oxidative stress and inflammation are the major contributors with poor control and diabetic complications [30]. Shen et al. showed that brachial ankle pulse wave velocity (baPWV), an early marker of atherosclerosis, was significantly higher in subjects with HbA1C between 5.7%-6.4%and IFG as compared to subjects with normoglycaemia [31]. These results presented additional evidence to support the hypothesis that early development of adverse vascular changes already existed prior to the development of overt diabetes, suggesting that the strict glycemic control in pre-diabetic subjects might achieve a positive long-term protection against atherosclerosis.
The mechanistic role of type 2 ryanodine receptor/Ca2+ release channel (RyR2), which is expressed on the endoplasmic reticulum (ER) of pancreatic β cells in type 2 DM, is accompanied by chronic intracellular Ca2+ leak via RyR2 channels that causes store depletion, triggering ER stress and mitochondrial dysfunction, leading to reduced ATP synthesis and eventually decreased glucose stimulated insulin release, indicating an altered metabolism-secretion coupling. Impaired mitochondrial fitness also leads to increased production of ROS, which trigger redox modifications of RyR2 alongside calstabin2 dissociation, thereby exacerbating the Ca2+ leak [12]. One limitation of the study is that the genetically altered mice used are not pancreatic β cell specific, but general knockins. On the one hand, this accurately reflects the human condition, as the CPVT patients are heterozygous for RyR2 mutations which are expressed throughout the body. However, the possibility that leaky RyR2 in other organs also contribute to the altered glucose metabolism observed in humans and mice with RyR2 mutations cannot be excluded. Also, an arginine test was not performed to assess the maximal insulin response. Such a test could have helped distinguish between the role of RyR2 in acute function versus the maintenance of β cell mass [32] (Table 1).
| S. No | Reference | Objective | Result | Inference |
|---|---|---|---|---|
| 1 | Royetal. [13] | Insulinadministrationdecreasesfibronectinexpression | Fibronectinlevels↑ininsulintreatedthanuntreated | Hyperglycemiceffectinearlystagespersistbeyondwithdrawal |
| 2 | Hammesetal. [14] | Islettransplantationreducesdiabeticcomplications | After6weeks-preventedendothelialcellproliferation;after12weeks-partialeffect | Metaboliccontrolinearlystagespreventeffectofmetabolicmemory |
| 3 | Kowluru et al. [7] | Effectofgoodglycemiccontrolonoxidativeandnitrativestress | PC-↓GSH,↑LPO,NO,iNOS;PC2GC7-↓GSH,↑LPO;70%↓NO,iNOS | ChangesduringPCnotreversibleduringGC |
| 4 | EngermanandKern [15] | Extentofglycemiccontrolondiabeticneuropathy | PC-capillaryaneurysm;histologicallesion;GC-nochanges;PC-GC-resiststreamentanddiseaseprogresses | Earliercontroldecreasespostcomplications |
| 5 | Yoreketal. [8] | Impactofdurationofhyperglycemiaandtreatmentonneuropathyendpoints | Conductionvelocityofsensoryandmotornervesdecreased | Bloodglucosedecreasedoesnotpreventneuropathyendpoints |
| 6 | Tanetal. [16] | RoleofactivationofRAGEinvascularcomplications | sRAGE↑-proteinuria | FurtherprospectivestudyneededtoexplainimportanceofsRAGE |
| 7 | Devangelio et al. [17] | Upregulationofligand-RAGEaxiswithimpairmentofNOsynthesis,duetoincreasedoxidativestress | sRAGE↓,ADMA↑-PCdiabetes;sRAGE↑,ADMA↓-GC | Metaboliccontrol-decreasesabnormalities |
| 8 | MishraandSingh [18] | effectofglycemiccontrolonlipidprofileandbloodviscosity | Malondialdehyde↑-PC | GC-↓lipidprofile,viscosityofblood |
| 9 | Kostolanskaetal. [19] | oxidativestressparameterswereevaluated | ↑AGE,↑HbA1c,↑fructosmamine-PC | oxidativeandglycativeparametersvarywithextentofglycemiccontrol |
| 10 | Nakamuraetal. [20] | sRAGElevelscorrelatedwithAGE,VCAM-1/ICAM-1 | AGE,sVCAM-1-deteminantofsRAGEinDM | sRAGE↑dependingoncirculatingAGE |
| 11 | Motawietal. [21] | effectofglycemiccontrolonsRAGE | sRAGE,GSH-↓,VCAM-1↑-PC | GC-↑sRAGEand↓VCAM-1 |
| 12 | Brinkmannetal. [22] | influenceofglycemiccontrolandphysicalfitnessonoxidativestress | positivecorrelationbetween8-iso-PGFandHbA1c | Physicaltrainingimprovesperoxiredoxinsystem |
