- Biomedical Research (2015) Volume 26, Issue 4
HPLC determination of oleanolic acid content in Hedyotis diffusa Willd. and its anti-HepG-2 cell activity
Lin Sun, Jueshi Liu and Hua Xiang*Interventional Vascular Surgery,Hunan Provincial People?s Hospital, Changsha 410005, China
- *Corresponding Author:
- Hua Xiang
Interventional Vascular Surgery
Hunan Provincial People?s Hospital Changsha 410005, China
Accepted date: July 07 2015
Abstract
Objective To establish the method for quantitative determination of oleanolic acid in Hedyotis diffusa Willd., and study its inhibitory effect on human hepatoma HepG-2 cells. Methods HPLC with a column of Angilent ZORBAX Eclips Plus C18 (4.6 × 250 mm, 5 um) is used; column temperature: 20?. Detection wavelength: 210nm. Anti-hepatoma activity of Hedyotis diffusa Willd. is analyzed by observing changes in cell morphology under inverted microscope and by MTT assay. Results Oleanolic acid shows a good linearity within an 11.06-154.84 ug range; its recovery is 98.51%, with a RSD of 1.18% (n = 6). Under inverted microscope, cells in the control group are grown adherently, with intact membranes. Growth density of cells in the Hedyotis diffusa Willd. test groups becomes gradually low with increasing drug concentration. Cell surface is wrinkled. In the high concentration group, most cells are disrupted, cell morphology is not intact, and number of adherent cells is reduced. MTT assay results show that the viability of HepG2 cells decreases with increasing concentration of Hedyotis diffusa Willd.; inhibition rate reaches 52.67% in the high dose group after treating HepG2 cells for 48 h. HepG2 cell inhibition rate exhibits rather obvious dose-response relationship. Conclusion HPLC method is accurate and reliable for the determination of oleanolic acid content in Hedyotis diffusa Willd.; Hedyotis diffusa Willd. can effectively inhibit the proliferation of human hepatoma HepG-2 cells.
Keywords
Hedyotis diffusa Willd.; human hepatoma HepG-2 cell; MTT assay
Baihua Sheshecao is the whole plant of Hedyotis diffusa Willd. in the genus Hedyotis of the family Rubiaceae, which is also known as Sheshecao, Heshecao. Jiejiejieruicao, etc. The herb is distributed mainly in China's Jiangsu, Zhejiang, Guangdong, Guangxi, Anhui, Yunnan, Fujian, Hunan and Hubei provinces [1], which is harvested mainly in Summer and Autumn. Its whole plant is used as medicine.
Scholars at home and abroad have made extensive studies on chemical constituents of Hedyotis diffusa Willd., and confirmed that its major chemical constituents are anthraquinones [2], terpenoids, flavonoids [3], sterols, organic acids, polysaccharides, alkaloids, etc.; besides, the herb also contains some trace elements, amino acids and volatile components [4,5].
Pharmacological studies have shown that Hedyotis diffusa Willd. has anti-tumor [6,7], anti-microbial & antiinflammatory [8], immunomodulatory & anti-oxidant [9], anti-aging, hepatoprotective and choleretic effects [10-12]. Because of repeated successful treatment of abdominal tumors with Hedyotis diffusa Willd. in folk medicine, researchers have conducted in-depth study on its antitumor activity. Studies have shown that total flavonoids, total polysaccharides and triterpenoids in Hedyotis diffusa Willd. have marked tumor inhibitory activities.
According to statistics, there are not many patent preparations containing Hedyotis diffusa Willd.; China Food and Drug Administration website lists only seven companies having Hedyotis diffusa Willd. injections, showing great development potential of the herb. Hedyotis diffusa Willd. preparations should be developed in multifaceted, multiform way, in order to meet the increasing clinical demand.
Materials
Instruments and reagents
Agilent 1290 Infinity LC system with UV detector. FBS (HYCLONE); MTT reagent (Sigma). ECLIPSE TE2000- U inverted microscope (Nikon, Japan); CO2 incubator (NBS, USA).
Herb and cell
Hedyotis diffusa Willd. was purchased from the medicine market in Anguo, which was identified as Hedyotis diffusa Willd. Hepatoma HepG-2 cells were purchased from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Methods
Chromatographic conditions
Column: Angilent ZORBAX Eclips Plus C18 (4.6 × 250 mm, 5 um), column temperature: 20°C. Detection wavelength: 210 nm. Injection volume: 5 ul (reference), 5 ul (sample). Number of theoretical plates of column: not less than 5,000 calculated based on the peak of oleanolic acid.
