Research Article - Biomedical Research (2017) Volume 28, Issue 1
Hybrid compounds from thiosemicarbazone and triazole as antidiabetic agents and their antioxidant potentials
Ademola Ayeleso1, Jitcy Joseph2, Yonas Belay3, Henok Kinfe3, Sithandiwe Mazibuko4, Oluwafemi Oguntibeju5 and Emmanuel Mukwevho1*1Department of Biological Sciences, North West University, Private Bag X2046, Mmabatho 2735, South Africa
2Department of Biochemistry, University of Johannesburg, P.O. Box 524, Auckland Park 2006, South Africa
3Department of Chemistry, University of Johannesburg, P.O. Box 524, Auckland Park 2006, South Africa
4Diabetes Discovery Platform, South African Medical Research Council, P.O. Box 19070, Tygerberg 7505, South Africa
5Oxidative Stress Research Centre, Department of Biomedical Sciences, Cape Peninsula University of Technology, Bellville 7535, South Africa
- *Corresponding Author:
- Emmanuel Mukwevho
Department of Biological Sciences
North West University
South Africa
Accepted on June 10, 2016
Abstract
Diabetes mellitus is a metabolic disease that threatens and reduces the quality of life. Eight hybrids (1ah) of thiosemicarbozone and triazole were screened for their effects on genes related to type 2 diabetes as well as their antioxidant activity. The influence of the hybrids on glucose transport genes (Glut-4, Mef2a and Nrf-1) was carried out using quantitative real time polymerase chain reaction (PCR). Antioxidant assays were carried out using established techniques. Hybrids 1b, 1d, 1e and 1g exhibited high expression of Glut-4 gene relative to insulin and control. All the hybrids tested except 1h and 1f expressed the Nrf-1 while only 1h did not express Mef-2a relative to control. Among all the compounds, 1b showed the highest 1-diphenyl-2-picryl- hydrazyl (DPPH) radical scavenging ability and Trolox Equivalent Antioxidant Capacity (TEAC) values. In terms of Ferric Reducing Antioxidant Power (FRAP) and Oxygen Radical Absorbance Capacity (ORAC), 1c and 1d had the highest values, respectively. In all the antioxidant assays carried out, 1a was shown to have the lowest antioxidant activities. Hybrids 1d and 1g showed consistent pattern of glucose transport pathway gene transcription with all the hybrids showing antioxidant potentials though at varying extents. These hybrids could be potential candidates eliciting antidiabetic and antioxidant effects.
Keywords
Thiosemicarbazone, Triazole, Free radical, Oxidative stress, Antioxidant, Diabetes.
Introduction
Diabetes mellitus is a metabolic disorder in which a combination of hereditary and environmental factors results in abnormally high blood sugar levels [1] and it is associated with absolute or relative deficiency in insulin secretion or insulin action [2,3]. Diabetes is characterized by hyperglycemia, lipoprotein abnormalities, raised basal metabolic rate, defect in enzymes, and high oxidative stress which induces damage to pancreatic beta cells [1]. It is a common disorder that affects people at all ages which include children, adolescents and young adults, and also sometimes in older people [4,5]. The increasing occurrence of diabetes around the world constitutes a global public health problem and it is predicted that by 2025, the world will have an increase of 72% of diabetic patients [4]. Diabetes is divided into two types; i) type 1 diabetes, which accounts for less than 10% of all diabetic cases and results from the autoimmune destruction of beta cells with patients depending on exogenous insulin and, ii) type 2 diabetes, which affects about 90% of all people with diabetes and is characterized by defects in insulin secretion and action [6].
Free radicals belong to a group of reactive oxygen species (ROS) [7] which are unstable, short-lived and have one or more unpaired electrons [8]. They attack important cellular macromolecules such as protein, lipid and DNA and cause cell damage [9]. Antioxidants help to scavenge free radicals in the body [10] and act through inhibiting initiation and generation steps that leads to termination of the chain reaction and delay the oxidation process [11]. They exhibit their antioxidant activities by donating hydrogen atoms or the single-electron transfer to a radical [12,13]. Antioxidant systems, both enzymatic and non-enzymatic help to balance ROS in order to keep their level constant in living organisms and an imbalance by over production of ROS and/or reduction of antioxidants leads to oxidative stress [14]. Oxidative stress, an imbalance between the production of free radicals and the ability the body’s antioxidant system to fight back, has been implicated in the pathogenesis of many chronic diseases. Antioxidants alleviate oxidative stress, the adverse effects of free radical [15] and reportedly help in slowing down aging process and fight diseases such as diabetes mellitus, hypertension and cancer [16,17].
Semicarbazone is synthesized by the condensation of semicarbazide and aldehyde/ketone [18]. Thiosemicarbazone is an analog of a semicarbazone which contains a sulphur atom in place of the oxygen atom. A considerable number of thiosemicarbazones derivatives have been shown to have a wide spectrum of biological activities which include antidiabetic [19], antiviral [20], anticancer [21], antibacterial [22,23], antifungal [22], and antimalarial [24] activities. Triazoles are heterocyclic compounds featuring five member ring of two carbon atoms and three nitrogen atoms as part of the aromatic five-member ring with molecular formula C2H3N3 [25]. Triazole derivatives have also been reported to have antidiabetic [19], antifungal [26,27], antibacterial [26], anticancer [28,29], antimalaria [30], and anti-inflammatory [31] benefits.
The semicarbazone is an electron withdrawing group and exhibits antioxidant activity and its favourable substitution may increase its free radical scavenging effect [32,33]. The synthesis of high nitrogen containing heterocyclic systems has been attracting increasing interest over the past decade because of their utility in various applications [25]. Novel therapeutic strategies support the use of the ROS scavengers, chelators of transition metal ions or modulators of a cell signaling for the treatment of diseases [34-36]. In our previous studies, the antimalaria [37] and anti-obesity [38] activities of these newly synthesized hybrid compounds have been investigated. Currently, drugs in use to treat or manage type 2 diabetes which include metformin and the glitazones i.e. rosiglitazone have several negative side-effects [39,40]. Metformin, for instance, is associated with gastric disturbances while rosiglitazone is known to increase the risk of heart attack and stroke by as much as 43% [41]. There is therefore an urgent need for better and new classes of drugs that can possibly treat or better manage the disease than those currently in use. The present study was carried out to assess the influence of the hybrids from thiosemicarbazone and triazole on genes related to type 2 diabetes as well as investigating their antioxidant potentials.