| 13 | Aydin et al. [23] | StatusofoxidativestressandnitricoxiderelatedparametersintypeIIdiabetesmellitus(DM) | Cu-ZnSOD,TBARS-↑;cGMP-↓ | cGMP-↓;goodmarkerforendothellialdysfunction |
| 14 | Karimaetal. [24] | Roleofinflammationandoxidativestress | PC-↑PKC,DG,superoxide | correlationbetweenHbA1candperiodontitis |
| 15 | Cakatay [25] | roleofplasmaproteincarbonyl(PCO)andAdvancedoxidationproteinproductlevelsofdiabeticpatients(AOPP) | LHP,AOPP↑-PC; | IncreaseinplasmaPCO,AOPP,andLHP-PC-diabeticcomplicaitions |
| 16 | KorpackaKMetal. [26] | OxidativestressmarkerintypeIDM | AOPP,TBARS↑,TAS-↓ | AOPP,TAS-susceptibleindicatorinrelatives |
| 17 | Piwowaretal. [27] | IMA-type2DM | IMA-↑inPC | HigherIMAprovokedduringoxidativestress |
| 18 | Raffaelli et al. [28] | EffectofSyzygiumcumini(L.)(Sc)onplatelets | NO,SOD-↑;LPO-↓ | Syzygiumcumini(L.)canbeusedinmanagementofdiabetesandprotectiveeffectinoxidativedamageprogression |
| 19 | Lausten et al. [29] | Causeforpoorglycemiccontroldespiteinsulinadministration | TBARS-↓;Increasedaerobicandanaerobicmetabolicflux-insulintreated | Metabolicdisturbancesindiabetickidneyduringpoorcontrolarelimitedbysubstrateavailability |
| 20 | GohelandChacko [30] | SerumGGTandhsCRP-type2DM | ↑serumGGTandhsCRP-PC | Oxidativestress-majorfactorinDMcomplications |
| 21 | Cicconeetal. [11] | PrediabeticsandfamilyhistorypredisposetoCVrisk | Increaseinc-IMT,baPWV | Inflammation,↑glucose-impairvascularendothelium,atherosclerosis |
| 22 | Santullietal. [12] | RyR2/Calciumreleasechannel | CalciumleakviaRyR2channel;decreasesATPproductionfrommitochondria;↑ROS | RyR2-crucialroleininsulinsecretion;glucoserelease |
Table 1: Outline of important parameters and conclusions from the context in review
Conclusion
Impaired fasting glucose and impaired glucose tolerance predisposes to development of vascular complications and the hyperglycemia that develops as a consequence of poor metabolic control, at the early stages of diabetes releases excess superoxide radicals that impairs the mitochondria . Advanced glycation end products and elevated RAGE expression activates a series of signal at the cellular level that increases the risk of microvascular and macrovascular complications. The metabolic memory established in the initial phase cannot be reversed by good glycemic control in the later stages of the disease by therapeutic measures. Hence a severe intensive treatment in the early stages of IFG/IGT after suitable diagnosis is essential to prevent the progression to diabetic state and the metabolic disturbances which develop further in the course of the disease.
References
- Wild S, Roglic G ,Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27 (5) 2004; 1047-1053.
- Drugs for Diabetes, 2005. Treatment Guidelines from the Medical Letter, 57-62.
- White NH, Sun W, Cleary PA, Danis RP, Davis MD, Hainsworth DP, Hubbard LD, Lachin JM, Nathan DM . Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the diabetes control and complications trial. Arch Ophthalmol 2008; 126: 1707-1715.
- Drzewoski J, Kasznicki J, Trojanowski Z. The role of metabolic memory in the natural history of diabetes mellitus. Pol Arch Med Wewn 2009; 119: 493-500.
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM,Orchard TJ Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643-2653.
- Colagiuri S, Cull CA, Holman RR, UKPDS Group. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes? U.K. prospective diabetes study 61. Diabetes Care 2002; 25: 1410-1417.
- Kowluru RA. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes 2003; 52: 818-823.
- Yorek MS, Obrosov A, Shevalye H, Lupachyk S, Harper MM, Kardon RH, Yorek MA .Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J mice. J Peripher Nerv Syst 2014; 19: 205-217.
- Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest 1997; 100: 115-126.
- He Z, King GL. Microvascular complications of diabetes. Endocrinol Metab Clin North Am 2004; 33: 215-238.
- Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F. Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy: A Review. J Diabetes Metab 2014; 5: 364.