Selection of detection wavelength
Oleanolic acid reference solution was spectrally scanned within a 200-300 nm range, and maximum absorption occurred at 206 nm. Taking into account the end absorption, detection wavelength was set as 210 nm.
Linearity range
Preparation of test solution
Crude drug was pulverized into a coarse powder, and passed through a 60-mesh sieve, 2 g of which was then accurately weighed, and Soxhlet extracted with ethyl ether for 5 h. After the solvent was removed to dryness, the residue was extracted with 20 mL of petroleum ether twice. Afterwards, petroleum ether layer was discarded, and the residue was evaporated to dryness, diluted to the mark with methanol, and ultrafiltered to give the test solution.
Linearity range
Oleanolic acid reference substance which was dried to constant weight at 105°C was accurately weighed, and prepared into a 110.6 ug·mL-1 reference solution. 1, 2, 4, 6, 8, 10, 12 and 14 uL of the reference solution were precisely drawn, and injected into the chromatography system, respectively, and peak areas were measured under the above chromatographic conditions. Regression analysis was performed by peak area integral versus injection volume, and regression equation was obtained as: Y = 50158x + 2348.1, r = 0.9995. Linearity range was 1.266ug·mL-1~7.596ug·mL-1. The results revealed that the oleanolic acid had a good linearity within an 11.06- 154.84 ug range.
Precision test
Oleanolic acid control solution was injected repeatedly 5 times for determination according to the above chromatographic conditions. RSD of oleanolic acid peak area was found to be 0.8%, indicating good precision of the instrument.
Stability test
The above test solution was injected at 1, 2, 4, 8, 16 and 24 h, respectively, and RSD was calculated to be 1.18%. The results showed that the test solution was stable within 24 h.
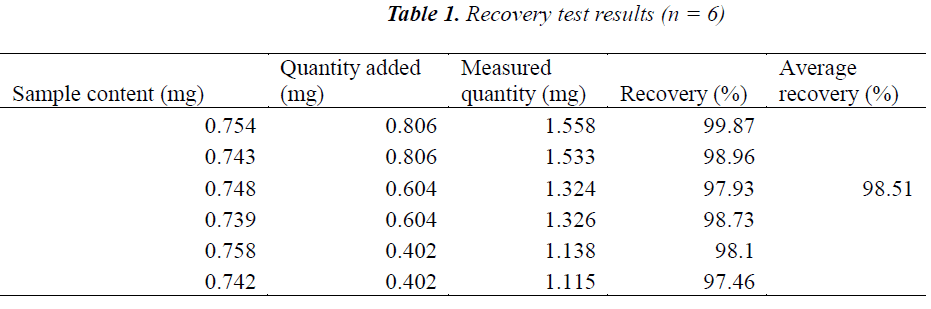
Recovery test
Six aliquots of 3 g of samples with known oleanolic acid content were accurately weighed, added separately with 0.806, 0.604 and 0.402 reference substances, prepared as per the above preparation method of test solution, and determined according to the above chromatographic conditions. The results showed that the average recovery of oleanolic acid was 98.51%, with a RSD of 1.18% (n = 6), indicating that the method was accurate. The results are shown in Table 1.
Reproducibility test
Five aliquots of 3 g of Hedyotis diffusa Willd. samples of the same batch produced in Zhejiang were determined according to the method described above, and oleanolic acid contents were calculated, respectively. The results showed that RSD was 0.8%, indicating good reproducibility of the above method.
Sample determination
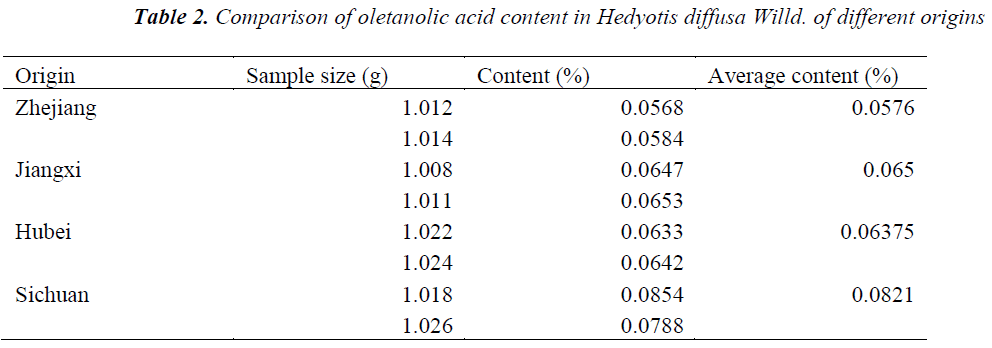
Hedyotis diffusa Willd. samples of different origins were accurately weighed, prepared as per the above preparation method of test solution, and quantitatively determined under the above chromatographic conditions, respectively, followed by calculation of oletanolic acid contents. The results are shown in Table 2.