Materials and Methods
Synthesis of hybrid compounds
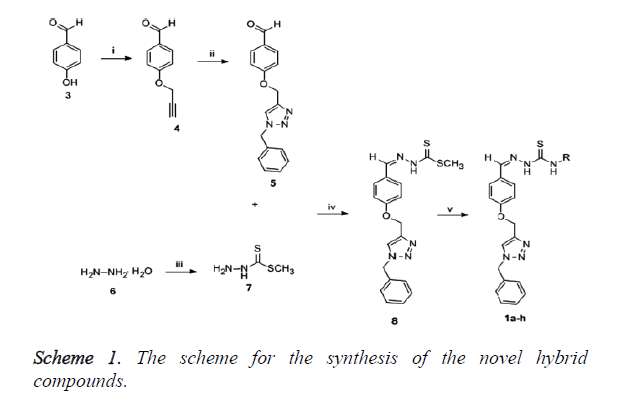
The hybrids (1a-h) were synthesized according to a published protocol as outlined in Scheme 1 [37,38]. Briefly, the synthesis started with alkylation of commercially available 4- hydroxybenzaldehyde with propargyl bromide in the presence of K2CO3 to give 4 which incorporates an alkynyl group which is required for click chemistry. Compound 4 was subjected to click chemistry with freshly prepared benzyl azide to give 1,4- disubstituted triazole 5. Methylhydrazinecarbodithioate 7, that was prepared in a one pot synthesis from the condensation of hydrazine monohydrate and methyl iodide reacted with triazole 5 under Schiff’s base condensation reaction conditions to produce compound 8 as presented in Scheme 1 and this went through nucleophilic substitution reactions with a series of primary amines to produce the hybrid compounds (1a-h) as shown in Table 1. The reagents and conditions which are involved in the synthesis include (i) K2CO3, propargyl bromide, acetone, reflux, 2.5 h, 92%; (ii) BnN3, CuSO4.5H2O, sodium ascorbate, DMF: H2O (4:1), 60°C, 3 h, 80%; (iii) CS2, KOH, CH3I, H2O: isopropanol (1:1), RT, 4 h, 90%; (iv) 5, 7, MeOH, reflux, overnight, 89%; (v) RNH2, MeOH, reflux, 24 h, 62-85% (Figure 1).
| Hybrid Compounds | Amines (R-NH2) |
|---|---|
| 1a | PhCH2NH2 |
| 1b | CH3CH2CH2CH2CH2NH2 |
| 1c | PhCH2CH2NH2 |
| 1d | (CH3)2NCH2CH2NH2 |
| 1e | HOCH2CH2CH2CH2CH2CH2NH2 |
| 1f | CH3CH2CH2CH2CH2CH2CH2CH2NH2 |
| 1g | CH3CH(CH3)CH2NH2 |
| 1h | HOCH2CH2NH2 |
Table 1. Series of primary amines that provided the new hybrid compounds. The hybrid compounds were labelled 1a-1h as shown in the table below.
Cell culture protocol
In the study, 3T3-L1 adipocytes cell lines (mouse) were cultured using Dulbecco's modified Eagles medium (DMEM) (GIBCO, USA) supplemented with 10% Foetal calf serum (BioWest, France) and 1% penicillin/streptomycin/fungizone (GIBCO, USA) at 37°C with 5% CO2 and 95% humidity. Cells were maintained in continuous passage by trypsinization of subconfluent cultures with Trypsin/Versene (Highveld, RSA). Differentiation was induced by introduction of medium containing 2% Foetal calf serum and 2% penicillin/ streptomycin/fungizone when pre-adipocytes were 80% confluent. Cells were kept in this medium for 5 days until adipocytes were well formed. The resultant adipocytes were then treated with 5 μl of the hybrid compounds (10 mg/ml in DMSO) for 4 days. After 4 days treatment, the medium was changed with non-supplemented DMEM medium, incubated for 4 hours.
Quantitative real-time PCR
Total RNA was extracted from the cells using QIAzol lysis reagent (QIAGEN Sciences, USA) and RNA clean and Concentrator-25 (Inqaba Biotech, SA). Double stranded cDNA was synthesized from 3 μg of total RNA using Superscript Reverse Transcriptase III (Invitrogen, USA). Real time PCR performed in triplicate using Rotor gene-3000 Quantitative real-time PCR machine using Sensi Mix SYBR No-ROX One- Step Kit (Bioline, UK). Primers used are mouse Glut-4 gene (Forward primer- 5' GCA GCG AGT GAC TGG AAC A 3'; Reverse primer- 5'CCA GCC ACG TTG CAT TGT AG 3'), Nrf-1 gene (Forward primer- 5' AAA CAC AAA CTC AGG CCA CC 3'; Reverse primer-5' CCA TCA GCC ACA GCA GAG CA 3') and Mef2a gene (Forward primer-5' GTG TAC TCA GCA ATG CCG AC 3'; Reverse primer-5' AAC CCT GAG ATA ACT GCC CTC 3'). The amplification occurred in a three-step cycle: denaturation at 95°C for 5 sec, annealing at 60°C for 10 sec and extension at 72°C for 15 sec. Relative mRNA expression normalized to mouse Actin reference gene (Forward primer- 5' GAG ACC TTC AAC ACC CCA GCC 3'; Reverse primer- 5' GGA GAG CAT AGC CCT CGT AG 3') and calculated according to relative standard method.
In vitro glucose uptake in 3T3-L1 adipocytes
To assess glucose uptake, the fully differentiated adipocytes were exposed to the respective representative compounds (1c, 1f, 1g, 1h) at lower concentrations of 0.05, 0.5, 5 and 50 μg/ml. Insulin (1 μM) was included as a positive control and metformin (1 μM) as a reference drug control. The vehicle control containing 0.01% DMSO was also included and plates were incubated for 3 hours. Since 1g was the compound that exhibited the highest gene expression in our previous publication [38], we assessed glucose transport using fewer selected compounds including 1g where highest gene expression was observed with glucose transport genes. After incubation with these compounds, glucose uptake in 3T3-L1 adipocytes was determined using pulse-labeling with [3H]-2- deoxy-D-glucose (3H-2-DOG) in glucose-free DMEM containing the extracts for 15 min.