- Santulli G, Pagano G,Sardu C, Xie W, Reiken S, D’Ascia SL ,Cannone M, Marziliano N, Trimarco B, Guise TA, Lacampagne A, Marks AR. Calcium release channel RyR2 regulates insulin release and glucose homeostasis . J Clin Invest 2015; 125: 1968-1978.
- Roy S, Sala R, Lorenzi ECM. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory (basement membranes/collagen IV/kidney/heart/endothelial cells). Proc Natl Acad Sci USA 1990; 87: 404-408.
- Hammes HP, Klinzing I, Wiegand S, Bretzel RG, Cohen AM, Federlin K. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen rat. Invest Ophthalmol Vis Sci 1993; 34: 2092-2096.
- Engerman RL, Kern ST, Progression of Incipient Diabetic Retinopathy During Good Glycemic Control. Diabetes 1987; 36: 808-812.
- Tan KC, Shiu SW, Chow WS, Leng L, Bucala R, Betteridge DJ. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia 2006; 49: 2756-2762.
- Devangelio E, Santilli F, Formoso G, Ferroni P, Bucciarelli L, Michetti N, Clissa C, Ciabattoni G, Consoli A, Davì G. Soluble RAGE in type 2 diabetes: association with oxidative stress. Free Radic Biol Med 2007; 43: 511-508.
- Mishra N, Singh N. Blood viscosity, lipid profile, and lipid peroxidation in type-1 diabetic patients with good and poor glycemiccontrol. N Am J Med Sci 2013; 5: 562-566.
- Kostolanská J, Jakus V, Barák L. HbA1c and serum levels of advanced glycation and oxidation protein products in poorly and well controlled children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2009; 22: 433-442.
- Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita-Nakamura Y, Takeuchi M, Inoue H, Imaizumi T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvasc Res 2008; 76: 52-56.
- Motawi TM, Abou-Seif MA, Bader AM, Mahmoud MO. Effect of glycemic control on soluble RAGE and oxidative stress in type 2 diabetic patients. BMC Endocr Disord 2013; 13: 32.
- Brinkmann C, Neumann E, Blossfeld J, Frese S, Orthmann P, Montiel G, Bloch W, Brixius K. Influence of glycemic status and physical fitness on oxidative stress and the peroxiredoxin system in the erythrocytes of non-insulin-dependent type 2 diabetic men. Exp Clin Endocrinol Diabetes 2011; 119: 559-564.
- Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, I?imer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clin Biochem 2001; 34: 65-70.
- Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH, Malabanan A, Trackman PC,Badwey JA, Van Dyke TE. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. Leukoc Biol 2005; 78: 862-870.
- Cakatay U. Protein oxidation parameters in type 2 diabetic patients with good and poor glycaemic control. Diabetes Metab 2005; 31: 551-557.
- Korpacka KM, Salmonowicz B, Boehm D, Berdowska I, Zielinski B, Patryn E, Noczynska A, Gamian A. Diagnostic potential of oxidative stress markers in children and adolescents with type 1 diabetes. Clin Biochem 2008; 41: 48-55.
- Piwowar A, Knapik-Kordecka M, Warwas M.Ischemia-modified albumin level in type 2 diabetes mellitus-Preliminary report. Dis Markers 2008; 24: 311-317.
- Raffaelli F, Borroni F, Alidori A, Tirabassi G, Faloia E, Rabini RA, Giulietti A, Mazzanti L,Nanetti L, Vignini A. Effects of in vitro supplementation with Syzygium cumini (L.) on platelets from subjects affected by diabetes mellitus. Platelets 2015; 26: 720-725.
- Laustsen C, Lipso K, Ostergaard JA, Nørregaard R, Flyvbjerg A, Pedersen M, Palm F, Ardenkjaer-Larsen JH. Insufficient insulin administration to diabetic rats increases substrate utilization and maintains lactate production in the kidney. Physiol Rep 2014; 2: e12233.
- Gohel MG, Chacko AN. Serum GGT activity and hsCRP level in patients with type 2 diabetes mellitus with good and poor glycemic control: An evidence linking oxidative stress, inflammation and glycemic control. J Diabetes Metab Disord 2013; 12: 56.
- Shen L, Zhang YG, Liu M, Qiang DC, Sun XL. Increased arterial stiffness in subjects with pre-diabetes among middle aged population in Beijing, China. Biomed Environ Sci 2013; 26: 717-725.
- Robertson RP. Assessment of β-cell mass alpha- beta-cell survival function by arginine stimulation in human autologous islet recipients. Diabetes 2014; 64: 565-572.