Inhibitory effect of Hedyotis diffusa Willd. on hepatoma HepG-2 cell proliferation
Cell culturing
Hepatoma HepG-2 cell lines were cultured statically in a 37°C, 5% CO2 incubator with RPMI 1640 medium containing 10% FBS, penicillin (100m L/L) and streptomycin (1 mg/ml). Logarithmic phase cells were harvested for experiment.
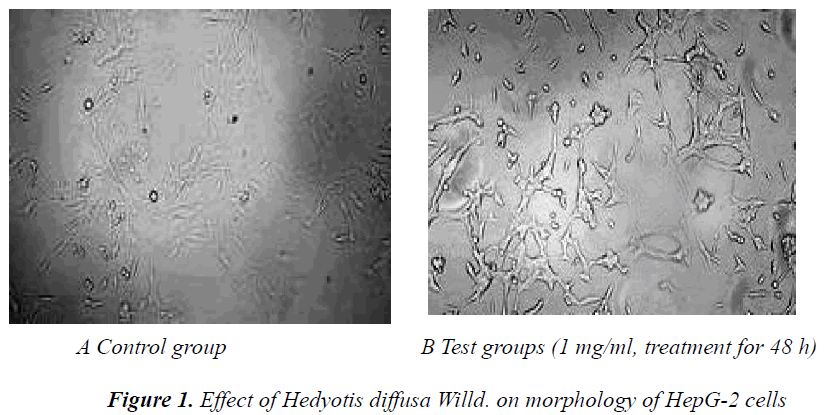
Effect of Hedyotis diffusa Willd. on cell morphology Logarithmic phase cells were seeded in 6-well plates at a 5Ã104/ml density for growth. After the HepG-2 cells were adherent, they were cultured with medium containing Hedyotis diffusa Willd. extract in a 37°C, 5% CO2 incubator for 48 h, followed by observation and photography under an inverted microscope.
Under inverted microscope, cells in the control group were grown adherently, with intact membranes. Growth density of cells in the Hedyotis diffusa Willd. test groups became gradually low with increasing drug concentration. Cell surface was wrinkled. In the high concentration group, most cells were disrupted, cell morphology was not intact, and number of adherent cells was reduced. The results are shown in Figure 1.
Inhibitory effect of Hedyotis diffusa Willd. on HepG-2 cell proliferation
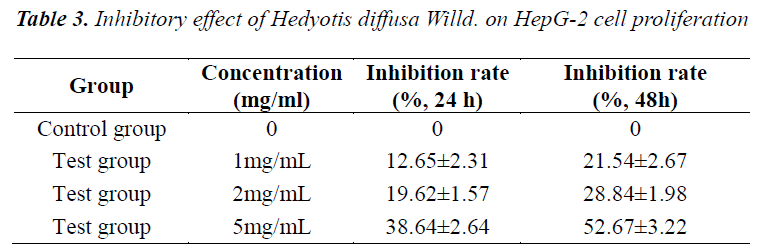
Actively growing exponential phase cells were digested with 0.25% trypsin, prepared into a 5Ã105/mL cell suspension, and seeded in 96-well plates at 1Ã104 cells per well. After the HepG-2 cells were treated with Hedyotis diffusa Willd. extracts (concentrations of 1 mg/mL, 2 mg/mL and 5 mg/mL) for 48 h, 20 µL of 5 mg/mL MTT solution was added to each well, and the culturing was continued for an additional 4 h. Then, supernatant was discarded, and each well was added with 150 µL of DMSO to terminate the reaction. Absorbance (A) of each well was measured at 570 nm using a microplate reader. The experiment was repeated three times, and growth inhibition rate was calculated.
MTT assay results showed that the viability of HepG2 cells decreased with increasing concentration of Hedyotis diffusa Willd.; inhibition rate reached 52.67% in the high dose group after treating HepG2 cells for 48 h. HepG2 cell inhibition rate exhibited rather obvious dose-response relationship. The results are shown in Table 3.
Discussion
Liver cancer, i.e. liver malignancy, can be divided into two categories: primary and secondary. Primary liver malignancy originates in the hepatic epithelial or mesenchymal tissues; the former is known as primary liver cancer, which is a greatly harmful malignancy with high incidence in China; while the latter is called sarcoma, which is relatively rare compared with primary liver cancer. Secondary or metastatic liver cancer refers to the invasion of malignancy of multi-organ origin into the liver, which is usually seen in liver metastases of malignancies from organs like stomach, biliary tract, pancreas, colorectum, ovary, uterus, lung and breast.