Liquid Scintillation counting for glucose uptake
Liquid scintillation counting (2200 CA, ParkardTricarb, USA) was used to measure intracellular 3H-2-DOG. Activity of the compounds were determined as fmol (3H-2-DOG CPM/mg protein) and expressed below as 100% relative to the DMSO control. Insulin and metformin were expressed as % relative to the media control.
ABTS radical scavenging activity
2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activity was done according to a method described by Re et al. [42]. ABTS+ solution was prepared a day before use by mixing ABTS salt (8 mM) with potassium persulfate (3 mM) and kept in the dark. The ABTS+ solution was further diluted with distilled water before use. Twenty five microlitres (25 μl) of the sample was mixed with 300 μl ABTS+ solution in a 96-well clear microplate. The plate was read after 30 min incubation at room temperature in a Multiskan Spectrum plate reader (Thermo Fisher Scientific, USA) at 734 nm in triplicates. Trolox was used as the standard and result expressed as μmol TE/g sample.
Ferric reducing antioxidant power assay
The FRAP assay was carried out using the method described by Benzie and Strain [43]. Briefly, 10 μl of the sample was mixed with 300 μl FRAP reagent in a 96-well clear plate. The FRAP reagent was a mixture (10:1:1, v/v/v) of acetate buffer (300 mM, pH 3.6), tripyridyl triazine (TPTZ) (10 mM in 40 mM HCl) and FeCl3.6H2O (20 mM). After incubation at room temperature for 30 min, the plate was read at a wavelength of 593 nm in a Multiskan Spectrum plate reader (Thermo Fisher Scientific, USA) in triplicates. Ascorbic acid (AA) was used as the standard and the result expressed as μmol AAE/g sample.
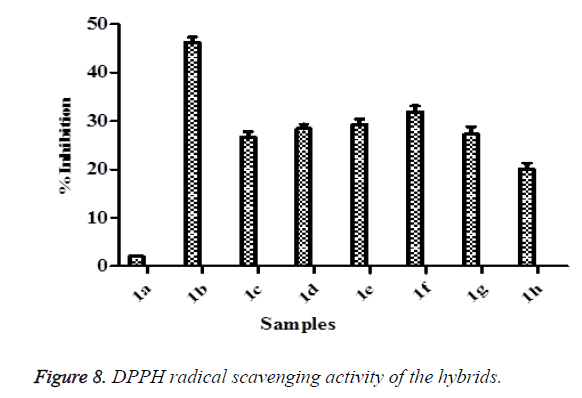
DPPH radical scavenging activity assay
DPPH free radical scavenging activity was conducted using a method described by Zheleva-Dimitrova [44] with slight modifications. Briefly, 10 μl of the sample was reacted with 190 μl of DPPH solution in a 96-well clear plate. The sample was left for 30 min at room temperature and the plate was read using a Multiskan Spectrum plate reader (Thermo Fisher Scientific, USA) at 517 nm in triplicates. Free radical scavenging activity of the samples was expressed according to the equation below.
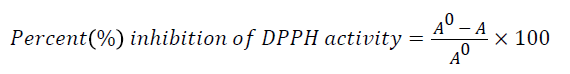
where A0 is the absorbance of DPPH• in solution without an antioxidant, and A is the absorbance of DPPH.
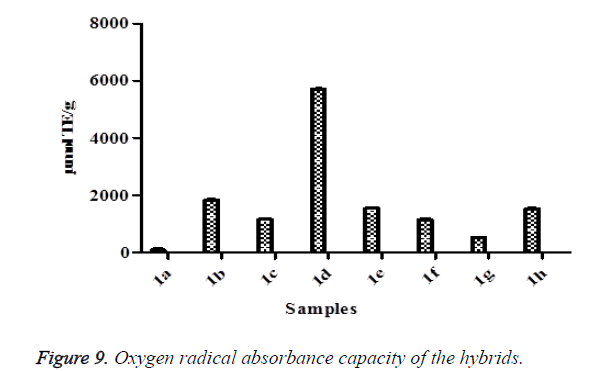
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was carried out according to the method of Ou et al. [45] using a fluorescence plate reader (Thermo Fisher Scientific, Waltham, Mass., USA). The reaction consisted of 12 μl of the samle and 138 μl of fluorescein (14 μM), which was used as a target for free radical attack. The reaction was initiated by the addition of 50 μl AAPH (768 μM) and the fluorescence (emission 538 nm, excitation 485 nm) recorded every 1 min for 2 hours in triplicates. Trolox was used as the standard and the result expressed as μmol TE/g sample.
Results and Discussion
Glut-4 is an insulin-responsive glucose transporter that regulates glucose uptake [46,47]. Glut-4 transports glucose from blood to the cells thereby maintaining glucose homeostasis (euglycemia). Some reports have indicated that Glut-4 levels are low in diabetes mellitus [48-52] hence, up-regulation of Glut-4 transcription is seen as a means to alleviate type 2 diabetes [53-56]. Glut-4 gene is regulated by myocyte enhancing (Mef2) transcription factor which binds to its cis-elements as a hetero-dimer (Mef2a/D) resulting in Glut-4 expression [55,56]. Recent studies showed that Glut-4 expression is also regulated by nuclear respiratory factor (Nrf-1), a mitochondrial transcription factor, which controls Mef2a gene to regulate Glut-4 [57,58]. Although exercise and diet can potentially protect individuals from type 2 diabetes, the prevalence of type 2 diabetes continues to rise worldwide driving a need for new more effective therapeutics in the management or treatment of the disease [59]. These could include therapeutics that mimic the induction of genes comparable to those responses brought about by regular exercise. Thus, owing to their essential roles in blood sugar metabolism, these genes have become targets for pharmacological therapeutics in the management and treatment of type 2 diabetes.
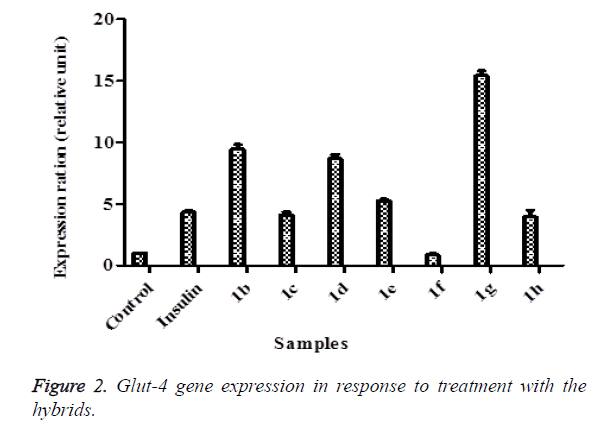
After successful synthesis of hybrids 1a-h, we investigated their effect on Glut-4 gene expression in 3T3-L1 adipocytes using quantitative real time PCR (qPCR). Expression of Glut-4 gene in relation to test compounds was compared with that of insulin as a standard drug that induces Glut-4 gene expression. Upon treatment with 1a, the cells lifted from the plate hence, they could not be used for RNA extraction. As shown in Figure 2, it is evident that 1b, 1d, 1e and 1g exhibited high expression of Glut-4 gene relative to insulin and control. 1g showed a more pronounced increase (4 fold of the insulin and 15 folds of the control) followed by 1b and 1d with 2 folds of the insulin under the experimental conditions employed. On the contrary, 1f did not exhibit any influence on Glut-4 gene expression. The Glut-4 gene is now seen as one of the potential targets for therapeutic modalities aimed at alleviating type 2 diabetes because it is the rate-limiting step in glucose transport and maintenance of normal blood glucose levels (euglycemia). Increase in Glut-4 gene in this study by these hybrid compounds more than insulin that is usually used to treat diabetes has huge potential in the treatment and management of diabetes.
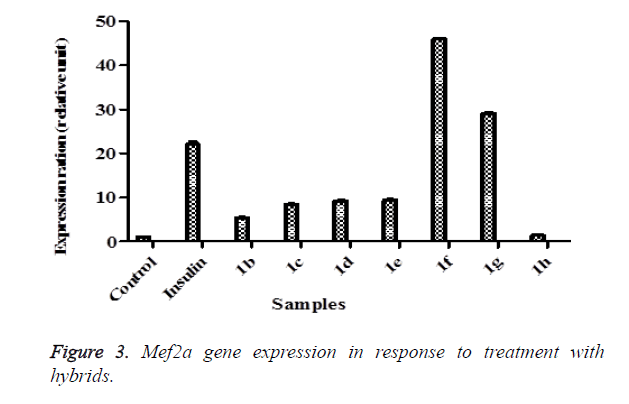
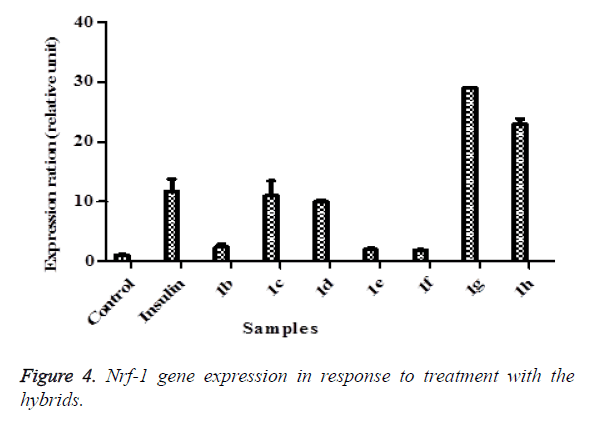
Hybrids 1b-h were further investigated if they can express Mef2a gene which is the transcription regulator of Glut-4. Interestingly, all except 1h expressed the Mef2a (Figure 3) relative to control. The effect of 1f was almost 50 folds higher than the control while 1g displayed 31 folds more expression. The increase in Mef2a followed the same pattern observed with that seen with the Glut-4 gene. These results further reinforce systematic increase in the transcriptional cascade involved in glucose transport mechanisms. Deletion of the Mef2a binding sites in the Glut-4 gene has shown to abolish Glut-4 transcription indicating the importance of Mef2a in glucose transport. The consistent increase of both Glut-4 and Mef2a by these compounds shows they can be potential agents in mechanisms involved in improving glucose transport. To ascertain further the increases observed above with Glut-4 and Mef2a genes, we assayed the effects of the hybrids on Nrf-1 and the results showed that 1c, 1d, 1g and 1h greatly stimulated Nrf-1 expression compared to control (Figure 4) with 1g and 1h even stimulating more than insulin. As it is evident from the results, the consistent higher stimulating effect of 1g followed by 1d on the three genes suggests that the presence of a non-polar short-branched chain of the amine moiety might be important in the up-regulation of Glut-4, Mef2a and Nrf-1 genes. The increase in Nrf-1 also strengthens the increases in Glu-4 and Mef2a. Reports have shown that Nrf-1 binds and controls Mef2a which in turn regulate Glut-4. This means Nrf-1 regulate Glut-4 indirectly by controlling Mef2a.