Etiology and exact molecular mechanisms of primary liver cancer are not fully understood yet. Currently, its incidence is believed to be a complex multifactorial, multistep process, which is impacted by environmental and hereditary factors. Epidemiological and experimental studies have shown that the incidence of liver cancer is associated with HBV and HCV infection, aflatoxin, water contamination, alcohol, cirrhosis, sex hormones, nitrosamines, trace elements, etc.
Liver cancer can be asymptomatic in early stage. Once obvious symptoms occur, about one-third are already in advanced stage. Patients will feel swelling pain in the liver area, especially after meals, and will have anorexia, hepatomegaly, right upper abdominal mass, unexplained weight loss, abdominal distension, diarrhea, intermittent fever, fatigue or loss of appetite.
According to the statistics of IARC, there are about 500,000 new cases of liver cancer worldwide every year, of which more than a half occurs in China; the proportion is depressing. Despite the advanced clinical diagnostic and treatment methods for liver cancer, over 60% of liver cancer patients, especially advanced stage patients, cannot get effective treatment each year, whose quality of life is poor, and life and health are seriously threatened.
In this study, method for the determination of oleanolic acid content in Hedyotis diffusa Willd. is established using HPLC with oleanolic acid as the reference. The results show good linearity within the experimental concentration range, as well as good stability and reliability of the method.
During the investigation of the inhibitory effect of Hedyotis diffusa Willd. on human hepatoma HepG2 cell proliferation, MTT assay and microscopy are used. Different concentrations of Hedyotis diffusa Willd. extracts have good inhibitory effects on proliferation of HepG2 cells, showing a dose-response relationship. Its specific mechanisms of inhibition will be confirmed gradually in future research.
References
- Zhang Y, Chen Y, Fan C, Ye W, Luo J. Two new iridoidglucosides from Hedyotisdiffusa.Fitoterapia 2010; 81: 515-517.
- Kang Xingdong, Li Xian, Mao Yu, Zhao CC, MengDL.Chemical constituents of HedyotisdiffusaWilld.Journal of Shenyang Pharmaceutical University 2007; 24: 479-481.
- Si JY, Chen Dihua, Pan Ruile, Zhao XH. Study on chemical constituents of HedyotisdiffusaWilld. Natural Product Research and Development 2006; 3: 942-946.
- Zhang YY, Luo JB. Studies on the Chemical Constituents in Herb of Hedyotisdiffusa. Journal of Chinese Medicinal Materials 2008; 31: 522-544.
- Cui J, Wang SC, Shi SS, Wang ZT .Structural Characterization of a Glucan Isolated from Hedyotisdiffusa Wild. J Chin Mater 2006; 29: 912-916.
- Hu L, Wang HQ, Cheng XY, ;Cui NJ, Hu CX, Li JG, Ye LP. Effect of HerbaHedyotisDiffusae on Expression of Heat Shock Protein 70 in Hepatoma Cell Line H22.Journal of Guangzhou University of Traditional Chinese Medicine 2007; 24: 44-46.
- Qu Y, Huang JM, Shan CG. The significance of immunohisto chemical testing p53 expression in nasopharyngeal lesions. Mod J IntegrTradit Chin West Med 2003; 11: 448-449.
- BianCaimiao. Study on the Anti-microbial Effect of the Oldenlandiadiffusa (Willd.) Roxb. Extract. Lishizhen Medicine and MateriaMedica Research 2005; 16: 991- 992.
- Li R, Zhao HR, Lin YN. Anti-tumor Effect and Protective Effect on Chemotherapeutic Damage of Water Soluble Extracts from Hedyotisdiffus. J Chin Pharm Sci 2002; 11: 54-58.
- Krajewska M, Fenoglio-preiser CM. Immunohis to chemical analysis of Bel-2 family proteins in adenocareinomas of the stomach. Am J Pathol 1996; 140: 1449-1452.
- Yang X, HE H, Yang W. Effects of HSP70 anti-sense oligonuclcotide on the proliferation and apoptosis of human hepatocellular carcinoma cells. J HuazhongUnivSciTechnolog Med Sci 2010; 30: 337-343.
- Schattenberg JM, Schuchmann M, Gallep LFPR. Cell death and hepatocarcinogenesis:Dysregulation of apoptosis signaling pathways. J GastroenterolHepatol 2011; 26 (Suppl l): 213-219.