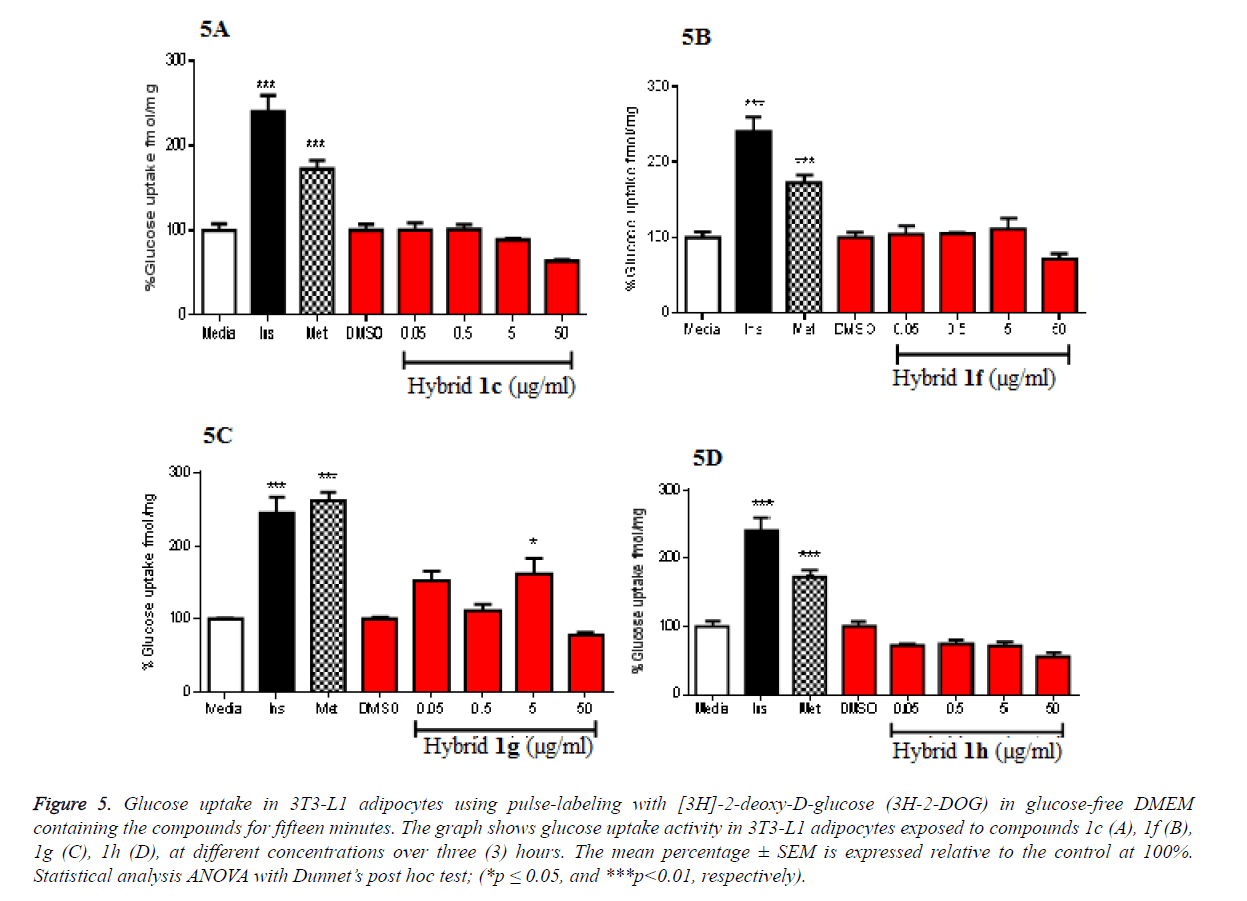
From the observed increased transcription of the genes involved in glucose transport metabolism, we decided to investigate if this would have any influence on the functional assays to determine if the observed increased in gene expression corresponds to improved glucose transport. This is because gene transcription does not always translate to enhanced functionality of a particular gene. Results from the assay indicated that only 1g showed increased glucose transport (Figure 5). Using lower concentrations of the hybrids, the increased glucose transport induced by 1g corresponded with the observed gene transcription with Glu-4, the major glucose transporter in skeletal and adipose tissues. The positive controls, metformin and insulin also increased glucose transport as expected. These are very promising results warranting further investigation on the mechanisms through which this compound (1g) enhances gene transcription of genes involved in glucose transport concomitantly with corresponding glucose transport in these cells. However, other tested compounds did not show increase in glucose transport in relation to the gene transcription (Figures 2 and 5).
Figure 5. Glucose uptake in 3T3-L1 adipocytes using pulse-labeling with [3H]-2-deoxy-D-glucose (3H-2-DOG) in glucose-free DMEM containing the compounds for fifteen minutes. The graph shows glucose uptake activity in 3T3-L1 adipocytes exposed to compounds 1c (A), 1f (B), 1g (C), 1h (D), at different concentrations over three (3) hours. The mean percentage ± SEM is expressed relative to the control at 100%. Statistical analysis ANOVA with Dunnet’s post hoc test; (*p ≤ 0.05, and ***p<0.01, respectively).
ROS are produced during both normal and altered cellular metabolism and they include singlet oxygen (oxygen molecule in the electronically excited state), superoxide anion radical, peroxy radical, alkoxy radical, hydrogen peroxide [60-62]. ROS can both be beneficial and harmful to the physiology of cells [62]. As previously mentioned, excessive production of ROS over the body antioxidant system results in the destruction of the cell components and causes oxidative stress. Oxidative stress has been implicated in a wide array of diseases which include inflammation, neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease), atherosclerosis, diabetes and cancer [62,63]. Scavenging of free radicals is one of the well-recognized mechanisms by which antioxidants inhibit lipids and other biomolecules oxidation [13].
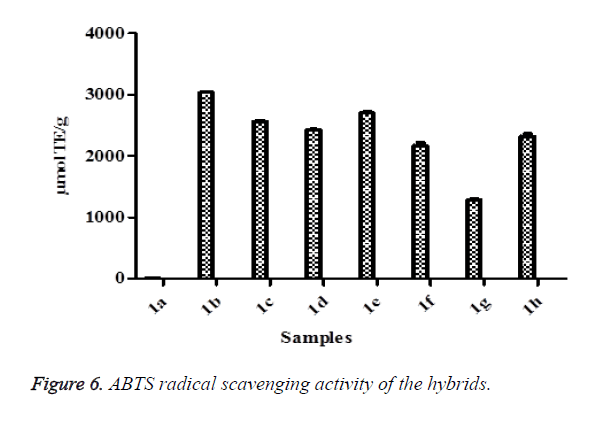
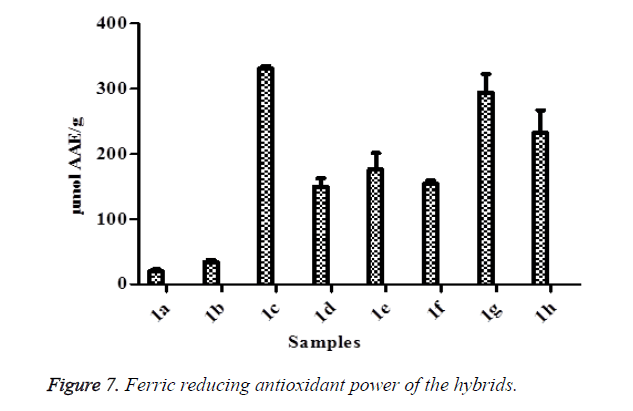
ABTS method involves a technique that produces a blue/green ABTS+ chromophore through the reaction of ABTS and potassium persulfate [7,64]. It is dependent on the inhibition of the absorbance of radical cation ABTS [65]. The decolorization of ABTS radical in a sample shows the capacity of an antioxidant to donate electron or hydrogen atoms to inactivate the radical cation [66,67]. In this study, the results indicated that all the hybrid compounds had ABTS radical scavenging activities. In this study, the hybrid compound 1e was the highest while 1a was the lowest (Figure 6). These results confirmed the potency of the hybrid compounds to protect against oxidative damage. FRAP assay is a novel method for the assessment of antioxidant power where the ferric reducing ability of samples are investigated [68]. The reduction of ferric to ferrous ion at low pH generated coloured ferrous-tripyridyltriazine complex. This method is based on the reaction between antioxidant potentials and Fe3+-TPTZ complex to produce blue color Fe2+-TPTZ form [69]. In this study, all the hybrids compound showed FRAP potentials through their reducing abilities (Figure 7) as evident in the conversion of ions Fe3+ to Fe2+ by the formation of blue color. Among the FRAP values for the hybrid compounds, 1c was the highest while 1a was the lowest. The higher the FRAP value, the greater is the antioxidant activity. The change in absorbance is directly related to the total reducing power of the electron-donating antioxidants present in the samples.
DPPH radical scavenging assay is a common method of assessing intrinsic free radical scavenging activity in a cell free system with 2,2-diphenyl-1-picryhydrazyl (DPPH), a free-radical generating compound [15]. It has many advantages than other methods which include as good stability, credible sensitivity, simplicity and feasibility [70-72]. This method is based on the formation of the DPPH-H non radical form in the presence of hydrogen donating antioxidants at 517 nm [69]. DPPH radical is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule [15,73]. In this study, DPPH scavenging activities were found in all the hybrid compounds (Figure 8). The degree of decrease in absorbance values is indicative of the antioxidant power of the compounds. From the results, the highest DPPH inhibition value was 1b while the 1a was the lowest. The exhibited antioxidant activities of these hybrid compounds on DPPH radical scavenging may be attributed to their hydrogen donating abilities. ORAC assay entails the use of fluorescent probe known as fluorescein and the loss of fluorescein over time due to peroxyl radical formation by the breakdown of AAPH is an indication of oxidative damage [74]. In this study, all the hybrid compounds showed ORAC values (Figure 9). The highest ORAC value was 1d while the lowest was 1a. The ORAC results confirmed the ability of the hybrid compounds to donate hydrogen atoms which resulted into the quenching of generated peroxyl radicals.
Conclusion
A series of thiosemicarbazone-triazole hybrids were evaluated for their effect on Glut-4, Mef2a and Nrf-1 genes. There was consistency in the pattern of glucose transport pathway gene transcription by hybrids 1d and 1g with 1g showing the most pronounced increase. Other compounds studied did not show a consistent up-regulation of all the relevant glucose transport genes investigated. This present study suggests that 1g could be potential therapeutic agent to treat type 2 diabetes. The antioxidant activities of hybrids were examined for the first time in this study. The results showed that all the hybrid compounds have antioxidant potentials at varying degrees. As a result, they could play important protective roles to counteract the onset and progression of several diseases of the free radical aetiology such as diabetes mellitus. It can be speculated that the interactions of these hybrid compounds with free radicals and their abilities to donate electrons depend on their structures and hence, further research studies in the understanding of the mechanisms through which they exert their antioxidants effects are needed.
Acknowledgment
We would like to thank the National Research Foundation of South Africa (NRF) for providing funding for this research, Grant numbers: 76194 and 88062 granted to Prof Emmanuel Mukwevho. We also thank the Cape Peninsula University of Technology, South Africa for funding through Prof Oluwafemi Oguntibeju.
References
- Anusooriya P, Malarvizhi D, Gopalakrishnan VK, Devaki K. Antioxidant and antidiabetic effect of aqueous fruit extract of Passiflora ligularis juss. on streptozotocin induced diabetic rats. Int Scholar Res Notices 2014.
- Ramadas AKF, Quek KF, Chan CKY, Oldenburg B: Web-based interventions for the management of type 2 diabetes mellitus: a systematic review of recent evidence. Int J Med Informat 2011, 80: 389-405.
- Tiwari BK, Kumar D, Abidi AB, Rizvi SI. Efficacy of composite extract from leaves and fruits of medicinal plants used in traditional diabetic therapy against oxidative stress in alloxan-induced diabetic rats. ISRN Pharmacol 2014.
- Lefèbvre P. Le diabète hier, aujourd'hui et demain. L'action de la fédération internationale du diabète. Revue médicale de Liège 2005.
- Florence NT, Benoit MZ, Jonas K, Alexandra T, Désiré DD, Pierre K, Théophile D. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae) aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol 2014; 151: 784-790.
- Szkudelski T, Szkudelska K: Anti-diabetic effects of resveratrol. Ann N Y Acad Sci 2011; 1215: 34-39.
- Ayeleso AO, Oguntibeju OO, Brooks NL. In vitro study on the antioxidant potentials of the leaves and fruits of Nauclea latifolia. Sci World J 2014.
- Aruoma OI. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol1994; 32: 671-683.
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 2010; 4: 118-126.
- Goswami N, Chatterjee S. Assessment of free radical scavenging potential and oxidative DNA damage preventive activity of Trachyspermum ammi L. (Carom) and Foeniculum vulgare Mill. (Fennel) seed extracts. Bio Med Res Int 2014.
- Khalighi-Sigaroodi F, Ahvazi M, Hadjiakhoond A, Taghizadeh, M, Yazdani D, Khalighi-Sigaroodi S, Bidel S. Cytotoxicity and antioxidant activity of 23 plant species of leguminosae family. Iran J Pharm Res 2012; 11: 295-302.
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L: Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 2011; 50: 586-621.
- Berczyński P, Kładna A, Kruk I, Piechowska T, Aboul-Enein HY, Bozdağ-Dündar, O, Ceylan-Unlusoy M. Antioxidant activities of some new chromonyl-2,4- thiazolidinediones and chromonyl-2,4-imidazolidinediones having chromone cores. J Fluoresc 2013; 23: 1319-1327.
- Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative Stress and neurodegenerative disorders. Int J Mol Sci 2013; 14: 24438-24475.
- Kumar S, Kumar R, Dwivedi A, Pandey AK. In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. BioMed Res Int 2014.
- Liu RH: Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 2003; 78: 517S-520S.
- Oboh G, Ademosun AO, Ademiluyi AO, Omojokun, OS, Nwanna EE, Longe KO: In vitro studies on the antioxidant property and inhibition of -amylase, -glucosidase, and agiotensin 1-converting enzyme by polyphenol-rich extracts from cocoa (Theobroma cacao) bean. Pathol Res Int 2014.
- Ahsan MJ. Semicarbazone analogs as anticonvulsant agents: a review. Cent Nerv Syst Agents Med Chem 2013, 13: 148-158.
- Kadowaki S, Munekane M, Kitamura Y, Hiromura M, Kamino S, Yoshikawa Y, Saji H, Enomoto S. Development of new zinc dithiosemicarbazone complex for use as oral antidiabetic agent. Biol Trace Elem Res 2013; 154: 111-119.
- Glisoni RJ, Chiappetta DA, Moglioni AG, Sosnik A. Novel 1-indanone thiosemicarbazone antiviral candidates: aqueous solubilization and physical stabilization by means of cyclodextrins. Pharm Res 2011; 29: 739-755.
- Dilović I, Rubcić M, Vrdoljak V, Kraljević Pavelić S, Kralj M, Piantanida I, Cindrić M. Novel thiosemicarbazone derivatives as potential antitumor agents: Synthesis, physicochemical and structural properties, DNA interactions and antiproliferative activity. Bioorg Med Chem 2008; 16: 5189-5198.
- Kizilcikli I, Kurt YD, Akkurt B, Genel AY, Birteksöz S, Otük G, Ulküseven B: Antimicrobial activity of a series of thiosemicarbazones and their ZnII and PdII complexes. Folia Microbiol 2007; 52: 15-25.
- Khan SA, Yusuf M: Synthesis, spectral studies and in vitro antibacterial activity of steroidal thiosemicarbazone and their palladium (Pd (II)) complexes. Eur J Med Chem 2009; 44: 2270-2274.
- Usman A, Razak LA, Chantrapromma S, Fun HK, Sreekanth A, Sivakumar S, Kurup MRP. Bis[1-(pyridin-2-yl)ethanone-κN 4-phenylthiosemicarbazonato-κ2N4,S]manganese(II). Acta Cryst C 2002; 58: 461-463.
- Wakale VS, Pattan SR, Tambe V. Therapeutic importance of 1, 2, 4-Triazole: A review. Int J Res Pharmaceut Biomed Sci 2013; 4: 985-1001.
- Banday AH, Shameem SA, Ganai BA. Antimicrobial studies of unsymmetrical bis- 1,2,3-triazoles. Org Med Chem Lett 2012; 2: 13.
- Zou Y, Zhao Q, Liao J, Hua H, Yu S, Chai X, Xu M, Wua Q. New triazole derivatives as antifungal agents: Synthesis via click reaction, in vitro evaluation and molecular docking studies. Bioorg Med Chem Lett 2012; 22: 2959-2962.
- Bekircan O, Kahveci B, Kucuk M. Synthesis and anticancer evaluation of some new unsymmetrical 3,5- diaryl-4h-1,2,4-triazole derivatives. Turk J Chem 2006: 30: 29-40.
- Pokhodylo N, Shyyka O, Matiychuk V. Synthesis of 1,2,3-triazole derivatives and evaluation of their anticancer activity. Sci Pharm 2013, 81: 663-676.
- Berger O, Kaniti A, van Ba CT, Vial H, Ward SA, Biagini GA, Bray PG, O'Neill PM. Synthesis and antimalarial activities of a diverse set of triazole- containing furamidine analogues. ChemMedChem 2011; 6: 2094-2108.
- Pattan SR, Gadhave P, Tambe, V, Dengale S, Thakur D. Synthesis and evaluation of some novel 1,2,4-triazole derivatives for antmicrobial, antitubercular and anti- inflammatory activities. Indian J Chem 2012; 51B: 297-301.
- Dutta S, Padhye S, Priyadarsini KI, Newton C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg Med Chem Lett 2005; 15: 2738-2744.
- Singhal M, Paul A, Singh HP, Dubey SK, Songara RK. Synthesis and evaluation of antioxidant activity of semicarbazone derivatives. Int J Pharmaceut Sci Drug Res 2011; 3: 150-154.
- Adams JD, Odunze IN. Oxygen free radicals and Parkinson's disease. Free Radic Biol Med 1991; 10: 161-169.
- Aruoma OI: Characterization of drugs as antioxidant prophylactics. Free Radic Biol Med 1996; 20: 675-705.
- Kruk I, Bozdağ-Dündar O, Ertan R, Aboul-Enein HY, Michalska T. Hydroxyl and superoxide radical scavenging abilities of chromonyl-thiazolidine-2,4-dione compounds. Luminescence 2009; 24: 96-101.
- Kinfe HH, Belay YH. Synthesis and biological evaluation of novel thiosemicarbazone- triazole hybrid compounds antimalarial agents. S Afr J Chem 2013; 66: 130-135.
- Kinfe HH, Belay YH, Joseph JS, Mukwevho E. Evaluation of the influence of thiosemicarbazone-triazole hybrids on genes implicated in lipid oxidation and accumulation as potential anti-obesity agents. Bioorg Med Chem Lett 2013; 23: 5275- 5278.
- Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 2002; 277: 25226-25232.
- Lee MK, Olefsky JM: Acute effects of troglitazone on in vivo insulin action in normal rats. Metabolism 1995; 44: 1166-1169.
- Chen XL, Yang L, Zhai SD. Risk of cardiovascular disease and all-cause mortality among diabetic patients prescribed rosiglitazone or pioglitazone: a meta-analysis of retrospective cohort studies. Clin Med J 2002; 125: 4301-4306.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231-1237.
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 1996; 239: 70-76.
- Zheleva-Dimitrova DZ: Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Phcog Mag 2013; 9: 109-113.
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agr Food Chem 2001; 49: 4619-4626.
- Sparling DP, Griesel BA, Weems J, Olson L. GLUT4 enhancer factor (GEF) interacts with MEF2A and HDAC5 to regulate the GLUT4 promoter in adipocytes. J Biol Chem 2008; 283: 7429-7437.
- Charron MJ, Katz EB, Olson AL. GLUT4 gene regulation and manipulation. J Biol Chem 274: 3253-3256.
- Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP: Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest 1991; 87: 1072-1081.
- Kahn BB, Simpson IA, Cushman SW: Divergent mechanisms for the insulin resistant and hyper responsive glucose transport in adipose cells from fasted and refed rats. alterations in both glucose transporter number and intrinsic activity. J Clin Invest 1988; 82: 691-699.
- Sinha MK, Raineri-Maldonado C, Buchanan C. Adipose tissue glucose transporters in NIDDM. Decreased levels of muscle/fat isoform. Diabetes 1991; 40: 472-477.
- Sivitz WI, DeSautel SL, Kayano T, Bell GI, Pessin JE. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature 1989; 340: 72-74.
- Gibbs EM, Stock JL, McCoid SC, Stukenbrok HA, Pessin JE, Stevenson RW, Milici AJ, McNeish D. Glycemic improvement in diabetic db/db mice by overexpression of the Human insulin-regulatable glucose transporter (GLUT4). J Clin Invest 1995; 95: 15-18.
- Brozinick JT, McCoid SC, Reynolds TH, Nardone NA, Hargrove DM, Stevenson RW, Cushman SW, Gibbs EM. GLUT4 overexpression in db/db micedose-dependently ameliorates diabetes but is not a lifelong cure. Diabetes 2001; 50: 593-599.
- Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Kashyap S, Mandarino L. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. J Metab 2004; 53: 1233-1239.
- Oshel KM, Knight JB, Cao KT, Thai MV, Olson AL. Identification of a 30-base pair regulatory element and novel DNA binding protein that regulates the human GLUT4 promoter in transgenic mice. J Biol Chem 2000; 275: 23666-23673.
- Thai MV, Guruswamy S, Cao KT, Pessin JE, Olson AL. Myocyte enhancer factor 2 (MEF2)-binding site is required for GLUT4 gene expression in transgenic mice. Regulation of MEF2 DNA binding activity in insulin-deficient diabetes. J Biol Chem 1998; 273: 14285-14313.
- Ramachandran BG, Yu S, Gulick T. Nuclear respiratory factor 1 controls myocyte enhancer factor 2A transcription to provide a mechanism for coordinate expression of respiratory chain subunits. J Biol Chem 2008; 283: 11935-11946.
- Baar K, Song Z, Semenkovich CF, Jones TE, Han DH, Nolte LA, Ojuka OE, Chen M, Holloszy JO. Skeletal muscle over expression of nuclear respiratory factor 1 increases glucose transport capacity. FASEB J 2003; 17: 1666-1673.
- Kelly T, Yang W, Chen CS, Reynolds K, He KJ. Global burden of obesity in 2005 and projections to 2030. Int J Obesity 2008; 32: 1431-1437.
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human diseases. Int J Biochem Cell Biol 2007; 39: 44-84.
- Murakami K, Shimizu T, Irie K. Formation of the 42-mer amyloid radical and the therapeutic role of superoxide dismutase in alzheimer's disease. J Amino Acids 2011.
- Kładna A, Berczyński P, Piechowska T, Kruk I, Aboul-Enein HY, Ceylan-Unlusoy M, Verspohl EJ, Ertan R. Studies on the antioxidant activities of some new chromone compounds. Luminescence 2014.
- Termini J. Hydroperoxide-induced DNA damage and mutations. Mutat Res 2000; 450: 107-124.
- Osawa T, Kavakishi S, Namiki M, Kuroda Y, Shankal, DM, Waters MD. Antimutagenesis and Anticarcinogenesis Mechanisms II, Plenum. New York, NY, USA, 1990.
- Ammar A, Zhang H, Siddeeg A. In vitro antioxidant activity and total phenolic and flavonoid contents of alhydwan (Boerhavia elegana Choisy) Seeds. J Food Nutr Res 2014; 2: 215-220.
- Olajuyigbe OO, Afolayan AJ. Phytochemical assessment and antioxidant activities of alcoholic and aqueous extracts of Acacia mearnsii de wild. Int J Pharmacol 2011; 7: 856-861.
- El Abed N, Kaabi B, Smaali MI, Chabbouh M, Habibi K, Mejri M, Marzouki MN, Ben H. Chemical composition, antioxidant and antimicrobial activities of Thymus capitata essential oil with its preservative effect against listeria monocytogenes inoculated in minced beef meat. J Evid Based Complementary Altern Med 2014.
- Pushparaj FS, Urooj A. Antioxidant activity in two pearl millet (Pennisetum typhoideum) cultivars as influenced by processing. Antioxidants 2014; 3: 55-66.
- Ablat A, Mohamad J, Awang K, Shilpi JA, Arya A. Evaluation of antidiabetic and antioxidant properties of Brucea javanica seed. Sci World J 2014.
- Ozcelik O, Lee JH, Min DB. Effects of light, oxygen and pH on the absorbance of 2, 2- diphenyl-1-picrylhydrazyl. J Food Sci 2003; 68: 487-490.
- Jin J, Li Z, Zhang F. Scavenging function of mulberry vinegar extractives for 1, 1- diphenyl-2-picrylhydrazyl (DPPH). J Northwest Sci-Tech Univ Agri Forestry 34: 135-137.
- Prabhune AM, Jadhav, SN, Kadam DA, Nandikar MD, Aparadh VT. Free radical scavenging (DPPH) and ferric reducing ability (FRAP) of some commelinaceae members. IJBPAS 2013; 2: 1128-1134.
- Hatano T. Constituents of natural medicines with screening effects on active oxygen species-Tannins and related polyphenols. Nat Med 1995; 49: 357-363.
- Ranneh Y, Ali F, Fadel A. The antioxidant activity of cocoa polyphenolic extract-treated 3t3 fibroblast cells. Int J Toxicol Pharmacol Res 2014; 6: 14-19.